Abstract
Aim
We conducted a randomized placebo-controlled trial to assess the efficacy of Spirulina (SP) supplementation on disease activity, health-related quality of life, antioxidant status, and serum pentraxin 3 (PTX-3) levels in patients with ulcerative colitis (UC).
Methods
Eighty patients with UC were randomly assigned to consume either 1 g/day (two 500 mg capsules/day) of SP (n = 40) or control (n = 40) for 8 weeks. Dietary intakes, physical activity, disease activity, health-related quality of life, antioxidant status, erythrocyte sedimentation rate (ESR), and serum PTX-3 levels were assessed and compared between groups at baseline and post-intervention.
Results
Seventy-three patients (91.3%) completed the trial. We observed increases in serum total antioxidant capacity levels in the SP supplementation group compared to the control group after 8 weeks of intervention (p ≤ 0.001). A within-group comparison indicated a trend towards a higher health-related quality of life score after 8 weeks of taking two different supplements, SP (p < 0.001) and PL (p = 0.012), respectively. However, there were no significant changes in participant’s disease activity score in response to SP administration (p > 0.05). Similarly, changes in ESR and PTX-3 levels were comparable between groups post-intervention (p > 0.05).
Conclusions
SP improved antioxidant capacity status and health-related quality of life in patients with UC. Our findings suggest that SP supplementation may be effective as an adjuvant treatment for managing patients with UC. Larger trials with longer interventions periods are required to confirm our findings.
Similar content being viewed by others
Introduction
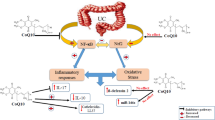
Ulcerative colitis (UC) is a prevalent type of Inflammatory Bowel Disease (IBD) characterized by chronic inflammation, ulcers in the distal part of the intestine, and clinically recurrent phases of aggravation and remission [1]. Although the etiology of UC is poorly elucidated, growing evidence has revealed that interactions between several components, including genetic variations in the intestinal microbiome, immune responses, and environmental factors, may play a role [2,3,4,5], UC represents an underlying cause of various other disorders, including intermittent diarrhea and constipation, cramping, abdominal, rectal, or joint pain, bleeding, and/or anemia [6,7,8]. Thus, identifying and treating pathophysiological factors can play an indispensable role in reducing UC-related complications.
Inflammatory markers, which involved the pathophysiology of UC, have received attention as a method for indirect assessment of UC and a potential therapeutic target for this condition [9]. To that end, acute phase inflammatory markers such as C reactive protein (CRP) or the erythrocyte sedimentation rate (ESR) in plasma are measured for this purpose [9, 10]. Emerging evidence also indicated that serum pentraxin-3 (PTX-3) is an important inflammatory marker for IBD [11, 12]. Short pentraxins (e.g., CRP, serum amyloid P) are produced by hepatocytes [13]; in contrast, PTX-3 as a long member of the pentraxin superfamily is produced by innate immunity cells in response to inflammatory cytokine and tissue damage [11, 12]. The PTX-3 is released mainly from neutrophils in inflamed colon tissue in UC patients, especially in crypt abscess injuries [14, 15]. Therefore, PTX-3 is an independent biomarker of disease activity produced at the site of inflammation, which may be helpful as a rapid disease activity biomarker to detect and treat primary local inflammation induced by epithelial damage and crypt abscess in patients with UC. Although immunosuppressants and anti-inflammatory medications are commonly used for patients with UC [16], these pharmacological treatments induce several adverse effects, including raised risk of infection, low bone mineral density, liver disease, tremor, eye disorders, gastrointestinal disease, pancreatitis, and antigen-antibody response [17, 18]. Thus, applying a comparably safer adjuvant treatment with fewer side effects and lower toxicities may be more favorable in UC management.
