Abstract
Croton macrostachyus is an important plant in traditional African medicine, widely utilized to treat a variety of diseases. In Kenya, HIV-infected patients use leaf and root decoctions of the plant as a cure for cough, back pain, bleeding, skin diseases, warts, pneumonia, and wounds. This study aimed to evaluate the anti-HIV activities and cytotoxic effects of extracts and chemical constituents isolated from C. macrostachyus. In our previous study we demonstrated that the hexane, CH2Cl2, ethyl acetate and methanol soluble fractions of a 1:1 v/v/ CH2Cl2/MeOH crude extracts of the leaves and stem bark of C. macrostachyus exhibited potent anti-HIV activities against HIV-1 with IC50 values ranging from 0.02–8.1 μg/mL and cytotoxicity effects against MT-4 cells ranging from IC50 = 0.58–174 μg/mL. Hence, hexane soluble extract of 1:1 v/v/ CH2Cl2/MeOH crude extract of the leaves of C. macrostachyus, that was more potent against HIV-1 at IC50 = 0.02 μg/mL was subjected to column chromatography leading to the isolation of 2-methoxy benzyl benzoate (1), lupenone (2), lupeol acetate (3), betulin (4), lupeol (5), sitosterol (6) and stigmasterol (7). Lupenone (2), lupeol acetate (3) and betulin (4) exhibited anti-HIV-1 inhibition at IC50 = 4.7 nM, 4.3 and 4.5 μg/mL respectively. The results obtained from this study support the potential of C. macrostachyus, as a source of anti-HIV constituents.
Similar content being viewed by others
Introduction
Since the beginning of the pandemic caused by the human immunodeficiency virus (HIV), more than 75 million people all over the world have become infected with the virus [1]. HIV remains a significant threat to public health around the world, as evidenced by the fact that it has resulted in the loss of 36.3 million lives [1]. The number of people that were infected with the virus in 2019 was close to 2 million [2]. More than 300,000 people died as a result of the outbreak in southern and eastern Africa in the same year that the global death toll caused by the virus reached more than half a million. There were 680,000 deaths attributed to HIV-related causes in the year 2020, while there were also 1.5 million new HIV infections. According to data collected from around the world, the number of individuals living with HIV is expected to reach 37.7 million by the end of the year 2020. More than two thirds of these people (25.4 million) reside in the WHO African Region [1].
After the discovery of zidovudine in 1987 [3, 4] there have been significant developments in the field of antiretroviral therapy (ART). Zidovudine was given as a monotherapy to patients with advanced, symptomatic disease at a dose of five times a day at that time. It wasn’t until the middle of 1996 that researchers realized that using three or more antiretroviral medications at the same time had significantly better results [4]. This combination therapy (comprising at least three ARV agents) has shown a significant decline in viral replication and improvement in patients’ quality of life [5]. Although ART has contributed significantly to reducing death due to HIV, toxicities of the drugs, long-term effects due to lifelong therapy, and drug resistance are challenging problems for the success of HIV care and treatment [6]. HIV/AIDS has a detrimental economic, psychosocial, and health-related impact on countries, households, and individuals around the world. As the number of people infected with HIV rises, medical treatment costs rise, and national resources shift to the HIV/AIDS program [7].
Furthermore, because HIV-infected individuals are susceptible to a variety of opportunistic infections (OI), which can reduce their productivity and shorten their lives, co-administration of other drugs to treat OI might result in drug interactions that can impair ARV therapy or induce severe side effects [8]. The development of drug resistance has also posed a great challenge for treating clients since many cases show that patients do not respond, even to second-line regimens. Moreover, this problem continues to be a danger to the worldwide struggle to end the epidemic by 2030 [9]. Hence, there is a need for the discovery of novel drugs. One of the systematic approaches to tackle these challenges is to discover new drugs by identifying bioactive antiretroviral compounds from natural products. Currently, Croton species have gained attention in providing different bioactive phytochemicals used to treat bacterial and viral infections [10], and many Croton plants are used in ethnomedicine. This study evaluated the antiretroviral activity of C. macrostachyus using in vitro approach.
