Abstract
Background
Diet quality is a significant determinant in the etiology of breast cancer (BrCa), but further studies are required to explore this relationship. Therefore, we tried to assess if diet quality, assessed using the Diet Quality Index-International (DQI-I), was related to BrCa among the Iranian population.
Methods
In the present case-control research, 134 women with a recent diagnosis of BrCa and 267 without BrCa were selected as case and control groups. Individual food intake data from a food frequency questionnaire was used to compute DQI-I. Also, the multivariable logistic regression models were utilized to evaluate the association between DQI-I and BrCa odds .
Results
We found a significant association between the last tertile of DQI-I and BrCa odds in the fully adjusted model (odds ratio (OR) = 0.30; 95% confidence interval (CI): 0.15–0.56). The subgroup analysis based on menopausal status also showed a significant decrease in BrCa odds in pre-and post-menopausal women (pre-menopausal: OR = 0.27; 95% CI: 0.10–0.70 – post-menopausal status: OR = 0.35; 95% CI: 0.13–0.92).
Conclusions
Our findings indicated that a higher DQI-I score was related to a lower chance of BrCa. According to our research, a healthy diet pattern is crucial for BrCa prevention.
Similar content being viewed by others
Introduction
The most prevalent cancer in women is breast cancer (BrCa), which has a significant mortality and morbidity rate in this group [1]. Data from the International Agency for Research on Cancer (IARC) shows 2.3 million new BrCa cases from 185 countries in 2018 [2]. The reports indicated 16,967 new BrCa cases and 4,810 deaths related to the disease in 2020 in Iran [3]. Also, based on Iran’s cancer registry data from 2008 to 2016, it is predicted that the incidence of BrCa in women will increase by 63% by 2025 [4].
Diet is one of the modifiable factors related to most cancer types, consisting of BrCa [5]. According to several studies, some foods and nutrients are considered protective or potential risk factors for BrCa. High carbohydrates, red and processed meats, and saturated fats are related to a higher progression and risk of BrCa [6,7,8]. In contrast, diets rich in fiber, minerals, omega-3 polyunsaturated fatty acids (PUFAs), antioxidants, fruits, vegetables, and vitamins have protective effects [9]. Although specific dietary factors have been shown to affect BrCa [5, 9,10,11], dietary patterns demonstrating the possible interactions between nutrients and foods can better evaluate the relationship between diet and the risk of BrCa [12, 13].
The diet quality index-international (DQI-I) is one of the dietary indices based on international dietary guidelines to investigate nutritional balance (0–10 score), moderation (0–30 score), variety (0–20 score), and adequacy (0–40 score), and the range of total index score is from 0 to 100, where greater scores demonstrate better diet quality [14, 15]. Recently, studies have indicated an inverse association between the DQI-I and risk factors for cardiovascular disease and cancer mortality [16, 17].
Considering the high prevalence of BrCa in Iran and the type of dietary patterns associated with higher consumption of saturated fats, refined grains, and the high percentage intake of energy from carbohydrates in the Middle East population, more studies on the association between BrCa and diet quality are necessary [3, 18]. The present research is one of the first to examine the association between diet quality and BrCa in a case-control design in Iranian women.
Methods
Study design
In the present case-control study, 136 women aged above 30 years with a recent BrCa diagnosis (less than six months) were recruited as a case group. For the control group, we recruited 272 women with non-neoplastic disorders and no long-term diet change who were not alcohol abusers. Based on a previous study by Ching et al., the study sample size was calculated (odds ratio (OR) = 0.47, α = 0.05, and β = 0.20) [19]. Patients with acute and non-neoplastic diseases hospitalized in the same hospital as the case group were selected from other hospital departments as a control group. The control group conditions were traumas and orthopedic disease, problems of the skin, nose, eyes or ear, and acute surgical conditions. We recruited all the participants from two Shohadaye Tajrish and Imam Hossein hospitals in Tehran (both case and control groups were selected from these hospitals). The study was conducted between September 2015 and February 2016. The number of people who withdrew from participating in the study at the same interview stage was less than 8%. In the final analysis, seven participants (five controls and two cases) were excluded because their caloric intake was out of three standard deviations (SDs) from the mean population calorie intake. Also, women with special diets or hormone replacement therapy or who were pregnant and lactating were excluded from the study. Ultimately, we conducted the final analysis on 134 cases and 267 controls.