Over recent decades, evidence has supported the benefits of select herbal therapies on UC due to their bioactive compounds’ healing or antioxidant characteristics. These therapies are generally considered safe for managing UC-related complications [19,20,21,22,23,24]. Namely, Spirulina (SP; Arthrospira platensis) which is a biomass of cyanobacteria (blue-green algae) [25, 26]. This alga, predominantly classified as a phytomedicine, has been widely consumed as a dietary supplement or a whole food. It is considered a good source of essential nutrients, including phytochemicals (carotenoids, phycocyanins), minerals (calcium, iron), amino acids, essential fatty acids, vitamins (vitamin B12, provitamin A), and fiber [27,28,29]. SP has anti-inflammatory, antioxidant, liver-protecting, antiviral, and microbiome-regulating properties and has been suggested as an effective adjuvant therapy for managing many disorders [27, 28, 30, 31]. SP’s antioxidant and anti-inflammatory effects are specifically remarkable in the management of chronic diseases, including IBD [32, 33]. Previous studies documented that SP supplementation decreases inflammatory cytokines, such as tumor necrosis factor (TNF)-α [34], interleukin-6 (IL-6) [35], CRP [36], ESR [37], and PTX-3 [38]. In addition, several studies reported that SP administration significantly affects oxidant and antioxidant parameters. For instance, SP supplementation has been shown to reduce malondialdehyde (MDA) levels [39] and increase total antioxidant capacity (TAC), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels [35, 40,41,42]. However, these studies mainly focused on the effect of SP on inflammatory and antioxidant factors per se, and the effect of this microalgae compound has been poorly elucidated on clinical outcome measures of patients with inflammatory conditions, including those with chronic colitis. Therefore, the purpose of this investigation was to evaluate the effects of SP supplementation on disease activity, health-related quality of life, serum antioxidant status, and PTX-3 levels in patients with UC.
Materials and methods
Participants’ characteristics
Eighty patients with UC (age: 38.64 ± 11.30 years, height: 166 ± 8.57 cm, and BMI: 25.81 ± 4.96 kg/m2) referred to the Imam Reza Hospital (Kermanshah, Iran) were enrolled in the study. Participants were included if they (1) were clinically diagnosed with UC using a colonoscopy exam, clinical records, and pathology assessment; (2) were between 18 and 65 years of age; and (3) exhibited symptoms of active mild to moderate UC disease (5 ≤ or ≤ 12 scores based on the Simple Clinical Colitis Activity Index [SCCAI]) [43]. Patients were excluded if they (1) had good or severe ulcerative colitis (SCCAI scores of < 5 or > 12); (2) were pregnant or breastfeeding; (3) consumed antidepressants, anxiety medications, antioxidants, omega-3, or other supplements in the past 3 months; (4) smoked or consumed alcohol; (5) had heart, liver, kidney, cancer, thyroid, parathyroid, or other gastrointestinal conditions; or (6) had poor compliance to SR supplementation protocol (consumed < 90% of their supplements during the intervention period).
Experimental protocol
This study was a double-blind and placebo-controlled randomized clinical trial. Prior to baseline measurements, participants were fully familiarized with all experimental procedures. Patients with active mild to moderate UC were randomly allocated to one of two groups: a SP supplementation (n = 40) or control (n = 40) utilizing simple randomization via a random number table. Prior to and following our eight-week study duration, measures of anthropometry, dietary intake, disease activity, health-related quality of life, serum antioxidant status, and PTX-3 levels were made. All patients were instructed to continue their usual lifestyles, including physical activity, dietary intake, and medication regimen throughout the study period. Compliance with the assigned intervention was evaluated through weekly phone calls and monitoring the number of supplement packages used. Patients, laboratory staff, researchers, and participants were blinded to the supplement allocation until the end of the trial period.
Randomization and blinding
The study employed a simple randomization method, facilitated by a random number table. Throughout the duration of the trial, the participants, laboratory personnel, and researchers were blinded to treatment assignment. At no time during the intervention were the investigators and/or participants aware of which treatment was being provided to study participants.