C. macrostachyus is a medium-sized tall tree or shrub that grows up to 30 mt [11, 12]. It is commonly known as a “broad-leaved Croton” or “rush foil.” It has various local/vernacular names- in Africa, including “bisana” in Ethiopia, “msinduzi, mutundu” in Kenya [12,13,14,15,16,17]. C. macrostachyus has a wide distribution in Africa, wherein in Kenya, it is found in the Karura Forest and many areas with significant rainfall [18]. C. macrostachyus is used as a remedy for a variety of illnesses. The plant possesses various medicinal properties [19] and treats constipation in Ethiopia, Cameroon, Rwanda, Kenya, Tanzania, Somalia, and Uganda. Usually, the decoction, macerated leaf, stem bark, or root is used [20,21,22]. In Kenya, leaf and root decoction is used by many patients, including HIV-infected patients, as a cure for cough, back pain, bleeding, skin diseases, warts, pneumonia, and wounds [22,23,24,25,26]. In Kenya, C. macrostachyus bark juice, leaf, and root decoction are used as a remedy for backache, bleeding, cancer, colds, cough, diarrhea, dysmenorrhoea, east coast fever, malaria, measles, obesity, pneumonia, ringworm, skin diseases, typhoid, warts, and wounds [27,28,29]. The leaves of C. macrostachyus are used by farmers in Kenya as biological pest control when mixed with tobacco (Nicotiana tobacuum L.) and boiled overnight [11]. In addition, roots of Cucumis ficifolius are often used in combination with C. macrostachyus bark as a remedy for abdominal and stomach pain [30]. Similarly, Allium sativum is given with C. macrostachyus to treat malaria [28, 29].
Antibacterial effects of C. macrostachyus against N. gonorrhea [31], B. cereus, E. coli and P. aeruginosa [32], and S. pyogenes [33] have been reported. In a related study, Obey et al., [25] reported that the ethyl acetate extract of stem bark of C. macrostachyus has good antibacterial activity against E. coli, S. typhi, K. pneumoniae, E. aerogenes, and L. monocytogenes. Taye et al., [33] demonstrated the antibacterial activity of methanol leaf extract against Streptococcus pyogenes with a minimum bacterial concentration (MBC) value of 7.81 mg/mL. The antimycobacterial activity of C. macrostachyus in an in vitro experimental study was reported by [34]. Semenya and Maroyi [35], also demonstrated the antimycobacterial activity of methanolic leaf extracts of C. macrostachyus with minimum inhibitory concentration (MIC) values ranging from 12.5 to 100 𝜇g/mL. This study demonstrated that C. macrostachyus has potential as an herbal medicine in the treatment and management of tuberculosis, a leading cause of death in sub-Saharan Africa [35]. Antimicrobial and antifungal effects of C. macrostachyus extracts have previously been reported [36, 37]. The isolated diterpenoid 12-oxo-Hardwick acid has efficacy against Candida albicans [38]. Ngo Bum et al [39] reported that decoctions of C. macrostachyus possess anticonvulsant effects [40]. The antimalarial efficacy of leaf and stem bark extracts of C. macrostachyus has been reported [41, 42]. Bantie et al [43] demonstrated a chemoprotective effect against malaria. The anthelmintic efficacy of seed extracts of C. macrostachyus has been reported by [44]. Kamanyi et al [45] demonstrated that extracts of stem bark of C. macrostachyus exhibited anti-inflammatory activity in experimental mouse models of inflammation [45]. Similar findings were also reported by Nguelefack et al., (2015) [46]. Methanol leaf extract of C. macrostachyus showed antioxidant activity with an IC50 value of 0.11 mg/ml. The documented antioxidant activities of C. macrostachyus leaf extracts were probably due to flavonoids and phenols that have been isolated from fruits, leaves, and roots [44, 47, 48]. Flavonoids and phenolic compounds found in plants have antioxidant properties [49]. In our previous study we demonstrated that the hexane, CH2Cl2, ethyl acetate and methanol soluble fractions of a 1:1 v/v/ CH2Cl2/MeOH crude extracts of the leaves and stem bark of C. macrostachyus exhibited potent anti-HIV activities against HIV-1 with IC50 values ranging from 0.02–8.1 μg/mL and cytotoxicity effects against MT-4 cells ranging from IC50 = 0.58–174 μg/mL [50]. The aim of this study was to isolate bioactive pure compounds from the hexane soluble extract of the leaves of C. macrostachyus, which was more potent against HIV-1 at IC50 = 0.02 μg/mL and analyze the cytotoxicity and anti-HIV activities of isolated pure compounds.