This study was approved by the National Nutrition and Food Technology Research Institute of Iran, and informed consent was taken from all the participants.
Data collection
Trained dietitians administered all measurements and questionnaires in the same interview. Also, a validated questionnaire was used to evaluate physical activity, and the results were reported as the metabolic equivalent of task-hours per day (MET-h/d) [20]. A non-stretchable tape meter fixed to a wall was used to measure their height (with an accuracy of 0.5 cm), and a digital scale with 0.1 kg accuracy was used to measure participants’ weight with minimum clothes and without shoes. Body mass index (BMI) was computed by dividing weight by the square of height (kg/m2). We also utilized a checklist to gather participants’ clinical information, lifestyle, and socio-demographic-economic including disease history (yes/no), bra wearing during the day and night (yes/no), age (year), marriage time (year), menopausal status (pre-and post-menopause), abortion history (yes/no), smoking history (yes/no), breastfeeding time (month), and taking medications and supplements (yes/no).
Dietary intake assessment
To assess participants’ food intake one year before diagnosis in the case group or hospitalization for the control group, a valid and reliable semi-quantitative food frequency questionnaire (FFQ) (168 items) was applied [21]. Participants were requested to indicate the frequency (daily, weekly, monthly, or yearly) of consuming each food item. Their consumption was then converted to frequency per day, and using the handbook for household measures; intake frequencies were converted to grams per day [22]. The composition table of the United States Department of Agriculture (USDA) food [23] was utilized to compute most foods’ calorie and nutrient content. We used the composition table of Iranian foods for the traditional Iranian foods that were not in the USDA database [24].
Diet quality assessment
We used DQI-I, consisting of four major dietary indicators, for diet quality assessment. The dietary components of DQI are as follows:
-
1.
Food variety with two components including various types of food groups (legumes and legumes products, eggs, meats and products of meat, vegetables, fruits, grains, milk, and dairy products) and the types of protein sources within the group (legumes and their products, milk, meats, eggs), which has a score between 0 and 20.
-
2.
Adequacy of foods used to assess protein, grains, fruits, vegetables, fiber, calcium, vitamin C, and ferric scored from 0 to 40 points.
-
3.
Moderation of diet (cholesterol, saturated fat, total fat, empty calorie foods, and sodium), with a score between 0 and 30 points.
-
4.
Diet balance (ratio of macronutrients and fatty acids), with a score of 0 to 10. The sum of the four above components is equal to the total score of DQI-I, which ranges from 0 to 100 (0 = the lowest and 100 = the highest dietary quality) [25].
Statistical analysis
We used the chi-square test for categorical variables and Mann-Whitney or independent samples T-test for continuous variables to ascertain the general characteristics difference between the case and control groups. Values are percentage and median (25th, 75th percentiles) or mean ± SD for categorical and continuous variables, respectively. Based on participants’ scores on DQI-I, we assigned them to tertiles. Analysis of variance (ANOVA) or Kruskal-Wallis were used to assess the nutrient intake across the tertiles of DQI-I. To evaluate the association between DQI-I and the odds of BrCa, the multivariable logistic regression models across the tertiles were applied. Variables like age, BMI, family history of cancer, family history of BrCa, energy, physical activity, taking vitamin D supplements, and menopausal status were adjusted to eliminate confounding effects (enter method). To carry out statistical analyses, SPSS (version 26.0, IBM Co., Chicago, IL) was used in the present study. We considered a p-value less than 0.05 as the significance level.
Results
As shown in Table 1, age, abortion history, family history of cancer, adequacy score, moderation score, DQI-I total score, wearing a bra during the day, and taking vitamin D supplements significantly differed between the two groups (P<0.05).
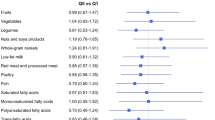
According to Table 2, monounsaturated fatty acids (MUFAs) (P = 0.010), PUFAs (P = 0.022), fruits (P = 0.019), vegetables (P = 0.031), and low-fat dairy (P = 0.025) intakes were significantly different between the case and control groups (the intake between the tertiles of case and control are shown in Supplementary Tables 1 and 2).
As shown in Table 3, there was a significant negative relationship between DQI-I and BrCa odds in both Model 1 (OR = 0.29; 95% confidence interval (CI): 0.16–0.51) and Model 2 (OR = 0.30; 95% CI: 0.15–0.56).