Spirulina supplementation
The SP group was supplemented with a 500 mg capsule of SP twice per day, before lunch and dinner. The control group received two placebo capsules containing 500 mg corn starch without chlorophyll during the same time periods. The placebo capsules had similar color, size, and shape compared to SP capsules. The selected dose and time of ingestion were based on prior investigations [44,45,46,47,48]. The SP powder was produced by the Javane Sabz Company, Shiraz, Iran. The chemical composition of Spirulina and placebo per 100 g is reported in Table 1. All chemical analytical procedures were completed in the Beh-Azma laboratory (Iran) in compliance with the assessment methods recommended by the Association of Analytical Communities. Both the SP and placebo capsules were prepared by researchers under sterile conditions, including the measurement of the weight and quality.
Outcome assessments
Height was evaluated via a nonelastic wall-mounted stadiometer, measured to the nearest 0.5 cm. Body mass was assessed with participants dressed in light clothes using a digital scale to the nearest 0.1 kg. Body mass index (BMI) was calculated using a formula: Weight (kg)/ the square of the body height (m2).
To assess the dietary intake of each patient, food diaries were collected for 3 days, including two weekdays and one weekend day. Nutrient intakes were assessed using the Nutritionist IV software (First Databank, San Bruno, CA) modified for Iranian foods. Physical activity levels were evaluated via a short form of the International Physical Activity Questionnaire (IPAQ) [49].
Patients’ disease activity was assessed using the SCCAI questionnaire score, which correlates closely to biochemical parameters of UC, and is valid and reliable for evaluating patients with this condition [50]. This questionnaire has various parts with a total score ranging from zero to 19. Higher scores indicate higher severity of UC symptoms during the past week. Moreover, the Short IBD Questionnaire (SIBDQ) score was used to assess health-related quality of life (HRQoL) in IBD patients. The validity and reliability of SIBDQ were first confirmed (r = 0.83) by Jowett et al. for patients with UC [51] and subsequently in an Iranian cohort [52]. Each question of SIBDQ has seven items and covers scores of 1 to 7.
Blood samples were obtained between 8:00, and 10:00 am after an overnight fast (12 hours) at baseline and post-intervention. The erythrocyte sedimentation rate (ESR) was determined by the Westergren assay. The blood samples were centrifuged (3500 rpm), and the aliquots were stored at − 80 °C before further analysis. Total antioxidant capacity (TAC) was assessed according to the ferric reducing antioxidant power (FRAP) method using a commercial kit (Kiazist, Iran). MDA level was evaluated based on the thiobarbituric acid reactive substance (TBARS) using a commercial kit (Kiazist, Iran). Superoxide dismutase (SOD) concentration was also measured according to the ability of Mn-SOD to inhibit the conversion of resazurin to resorufin accompanied by reducing superoxide radicals produced by the xanthine/xanthine oxidase system using a commercial kit (Kiazist, Iran). All intra- and inter-assay coefficients of variation were less than 10%. Finally, the serum PTX-3 levels were evaluated using the enzyme-linked immunosorbent assay (ELISA) with an intra- and inter-assay coefficient of variability (CV) less than 5% by a Human PTX-3 Test kit (Crystal, China) based on the manufacturer’s guideline.
Sample size calculation
We conducted an a priori calculation of sample size using the G*Power analysis software [53], accounting for a Type I error rate of 5% and a statistical power of 80%. A minimum detectable effect size (i.e., Δ of clinical response) of 0.3 was considered clinically plausible using data from previous clinical trials in patients with UC [54, 55]. Our calculated sample size was 33 participants in each group. Ultimately, 40 patients in each experimental arm were estimated to be sufficient after considering a 20% dropout rate.
Safety assessment
Adverse events that may or may not be associated with the study therapies, including abnormal gastrointestinal reactions, cardiovascular events, allergic reactions, and other medical conditions were recorded.