Materials and methods
General
Extraction and column chromatography (CC) was performed at the Pharmacology and Pharmacognosy department, United States International University, Kenya. Commercial silica gel (100–200, 200–300, and 300–400 mesh; Qingdao, China) was used for CC. Sephadex LH-20 (Amersham Biosciences) was also used for CC. All solvents used for CC were of analytical grade (Shanghai Chemical Reagents Co., Ltd.). CC was performed on polyamide columns (5 × 60 cm, 200 g) (Germany GmbH) over silica gel (Kieselgel 60 GF254, 15 μm, Merck, Germany). While Thin Layer Chromatography (TLC) was carried out on Kieselgel 60 F254 (Merck). Spots on UV active silica gel were detected under UV light (245 and 336 nm) and made visible using a concentrated sulphuric-anisaldehyde spray mixture and heating at 105 °C for 2 minutes. 1D and 2D NMR spectra were recorded in CDCl3 on a 400 MHz Bruker AVANCE NMR instrument at room temperature. Chemical shifts (δ) are expressed in ppm and were referenced against the solvent resonances at δH 7.26 and δC 77.23 ppm for 1H and 13C NMR for CDCl3. Structural assignments of the new compounds were made with additional information from 1H-1H COSY, HSQC, NOESY, and HMBC experiments. Mass spectra were recorded on a GC-MS Bruker MicroToF Mass Spectrometer by direct injection using a Bruker Bioapex-FTMS with electrospray ionization. The above analysis was performed at the Jodrell Laboratory, Royal Botanic Gardens Kew (UK).
Plant material
The leaves of C. macrostachyus were collected from a USIU botanical garden in June 2020. The collection of the medicinal plant was performed after obtaining the required ethical approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UON ERC), approval number KNH/ERC/A/154. Taxonomic identification was done by Ms. Lucy Wambui (botanist) and voucher specimen TEREFE E. /045 was deposited for C. macrostachyus at the United States International University herbarium for future reference. The leaves were thoroughly washed and dried in the shade. Then, the dried leaves were pulverized using a mortar and pestle at the Medicinal Plants Laboratory of United States International University-Africa (USIU-A). The leaves were ground to a fine powder using a hammer mill.
Extraction and isolation
The powdered plant leaves were extracted with 1:1 v/v dichloromethane: methanol solvent using the cold maceration technique. Maceration was done for 7 days with frequent agitation in an orbital shaker, and the extract was filtered. Extraction was repeated three times, and the filtrates of all portions were pooled. Finally, the extracts were concentrated using rotavapor at 30 °C to obtain dry extracts. The extract was weighed and packed in a glass vial and stored in a desiccator over silica gel until use.
The dried crude mass 1:1 v/v methanol:CH2Cl2 extract of the leaves of C. macrostachyus was dissolved in distilled water (200 mL) and successively partitioned using different solvents of increasing polarity (n-hexane, dichloromethane, ethyl acetate, and methanol) in separatory funnels (Fig. 1). The different solvent fractions were concentrated under reduced pressure using a rotary evaporator, and the resulting product was dried in an oven at 30 °C. The dried fractions were then transferred into separate vials and stored in a desiccator for further use. Bioassay-guided fractionation was performed on the partitions to determine the highest antiretroviral activity. Then, guided by their antiretroviral activity, the hexane fraction that showed the highest antiviral activity was partitioned using column chromatography, using 60–120 mesh silica gel, and eluted successively with varying concentrations of ethyl acetate and n-hexane (E:H). Fractions that were similar in TLC were pooled together. Each fraction was then evaluated for antiretroviral activity, and the fraction with the highest activity was further subjected to open column chromatography on 200–400 mesh silica gel. The obtain pure fractions, where necessary the fraction were subjected to column chromatography on Sephadex LH-20 as a stationary phase and methanol as the mobile phase. The eluents were monitored by thin-layer chromatography. The purity of the compounds were determined using thin-layer chromatography (TLC) on precoated aluminum-backed plates (silica gel 60 F254, Merck), and compounds were visualized using UV radiation at 254 nm, followed by an anisaldehyde spray reagent (1% p-anisaldehyde:2% H2SO4: 97% cold MeOH) and heating. Final purifications were carried out in selected solvent systems using preparative thin-layer chromatography (Merck 818,133) and gravity column chromatography (Merck Art. 9385), which used a 2 cm diameter column packed with silica gel.