The subgroup analysis based on menopausal status is shown in Table 4. As presented in the table, in the Model 1, we observed a significant reduction in the odds of BrCa in pre-and post-menopausal participants (pre-menopausal: OR = 0.21; 95% CI: 0.08–0.50 – post-menopausal status: OR = 0.35; 95% CI: 0.16–0.76). In addition, after adjusting for many potential confounders, this association remained significant (pre-menopausal: OR = 0.27; 95% CI: 0.10–0.70 – post-menopausal status: OR = 0.35; 95% CI: 0.13–0.92).
Discussion
According to our findings, higher DQI-I scores were related to a lower odds of BrCa. Furthermore, based on the fully adjusted model, the association remained strengthened, which shows that this relationship is robust. Also, after splitting according to menopausal status, a decreased risk of BrCa was observed with an increase in DQI-I score in post-menopausal and pre-menopausal women, with a greater reduction in pre-menopausal women.
Our literature review reveals that the current research is among the first to assess an association between DQI-I and the odds of BrCa. The present study’s findings are consistent with previous studies assessing the possible association between DQI and cancer risks. Park et al., Vulcan et al., and Vargas et al. demonstrated that higher diet quality scores are associated with a reduced risk of colorectal cancer [26,27,28]. In another study, Wang et al. found that more adherence to high diet quality predicted a lower risk of nasopharyngeal carcinoma [29]. Sohouli et al. demonstrated no association between any particular DQI-I quartile and BrCa. However, the trend was substantial across all quartile categories [30]. However, our results showed that people in the highest tertile compared to the lowest tertile of DQI-I scores had a significantly lower odds of BrCa. Inconsistent with our results, Godoy et al. reported no association between the DQI and its components with BrCa risk [31].
When women were stratified by menopausal status, higher DQI-I scores were related to a decreased risk of BrCa with a more decrease in pre-menopausal women. In fact, the findings showed that in higher DQI-I, the odds of BrCa in pre-and post-menopausal women decreases by 73% and 65%, respectively. Due to the limitation of studies on the relationship between DQI-I score and BrCa, the results of other dietary quality indices were reviewed. A prospective cohort study observed an inverse association between higher diet quality scores (alternate Mediterranean diet, DASH, and alternative healthy eating index–2010) and BrCa incidence in post-menopausal women. However, no association was observed between diet quality indices and BrCa incidence in pre-menopausal women [32]. In a systematic review, the association between diet quality scores and the risk of post-menopausal BrCa was inverse [33]. A case-control study revealed that pre-menopausal women had a decreased risk of BrCa in the highest quartile of the healthy pattern. In contrast, post-menopausal women’s diet patterns did not differ significantly from each other [34].
Menopause is not a cause of BrCa, but with ageing, the risk of developing women-related cancers such as ovarian, uterine, and breast increases [35]. As an unmodifiable risk factor, age leads to cellular and molecular changes in normal breast tissue that can lead to malignant transformation [36]. Two-thirds of newly diagnosed women with BrCa are post-menopausal, age 55 and older [37]. In the current study, the case group was older than the control group.
DQI-I is a diet quality indicator that assesses four diet quality components: diversity, adequacy, moderation, and balance [14]. A higher DQI-I score is related to a healthier dietary pattern containing bioactive substances and various vitamins and minerals that can reduce the risk of BrCa [29]. In this study, the higher total DQI-I score and the higher score of the adequacy and moderation components in the control group compared to the cases were consistent with our expectations.
Vitamin D plays a significant role in differentiation and subsequently influences the function and development of the mammary gland [38]. Vitamin D deficiency is related to the pathogenesis of different types of cancers like BrCa [39]. Although the results of studies on the dose of vitamin D and the risk of BrCa are conflicting, according to a dose-response meta-analysis, an increasing inverse relationship was observed above the threshold of 27 ng/mL in post-menopausal women but not in pre-menopausal. This effect reached a steady state in doses above 35 ng/mL [40]. In a randomized clinical trial, supplementation with calcium and vitamin D (400 IU vitamin D with 1 gr Ca /day) in post-menopausal women caused a significant decrease in the risk of both breast and invasive breast cancers by 14-20% [41]. The results of our study also revealed that the case group used vitamin D supplements less frequently than the control group. Due to the nature of the study design, there was no control over the subjects’ vitamin D intake.