Statistical methods
We presented continuous variables as mean ± standard deviation (SD) and expressed categorical variables as frequencies (percentage). The normality of data distribution was assessed using the Shapiro–Wilk test and Q–Q plot. For normally distributed variables, we used an independent sample t-test for comparison, and for non-normally distributed variables, the Mann–Whitney U test was employed. Categorical variables were analyzed using the chi-square test. The mean of markers between the SP and PL groups was compared using an independent t-test, and changes over time were evaluated utilizing a paired t-test. To examine the impact of the group on the markers in the post-test, controlling for the effects of baselines, we employed analysis of covariance (ANCOVA). If the homogeneity of variance was not met, we estimated the parameter with robust standard errors. The analyses were conducted using SPSS (version 26, Armonk, NY, USA) and STATA version 17 (Stata Corp LLC, TX, USA), and p-values less than 0.05 were considered statistically significant.
Results
Between May 2020 and January 2021, we screened 426 both female and male patients with UC. Of these, 346 were excluded for not meeting the exclusion criteria (n = 259) and for declining to participate (n = 87). Consequently, 80 participants with mild or moderate degrees of UC disease were enrolled in the current study and randomly assigned to the SP and control groups. Three patients withdrew before completing the study for personal reasons and four due to changes in their medication use (Fig. 1). Data are presented for the 73 patients (n = 36 and n = 37 in the SP and control groups, respectively) that completed our eight-week intervention. Participants’ compliance with their intervention was > 90% in both groups.
Participant’s characteristics
The mean age of participants in SP and PL group was 37.80 and 39.50 years which was homogenate by the group. Results showed that the difference of markers between SP and PL was not significant in pretest (baseline) (Table 2). However, the mean of markers Calcium (p = 0.007, ESCohen ′ s d = 0.64), Magnesium (p = 0.040, ESCohen ′ s d = 0.49) and B9 (p = 0.012, ESCohen ′ s d = 0.60) in post-test were significantly different by group (Table 3). In addition, there was a significant mean difference in marker (post – pre) (SIBDQ score) in both groups (SP: p = < 0.001, ESCohen ′ s d = 0.87, PL: p = 0.012, ESCohen ′ s d =0.43). (Table 4).
In the next step, the ANCOVA was used to evaluate the impact of the group on markers measured in post-test adjusting the baselines. Accordingly, the post means of protein (β = 1.22, 95% CI (0.87, 1.60), ESPartial Eta Squared = 0.41) and Iron (β = 1.16, 95% CI (0.56, 1.76), ESPartial Eta Squared =0.17) were significantly higher in SP compared to PL. However, the waist-to-height ratio (β = 1.00, 95% CI (0.95,1.05), ESPartial Eta Squared = 0.95), BMI (β = 1.00, 95% CI (0.99, 1.04), ESPartial Eta Squared = 0.99), physical activity (β = 1.00, 95% CI (0.99,1.01), ESPartial Eta Squared = 0.99), energy (β = 1.00, 95% CI (0.88,1.13), ESPartial Eta Squared = 0.78), vitamin B1 (β = 1.00, 95% CI (0.96,1.04), ESPartial Eta Squared = 0.97), Lycopene (β = 1.00, 95% CI (0.97, 1.03), ESPartial Eta Squared = 0.98), had equal mean value in SP and PL group. (Table 5).
Dietary intake and physical activity levels
As Table 3 shows, most nutrients remained unchanged over time except for calcium, magnesium, and vitamin B9 in SP group. In addition, the independent t-test (Table 3) indicated that the levels of all the nutrients were significantly different in the SP compared to the PL group, with the exception of linoleic acid, oleic acid, docosahexaenoic acid, α-Tocopherol, vitamin B6, vitamin D, fiber, galactose, and maltose and lutein (p > 0.05). Physical activity (Table 3) remained unchanged over time in both groups (p > 0.05).
Health-related quality of life and SCCAI score
The effect of SP administration on SIQBD and SCCAI scores in patients with UC is reported in Fig. 2. A within-group comparison indicated a trend towards a higher SIQBD score after 8 weeks of taking two different supplements, SP and PL, respectively (p < 0.001, ESCohen ′ s d = 0.87 and p = 0.012, ESCohen ′ s d = 0.43). However, there were no significant changes in participants SCCAI score in response to SP administration (p > 0.05).