Anti-HIV activities and cytotoxicity effects
The effects of the test compounds in preventing cytopathic effects that occur because of HIV-1 replication were evaluated by MTT colorimetric assay [51]. MT-4 cells suspended at 1 X 108 cells/ml were infected with 640 μL of HIV-1IIIB virus at 1.26 X108 TCID50/ml. After infection, 200 μl HIV-infected MT-4 cells (1 X105 cells/well) in growth media were added to each well. The plates were preincubated for 24 h at 37 °C to allow stabilization. Then, 50 μL of the test compounds (at a concentration of 4 mg/ml) were added to the first column of the well. With a multichannel pipette, 50 μL was transferred (in triplicate) from the wells labeled 1 to wells labeled 2. Such transfers were continued (serial dilution), moving from left to right, changing tips prior to mixing contents of the next column of wells. Finally, 50 μL was discarded from the wells in column 12. Different concentrations (800 to 8.192 × 105 μg/mL) of test compounds were prepared through serial dilution. Each dilution was tested in triplicates. The microtiter plates were incubated at 37 °C in a 5% CO2 incubator for 5 days. Two negative controls, infected untreated cells and uninfected untreated (mock) cells, and four positive controls (zidovudine, tenofovir, abacavir, and nevirapine) were also included. After 5 days of incubation, cell viability was determined by the MTT assay described [51]. All compounds were assayed in triplicate.
A dose-response curve was plotted to calculate the concentrations that reduced viral replication by 50% (IC50) [52,53,54]. The selectivity index (SI) of the test compounds was calculated as the ratio of 50% cytotoxic concentration (CC 50) to 50% effective concentration (EC50) [51].
A cytotoxicity test was conducted to evaluate the safety of the plant extracts by measuring cell death caused by the plant extracts. The assay was conducted using an MTT colorimetric assay [55, 56]. The MTT assay was based on the reduction of the yellow-colored tetrazolium salt MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) by NAD(P)H-dependent cellular oxidoreductase enzymes [57] to an insoluble dark-blue colored formazan that can be measured spectrophotometrically [55]. Formazan production indicates the number of viable cells; therefore, an increase or decrease in cellular viability results in a change in the amount of formazan formed, which indicates the degree of cellular cytotoxicity (CC50) caused by the plant extract. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was dissolved in PBS to obtain a final concentration of 5 mg/ml and filtered to sterilize and remove insoluble residue [58, 59]. The assay was carried out in 96-well, flat-bottomed microtiter plates. To each well, 200 μl of MT-4 cells (1 × 105 cells) in growth media was added. The plates were preincubated for 24 h at 37 °C to allow stabilization. Then, 50 μL of the test compounds (at a concentration of 4 mg/ml) were added to the first column of the well. With a multichannel pipette, 50 μL was transferred (in triplicate) from the wells labeled 1 to wells labeled 2, and such transfers were continued (serial dilution), moving from left to right, changing tips prior to mixing contents of the next column of wells. Finally, 50 μL was discarded from the wells in column 12. Different concentrations (800–8.192 × 105 μg/mL) of test compounds were prepared through serial dilution. Each dilution was tested in triplicates. The negative control (NC) wells contained 50 μl of MT-4 cells in 0.5% DMSO [52]. Positive control (zidovudine, tenofovir, abacavir and nevirapine) drugs were also added in triplicate. A 96-well microtiter plate containing the test compounds and positive and negative controls was incubated at 37 °C in a humidified atmosphere of 5% CO2 for 5 days. After incubation, 20 μl of MTT reagent (5 mg/ml MTT in phosphate-buffered saline) was added to each test well and control well. The plate was further incubated at 37 °C in a CO2 incubator for 4 hours. After 4 hours of incubation, 100 μl of DMSO was added to dissolve the dark-blue formazan crystals from surviving cells [60]. After the formazan crystals were dissolved completely, the resulting optical density (OD) readings were measured relative to the controls on an ELISA plate reader at 570 nm with a reference wavelength of 620 nm [58]. For each extract and pure compound tested, 3 triplicate determinations were performed. First, the percentage viability was calculated [51] then a dose-response curve was plotted to enable the calculation of the concentrations that reduced the number of viable cells by 50% (CC50). The concentration that determined cell viability above 80% (CC20) was chosen as the maximum non-toxic concentration (MNTC).
Results
Hexane soluble extract of 1:1 v/v/ CH2Cl2/MeOH crude extract of the leaves of C. macrostachyus
Repeated column chromatography and preparative thin-layer chromatography of hexane soluble fraction of the leaves of C. macrostachyus afforded 2-methoxy benzyl benzoate (1), lupenone (2), lupeol acetate (3), betulin (4), lupeol (5), sitosterol (6) and stigmasterol (7) (Fig. 2). The NMR spectrum of these compounds is available in the supplementary information.