Using logistic regression models with probable confounder adjustments is one of the study’s strengths. In addition, the validated FFQ represents the most types of foods that our study subjects consumed. To our knowledge, this is one of the first studies to investigate the association between DQI-I and BrCa. Nevertheless, there are several limitations that should be noted. The main limitation of the current study is its retrospective and case-control design, which makes it impossible to determine causality. Because of using FFQ, recall bias is probable. Also, using self-report methods can lead to over-or under-reporting. However, to minimize these problems, skilled and trained staff were employed to conduct the interviews.
Conclusions
In conclusion, the present study illustrated that a higher DQI-I score was related to a lower chance of BrCa. Also, a decreased odds of BrCa was found with an increase in DQI-I score in both post-and pre-menopausal women, with a higher decrease in pre-menopausal women. According to our research, a healthy diet pattern is crucial for BrCa prevention. Further investigations with different designs, especially prospective cohort studies, are required to support these results.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2021;124(1):13–26.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424.
Haghighat S, Omidi Z, Ghanbari-Motlagh A. Trend of breast Cancer incidence in Iran during a fifteen-year interval according to National Cancer Registry Reports. Iran J Breast Dis. 2022;15(2):4–17.
Roshandel G, Ferlay J, Ghanbari-Motlagh A, Partovipour E, Salavati F, Aryan K, Mohammadi G, Khoshaabi M, Sadjadi A, Davanlou M. Cancer in Iran 2008 to 2025: recent incidence trends and short‐term predictions of the future burden. Int J Cancer. 2021;149(3):594–605.
Vostakolaei FA, Broeders MJ, Mousavi SM, Kiemeney LA, Verbeek AL. The effect of demographic and lifestyle changes on the burden of breast cancer in iranian women: a projection to 2030. The Breast. 2013;22(3):277–81.
Kallianpur AR, Lee S-A, Gao Y-T, Lu W, Zheng Y, Ruan Z-X, Dai Q, Gu K, Shu X-O, Zheng W. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai breast Cancer Study. Breast Cancer Res Treat. 2008;107:123–32.
Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Premenopausal dietary fat in relation to pre-and post-menopausal breast cancer. Breast Cancer Res Treat. 2014;145:255–65.
Farvid MS, Stern MC, Norat T, Sasazuki S, Vineis P, Weijenberg MP, Wolk A, Wu K, Stewart BW, Cho E. Consumption of red and processed meat and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Int J Cancer. 2018;143(11):2787–99.
Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, Xepapadakis G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur J Nutr. 2018;57:2133–45.
Mattisson I, Wirfält E, Wallström P, Gullberg B, Olsson H, Berglund G. High fat and alcohol intakes are risk factors of postmenopausal breast cancer: a prospective study from the Malmö diet and cancer cohort. Int J Cancer. 2004;110(4):589–97.
Sieri S, Chiodini P, Agnoli C, Pala V, Berrino F, Trichopoulou A, Benetou V, Vasilopoulou E, Sánchez M-J, Chirlaque M-D. Dietary fat intake and development of specific breast cancer subtypes. JNCI: J Natl Cancer Inst 2014, 106(5).
Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20(2):125–38.
Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr. 2010;91(5):1294–302.
Guerrero MLP, Pérez-Rodríguez F, Hueda M. Diet quality indices for nutrition assessment: types and applications. Funct Food-Improve Health through Adequate Food. 2017;1:283–308.
Alipour Nosrani E, Majd M, Bazshahi E, Mohtashaminia F, Moosavi H, Ramezani R, Shahinfar H, Djafari F, Shab-Bidar S, Djazayery A. The association between meal-based diet quality index-international (DQI-I) with obesity in adults. BMC Nutr. 2022;8(1):1–11.
Lassale C, Gunter MJ, Romaguera D, Peelen LM, Van der Schouw YT, Beulens JW, Freisling H, Muller DC, Ferrari P, Huybrechts I. Diet quality scores and prediction of all-cause, cardiovascular and cancer mortality in a pan-european cohort study. PLoS ONE. 2016;11(7):e0159025.
Alkerwi Aa, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hébert JR. Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the Observation of Cardiovascular Risk factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr. 2015;113(2):259–69.
Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among iranian women. J Nutr. 2008;138(2):358–63.
Ching S, Ingram D, Hahnel R, Beilby J, Rossi E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr. 2002;132(2):303–6.
Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196–202.
Asghari G, Rezazadeh A, Hosseini-Esfahani F, Mehrabi Y, Mirmiran P, Azizi F. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran lipid and glucose study. Br J Nutr. 2012;108(6):1109–17.