Antioxidant status and inflammatory markers
The effect of SP supplementation on antioxidant status and inflammatory parameters in patients with UC is reported in Fig. 3. The within-group comparison revealed a significant decrease in serum MDA after 8 weeks of SP supplementation (p = 0.01, ESCohen ′ s d = 0.45), but their TAC, SOD, ESR, and PTX-3 remained unchanged post-intervention (p > 0.05). In contrast, the within-group comparison revealed no significant changes in serum antioxidant status and inflammatory parameters in the control group (p > 0.05). Our ANCOVA analyses revealed a significant increase in serum TAC after 8 weeks of intervention in the SP supplementation group vs. the control group (β = 0.83, 95% CI (0.60,1.10), ESPartial Eta Squared = 0.37). Moreover, no significant differences were observed in changes of MDA, SOD, ESR, and PTX-3 levels between groups from baseline to post-intervention (p > 0.05).
Adverse effects
Overall, SP supplementation was well tolerated by patients and did not yield any severe adverse effects (e.g., allergic reactions). However, 5 out of 36 patients (13.8%) reported mild adverse effects, evidenced by mild bloating early in the trial. All bloating events were resolved later during the intervention.
Discussion
The present trial evaluated the efficacy of SP supplementation on disease activity, health-related quality of life, serum antioxidant status, and PTX-3 levels in patients with UC. Our outcomes showed that; (1) SP supplementation improved serum TAC levels and stool frequency compared to the control group, (2) there were between-group significant differences in changes of health-related quality of life score, and (3) changes in ESR were comparable between groups.
Our observations indicate that SP supplementation may enhance antioxidant capacity and ameliorate oxidation status in patients with UC. Our observations corroborate the antioxidant properties of SP reported in recent studies [56]. Coskun et al. [57] and Abdel-Daim et al. [58] reported that SP intake led to a considerable enhancement of antioxidant potential and consequently reduced lipid peroxidation in rat models with acid-induced colitis. Similarly, Szulinska et al. [35] showed that 2 g/d of SP supplementation for a period of 3 months significantly promoted total antioxidant status in obese participants. Ismail et al. [59] also revealed that 1 g/d of SP administration for 2 months significantly reduced serum content of lipid peroxidation products and improved the antioxidant-related activity of enzymes, such as SOD and glutathione-s-transferase (GST) in patients with chronic obstructive pulmonary disease (COPD). Two unique pigments, the blue C-phycocyanin and yellow-to-red carotenoids are the most critical bioactivities influencing the antioxidant properties of SP [56, 60]. The SP-derived C-phycocyanin can have health-protective properties against oxidative stress harms through scavenging reactive oxygen species (ROS) and decreasing lipid peroxidation in liver microsomes [56, 60, 61]. Moreover, the yellow-to-red carotenoid content of SP acts as an antioxidant by decreasing oxygen-mediated lipid peroxidation and inhibiting the intracellular accumulation of ROS [56, 60, 62]. Accordingly, SP may alleviate UC symptoms by inhibiting oxidative stress and associated complications.
Another important finding from the current work is an improved health-related quality of life score in patients who received the SP supplementation compared to controls. An improved stool frequency score is among the most critical factors in improving the quality of life in patients with UC [63]. Besides pharmaceutical agents, various complementary therapies have been proposed to improve stool frequency and disease activity and increase the quality of life in patients with colitis [19, 64,65,66]. Of current therapies, the modification of the intestine microbiome by probiotics or symbiotics has received significant attention [64, 65, 67, 68]. Studies have documented that combination therapies containing Lactobacillus and Bifidobacterium strains and mesalazine decreased stool frequency and extended remission periods in patients with UC [67, 69]. Also, SP has prebiotic effects and can modulate gut microbiota [30, 70, 71]. In a dose-response model, Hu et al. [70] reported that oral SP supplementation altered the colonic microbial community in healthy mice. Similarly, Neyrinck et al. [30] reported that mice models supplemented with 5% SP had enhanced gut microbiota, especially regarding Roseburia and Lactobacillus strains. Further, extracellular products of SP significantly improve the growth and survival of the Lactobacillus and Bifidobacterium strains [71, 72]. The SP gut microbial modulating properties have been attributed to its high levels of phenolic bioactive, free amino acids, and nitrogenous compounds [71, 73].