Anti-HIV activities and cytotoxicity effects of the isolated compounds
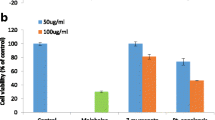
The cytotoxic and antiviral activity findings for the pure compounds isolated from C. macrostachyus and the control drugs are summarized in Table 1. Among the three isolated compounds from C. macrostachyus, lupenone (2) displayed the highest CC50 value of 32.46 ± 0.7 μg/mL (Table 1). Furthermore, a comparison between the pure isolated compounds and the control drugs showed that compounds 2, 3 and 4 had significantly (p < 0.001) higher CC50 values than AZT, ABC, and NVP, which indicates their safety and that high concentration levels are required to exert cytotoxic effects (Fig. 3). In addition, the maximum cytotoxic effect (Emaxc) of the compounds was not significantly different from the cytotoxic effect of AZT and NVP. Betulin (4) observed the highest anti-HIV activity, which inhibited virus-induced CPE by 76% with an IC50 value of 0.002 ± 0.04 μg/mL, which is much lower than the maximum nontoxic concentration (MNTC), which also indicates the safety and efficacy of the compound. Furthermore, all three compounds displayed anti-HIV activity at significantly (p < 0.05) lower IC50 values than TDF and NVP (Fig. 3), indicating their higher potency. In addition, the three compounds displayed significantly (p < 0.05) higher inhibition of viral-induced CPE (EmaxAV) than ABC. The antiviral activity of the tested compounds (EmaxAV) showed that both the control drugs (except ABC) and the tested compounds had approximately similar antiviral efficacy, as they showed non-significantly different EmaxAV values (Fig. 4). Furthermore, the results showed that the tested compounds had a higher selectivity index, indicating their efficacy at lower cytotoxic effects. As depicted in Fig. 5, the pure compounds (2, 3, and 4) displayed concentration-dependent inhibition of virus-induced CPE, as the % CPE inhibition increased with increasing concentrations of the pure compounds. Our finding on the anti-HIV activity of these pure compounds is in agreement with previous reports. A report by Chaniad et al [61] explained the efficacy of betulin (4) as a potent anti-HIV compound with an IC50 value of 17.7 ± 0.6 μM. Similarly, Esposito et al [62] reported that lupeol acetate and lupeol inhibited HIV-1 RT-associated RNase H function with IC50 values of 63 and 11.6 μM, respectively.
Cytotoxicity of pure compounds isolated from C. macrostachyus. The results are expressed as the mean of three independent experiments ± S.E.M. AZT, Zidovudine; TDF, Tenofovir; ABC, Abacavir; NVP, Nevirapine; Lupenone (2); Lupeol acetate (3); Betulin (4); CC50, 50% cytotoxic concentration; EmaxC, Maximum cytotoxic effect; C; control, ns, not significant, *Denotes p value < 0.05; **Denotes p value < 0.01, ***Denotes p value < 0.001
Anti-HIV activity of pure compounds isolated from C. macrostachyus. The results are expressed as the mean of three independent experiments ± S.E.M. AZT, Zidovudine; TDF, Tenofovir; ABC, Abacavir; NVP, Nevirapine; Lupenone (2); Lupeol acetate (3); Betulin (4); CC50, 50% cytotoxic concentration; EmaxC, Maximum cytotoxic effect; C; control, ns, not significant, *Denotes p value < 0.05; **Denotes p value < 0.01, ***Denotes p value < 0.001
Concentration-response curve analysis for the anti-HIV activity of pure compounds isolated from C. macrostachyus. Results presented in the curves are means ± S.E.M of three independent experiments. Cell viability % (red line) and the inhibition % of the virus-induced cytopathic effect (blue line) associated with control drugs and the tested extracts at the concentration level (800–8.192 × 105 μg/mL); Lupenone (2); Lupeol acetate (3); Betulin (4)
Conclusions
We conclude that the hexane soluble extract of 1:1 v/v/ CH2Cl2/MeOH crude extract of the leaves of C. macrostachyus, is potent against HIV-1 at IC50 = 0.02 μg/mL, and that 2-methoxy benzyl benzoate (1), lupenone (2), lupeol acetate (3), betulin (4), lupeol (5), sitosterol (6) and stigmasterol (7) are its major constituents. We demonstrated that lupenone (2), lupeol acetate (3) and betulin (4) exhibited anti-HIV-1 inhibition at 4.7, 4.3 and 4.5 nM respectively. These results are in agreement with previously reported ant-HIV activities of the known compounds. Chaniad et al [61] described the efficacy of betulin (4) against HIV with an IC50 value of 17.7 ± 0.6 μM, whereas Esposito et al [62] reported that lupeol acetate and lupeol inhibited HIV-1 RT-associated RNase H function with IC50 values of 63 and 11.6 μM, respectively. The current results and the described past anti-HIV effects of compounds found in C. macrostachys, ascertains the importance of the plant, as was previously reported by Maroyi (2017) [18].