Ghafarpour M, Houshiar-Rad A, Kianfar H, Ghaffarpour M. The manual for household measures, cooking yields factors and edible portion of food. In.: Tehran: Keshavarzi Press; 1999.
Haytowitz D, Lemar L, Pehrsson P, Exler J, Patterson K, Thomas R, Nickle M, Williams J, Showell B, Khan M. USDA national nutrient database for standard reference, release 24. US Department of Agriculture: Washington, DC, USA 2011.
Azar M, Sarkisian E. Food composition table of Iran. Tehran: National Nutrition and Food Research Institute, Shaheed Beheshti University; 1980.
Kim S, Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133(11):3476–84.
Park S-Y, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L. High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the multiethnic cohort. Gastroenterology. 2017;153(2):386–94. e382.
Vulcan A, Ericson U, Manjer J, Ohlsson B. A colorectal cancer diet quality index is inversely associated with colorectal cancer in the Malmö diet and cancer study. Eur J Cancer Prev. 2019;28(6):463–71.
Vargas AJ, Neuhouser ML, George SM, Thomson CA, Ho GY, Rohan TE, Kato I, Nassir R, Hou L, Manson JE. Diet quality and colorectal cancer risk in the women’s Health Initiative Observational Study. Am J Epidemiol. 2016;184(1):23–32.
Wang C, Lin X-L, Fan Y-Y, Liu Y-T, Zhang X-L, Lu Y-K, Xu C-H, Chen Y-M. Diet quality scores and risk of nasopharyngeal carcinoma in chinese adults: a case-control study. Nutrients. 2016;8(3):112.
Sohouli MH, Buckland G, Clark CC, Santos HO, Athayde FL, Sanati V, Janani L, Sajadian AS, Zarrati M. The relationship between diet quality indices and odds of breast cancer in women: a case–control study. BMC Womens Health. 2023;23(1):1–9.
Godoy LMd, Pinheiro MA, Godinho-Mota JCM, Vaz-Gonçalves L, Schincaglia RM, Martins KA. Souza LBd: Diet quality index and its components have not associated with the development of breast cancer risk assessed by the diet quality index: a case-control study. Revista Brasileira de Epidemiologia 2022, 25.
Haridass V, Ziogas A, Neuhausen SL, Anton-Culver H, Odegaard AO. Diet quality scores inversely associated with postmenopausal breast cancer risk are not associated with premenopausal breast cancer risk in the California teachers study. J Nutr. 2018;148(11):1830–7.
Potter J, Brown L, Williams RL, Byles J, Collins CE. Diet quality and cancer outcomes in adults: a systematic review of epidemiological studies. Int J Mol Sci. 2016;17(7):1052.
Sasanfar B, Toorang F, Nemati S, Mohebbi E, Azadbakht L, Zendehdel K. Dietary patterns and risk of breast Cancer among pre and post-menopausal women: a case-control study in Iran. J Nutr Food Secur 2022.
Surakasula A, Nagarjunapu GC, Raghavaiah K. A comparative study of pre-and post-menopausal breast cancer: risk factors, presentation, characteristics and management. J Res Pharm Pract. 2014;3(1):12.
Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol/Hematol. 2008;66(1):65–74.
Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92.
Atoum M, Alzoughool F. Vitamin D and breast cancer: latest evidence and future steps. Breast cancer: basic and clinical research. 2017;11:1178223417749816.
Gabryanczyk A, Klimczak S, Szymczak-Pajor I, Śliwińska A. Is vitamin D deficiency related to increased cancer risk in patients with type 2 diabetes mellitus? Int J Mol Sci. 2021;22(12):6444.
Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine. 2013;92(3):123.
Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94(4):1144–9.
Acknowledgements
We sincerely thank all field investigators, staff, and participants of the present study.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
F.P.Z, M.A.K, S.E, F.M, and M.N.; Contributed to writing the first draft. S.J and Z.H; Contributed to data collection; M.V, B.R, and Z.S; Contributed to all data and statistical analysis, and interpretation of data. M.N and B.R.; Contributed to the research concept, supervised the work, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Sciences. Informed consent was obtained from all subjects and their legal guardian(s).
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pourhabibi-Zarandi, F., Kahrizsangi, M.A., Eskandarzadeh, S. et al. Dietary quality index and the risk of breast cancer: a case-control study. BMC Women's Health 23, 469 (2023). https://doi.org/10.1186/s12905-023-02588-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02588-6