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ABC:
-
Abacavir
- AZT:
-
Zidovudine
- CC50 :
-
50% cytotoxic concentration
- CDD:
-
Methylene chloride soluble fractions of 1:1 CH2Cl2: MeOH extract
- CDE:
-
Ethyl acetate soluble fractions of 1:1 CH2Cl2: MeOH extract
- CDH:
-
Hexane soluble fractions of 1:1 CH2Cl2: MeOH extract
- CDM:
-
Methanol soluble fractions of 1:1 CH2Cl2: MeOH extract
- EmaxAV :
-
Maximum antiviral effect %
- EmaxC :
-
Maximum cytotoxic effect %
- IC50 :
-
50% antiviral effect concentration
- MNTC:
-
Maximum non-toxic concentration
- NVP:
-
Nevirapine
- SI:
-
selectivity index
References
WHO. HIV/AIDS. 2021 [cited 2022 May 16]. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids
UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet | UNAIDS. 2020 [cited 2021 Apr 17]. Available from: https://www.unaids.org/en/resources/fact-sheet
McLeod GX, Hammer SM. Zidovudine: Five years later. Ann Intern Med. 1992;117:487–501.
Sperling R. Zidovudine. Infect Dis Obstet Gynecol. 1998.
Erik. Antiretroviral drugs. Curr Opin Pharmacol. 2010;10:507–15.
Montessori V, Press N, Harris M, Akagi L, Montaner JSG. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170:229–38.
World Health Organization. Progress Report 2016, prevent HIV, test and treat all. WHO/HIV/2016.24. 2016.
Narayan LC, Rai VR, Tewtrakul S. Emerging need to use phytopharmaceuticals in the treatment of HIV. J Pharm Res. 2013;6(1):218–23.
UNAIDS. 5. The AIDS Epidemic Can Be Ended by 2030 With Your Help (15pps). 2016; Available from: http://www.nytimes.com/2013/08/21/opinion/global/the-aids-epidemic-can-be-ended.html?smid=fb-share&_r=1&&pagewanted=print
Saslis-Lagoudakis CH, Savolainen V, Williamson EM, Forest F, Wagstaff SJ, Baral SR, et al. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc Natl Acad Sci. 2012;109(39):15835–40 [cited 2021 Nov 2]. Available from: https://www.pnas.org/content/109/39/15835.
Edwards S, Tadesse M. and HI. Flora of Ethiopia and Eritrea: Canellaceae to Euphorbiaceae. Vol. 2.2. The National Herbarium, Addis Ababa and Uppsala University; 1995.
Umberto Q. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names ... - Umberto Quattrocchi - Google Books. CRC Press. 2012.
Dubale AA, Chandravanshi BS, Gebremariam KF. Levels of major and trace metals in the leaves and infusions of Croton macrostachyus. Bull Chem Soc Ethiop. 2015;29:11–26.
Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara Region, Ethiopia. J Ethnobiol Ethnomed. 2013;9:63.
Maroyi A. Ethnomedicinal uses and pharmacological activities of Croton megalobotrys Müll Arg: A systematic review. Trop J Pharm Res. 2017;16(10):2535–43.
Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124:69–78.
Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethnopharmacol. 2007;111:271–83.
Naturgucker N de. naturgucker.de. naturgucker. Occurrence dataset https://doi.org/10.15468/uc1apo accessed via GBIF.org on 2018-12-24. https://www.gbif.org/occurrence/1338462599. 2018.
Maroyi A. Ethnopharmacological Uses, Phytochemistry, and Pharmacological Properties of Croton macrostachyus Hochst. Ex Delile: A Comprehensive Review. Evidence-based Complement Altern Med. 2017.
Ndihokubwayo JB, Yahaya AA, Desta AT, Ki-Zerbo G, Odei EA, Keita B, et al. Antimicrobial resistance in the African Region: issues, challenges and actions proposed. Key Determinants for the African Region. Aff Health Monit. 2013;16:27–30.
Mazzanti G, Bolle P, Martinoli L, Piccinelli D, Grgurina I, Animati F, et al. Croton macrostachys, a plant used in traditional medicine: purgative and inflammatory activity. J Ethnopharmacol. 1987;19:213–9.
Pascaline J, Charles M, George O, Lukhoba C, Ruth L, D MS. Ethnobotanical survey and propagation of some endangered medicinal plants from south Nandi district of Kenya. J Anim Plant Sci. 2010;8:1016–43.
Jeruto P, Lukhoba C, Ouma G, Otieno D, Mutai C. An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J Ethnopharmacol. 2008;116(2):370–6 Available from: https://pubmed.ncbi.nlm.nih.gov/18215481/.
Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G. Traditional medicines among the Embu and Mbeere peoples of Kenya. African J Tradit Complement Altern Med. 2007.
Obey JK, von Wright A, Orjala J, Kauhanen J, Tikkanen-Kaukanen C. Antimicrobial Activity of Croton macrostachyus Stem Bark Extracts against Several Human Pathogenic Bacteria. J Pathog. 2016.
Okello SV, Nyunja RO, Netondo GW, Onyango JC. Ethnobotanical study of medicinal plants used by sabaots of mt. Elgon Kenya. African J Tradit Complement Altern Med. 2010.
Agisho H, Osie M, Lambore T. Traditional Medicinal Plants Utilization, Management and Threats in Hadiya Zone, Ethiopia. J Med Plants Stud. 2014;2:94–108.
Mesfin F, Demissew S, Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2009;5:28.
Bekele G, Reddy PR. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo Tribes in Abaya District. Univers J Plant Sci. 2015;3(1):1–8.
Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9:65.
Tefera M, Geyid A, Debella A. In vitro anti-Neisseria gonorrhoeae activity of Albizia gummifera and Croton macrostachyus. Pharmacologyonline. 2012.
Cyrus WG, Daniel GW, Nanyingi MO, Njonge FK, Mbaria JM. Antibacterial and cytotoxic activity of Kenyan medicinal plants. Mem Inst Oswaldo Cruz. 2008;103:650–2.
Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;1:370–5.
Gemechu A, Giday M, Worku A, Ameni G. In vitro Anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis Strains. BMC Complement Altern Med. 2013;13:291.
Semenya SS, Maroyi A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo Province, South Africa. Afr J Tradit Complement Altern Med. 2013;10(2):316–23 Available from: https://pubmed.ncbi.nlm.nih.gov/24146456/.
Desta B. Ethiopian traditional herbal drugs. Part II: Antimicrobial activity of 63 medicinal plants. J Ethnopharmacol. 1993;39:129–39.
Taniguchi M, Kubo I. Ethnobotanical drug discovery based on medicine men’s trials in the African savanna: Screening of East African plants for antimicrobial activity II. J Nat Prod. 1993;56:1539–46.
Tene M, Ndontsa BL, Tane P, Tamokou JDD, Kuiate J. Antimicrobial diterpenoids and triterpenoids from the stem bark of Croton macrostachys. Int J Biol Chem Sci. 2009.
Ngo Bum E, Ngah E, Ngo Mune RM, Ze Minkoulou DM, Talla E, Moto FC, et al. Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy Behav. 2012;24:319–23.
Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):1–10 Available from: https://ethnobiomed.biomedcentral.com/articles/10.1186/1746-4269-4-10.
Karunamoorthi K, Ilango K. Larvicidal activity of Cymbopogon citratus (DC) Stapf. and Croton macrostachyus Del. against Anopheles arabiensis Patton, a potent malaria vector. Eur Rev Med Pharmacol Sci. 2010;14:57–62.
Owuor BO, Ochanda JO, Kokwaro JO, Cheruiyot AC, Yeda RA, Okudo CA, et al. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J Ethnopharmacol. 2012;144(3):779–81 Available from: https://pubmed.ncbi.nlm.nih.gov/23041700/.
Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14:79.
Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J Ethnopharmacol. 2007;110:428–33.
Kamanyi A, Mbiantcha M, Nguelefack TB, Ateufack G, Watcho P, Ndontsa BL, et al. Anti-nociceptive and anti-inflammatory activities of extracts from the stem bark of Croton macrostachyus (Euphorbiaceae) in mice and rats. J Complement Integr Med. 2009;6.
Nguelefack TB, Dutra RC, Paszcuk AF, de Andrade EL, Calixto JB. TRPV1 channel inhibition contributes to the antinociceptive effects of Croton macrostachyus extract in mice. BMC Complement Altern Med. 2015;15:293.
Degu A, Engidawork E, Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016;16:379.
Amuamuta A, Mekonnen Z, Gebeyehu E. Traditional therapeutic uses and phytochemical screening of some selected indigenous medicinal plants from Northwest Ethiopia. Afr J Pharmacol Ther. 2015;4(3):80–5 Available from: http://journals.uonbi.ac.ke/ajpt/article/view/1359.
Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci Technol. 2014;34(1):16–23 Available from: http://www.scielo.br/j/cta/a/9X8CwkQF8pBHKXRBXLBrTzF/?lang=en.
Terefe EM, Okalebo FA, Deresse S, Muriuki J, Amuhaya EK, Munyendo LW, et al. Cytotoxicity and antiretroviral activity of the leaf and stem bark extracts of Croton macrostachyus. Pharm J Kenya. 2021;26(1):2021.
Terefe EM, Okalebo FA, Derese S, Muriuki J, Batiha GE-S. In Vitro Cytotoxicity and Anti-HIV Activity of Crude Extracts of Croton macrostachyus, Croton megalocarpus and Croton dichogamus. J Exp Pharmacol. 2021;13:971–9 Available from: https://www.dovepress.com/in-vitro-cytotoxicity-and-anti-hiv-activity-of-crude-extracts-of-croto-peer-reviewed-fulltext-article-JEP.
Weislow OS, Kiser R, Fine DL, Bader J, Shoemaker RH, Boyd MR. New soluble-formazan assay for HIV-1 cytopathic effects: Application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–86.
Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, et al. Rapid and automated tetrazolium-based calorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–21.
Gustafson KR, McKee TC, Bokesch HR. Anti-HIV cyclotides. Curr Protein Pept Sci. 2004;5:331–40.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, et al. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–21.
Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–52.
Szucs G, Melnick JL, Hollinger FB. A simple assay based on HIV infection preventing the reclustering of MT-4 cells. Bull World Health Organ. 1988;66:729.
ACTG Laboratory Technologist Committee. TCID50 (50% Tissue Culture Infectious Dose) Determination Quantitation of Viable HIV-1 Virions in Culture Supernatants. 2004;50(May):1–10.
Bahuguna A, Khan I, Bajpai VK, Kang SC. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J Pharmacol. 2017;12(2):115–8.
Chaniad P, Sudsai T, Septama AW, Chukaew A, Tewtrakul S. Evaluation of Anti-HIV-1 integrase and anti-inflammatory activities of compounds from betula alnoides buch-ham. Adv Pharmacol Sci. 2019;2019:2573965.
Esposito F, Mandrone M, Del Vecchio C, Carli I, Distinto S, Corona A, et al. Multi-target activity of Hemidesmus indicus decoction against innovative HIV-1 drug targets and characterization of Lupeol mode of action. Pathog Dis. 2017;75(6) Available from: https://pubmed.ncbi.nlm.nih.gov/28637198/.
Acknowledgments
EMT acknowledges United States International University- Africa, Princess Nourah bint Abdulrahman University, King Saud University, University of Nairobi, Kenya Medical Research institute and the Institute of Primate Research for their support toward the successful completion of the research work.
Institutional review board statement
The collection of the plant was performed after obtaining the required ethical approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UON ERC), approval number KNH/ERC/A/154.
Funding
This work was funded by United States International University- Africa Internal grant no. 10–2854, Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R62), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Researchers Supporting Project number (RSP-2021/26), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
E.M.T. Conceptualization, methodology, validation, Investigation, Formal analysis, Project administration, writing-original draft preparation, reviewing and editing, Project administration and fund acquisition; F.A.O, S.D., J.M., Conceptualization, methodology, validation, Investigation, writing- reviewing and editing, supervision; M.K.L., E.M.C. Investigation, Formal analysis, writing original draft preparation, reviewing and editing, G.S.B., A.G., N.H.A., E.A.E., S.A. validation, writing-reviewing and editing, funding. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was approved by Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UON ERC), approval number P992/12/2019. The experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Terefe, E.M., Okalebo, F.A., Derese, S. et al. In vitro anti-HIV and cytotoxic effects of pure compounds isolated from Croton macrostachyus Hochst. Ex Delile. BMC Complement Med Ther 22, 159 (2022). https://doi.org/10.1186/s12906-022-03638-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03638-6