Another mechanism by which SP affects intestinal health may be related to regulating adipokines [74,75,76]. In vitro, in vivo, as well as human models have proposed an interconnected and complex role for leptin, ghrelin, and resistin as pivotal mediators of pro-inflammation that may trigger UC [75, 77, 78]. In a mouse model study, Fujimoto et al. [79] reported that SP was associated with significantly lower leptin concentration. Likewise, Heo et al. [80] reported that SP administration attenuated leptin levels and other metabolites in serum. Moreover, Akbarpour et al. [81] showed a significant decrease in serum resistin concentrations following SP administration in patients with type 2 diabetes. This evidence suggests that the adipokine-regulating benefits of SP supplementation may play a role in managing patients with UC.
The SP has antiangiogenesis properties and fosters wound healing and health status in patients with UC [82, 83]. Angiogenesis is required to supply oxygen and nutrients to healing regions and is essential for lesion healing and tissue regeneration. However, angiogenesis attracts more inflammatory cells and cytokines, aggravating pro-inflammation in a vicious circle that exacerbates mucosal injury in patients with UC [84,85,86]. A critical factor in regulating the angiogenesis process in UC is the vascular endothelial growth factor (VEGF), which can be targeted for complementary treatment and ameliorating pathologic angiogenesis [84, 85]. To that end, Mahmoud et al. [82] have revealed SP antiangiogenic effects mediated by modulated VEGF expression in human colorectal carcinomas (HCC) -bearing mice. Similarly, Aldina et al. [87] showed antiangiogenic impacts of SP through decreasing VEGF expression in the cornea inflammation model in rats. However, other studies conducted by Zeinalian et al. [88] and Mehdinezhad et al. [89] demonstrated that SP did not alter serum VEGF in participants with obesity or diabetic rats. Due to the contradictory results in the available literature, further clinical trials are required to elucidate the potential role of angiogenesis and VEGF modulation in the improvement of disease activity and gut health after SP supplementation in patients with UC.
Several studies have suggested that using acute phase inflammatory markers, such as ESR and PTX-3 are non-invasive, safe, and available methods to reflect disease activity in patients with IBD [9, 11, 12, 15]. Our work revealed marginally significant changes in PTX-3 levels and non-significant changes in ESR after 8 weeks of SP administration compared to the control group. The PTX-3 is the longest member of the pentraxins family released mainly from neutrophils in inflamed colon tissue, especially in crypt abscess injuries of patients with UC [14]. The PTX-3-expressing cells and inflamed neutrophils have been shown to elevate proinflammatory reactions in the colon [14, 90]. Our study was conducted on patients with mild or moderate severity of UC who consumed anti-inflammatory medications, such as mesalazine, to attenuate pro-inflammation in the colon tissues [91]. These medications are known to modulate inflammatory reactions and their systemic biomarkers in patients with UC. Therefore, we may not have captured any net anti-inflammatory effect of SP supplementation by conducting a routine inflammatory profile test. Importantly, we are the first study to show the potential beneficial effect of SP supplementation on PTX-3 levels in individuals with UC.
Strengths and limitations
Among our strengths is that this is the first trial to examine the efficacy of SP supplementation in health status among free-living patients with UC. Furthermore, most participants who completed the trial indicated good compliance with their therapy. Additional strengths of this work include a homogeneous cohort with UC and accounting for patients’ physical activity and dietary intake levels during the intervention as critical confounding factors.
Our study had some limitations. We did not perform colonoscopy or tissue biopsy to evaluate the severity of UC disease due to the invasive nature of these procedures, which might have led to higher attrition rates. However, we applied a valid and reliable SCCAI questionnaire as an effective tool to assess disease status. Another limitation is our use of a per-protocol analysis, which is susceptible to confounding biases, making its findings less generalizable to the broader patient population than those obtained from intention-to-treat analysis. We also did not measure specific disease-related biochemical markers, such as C-reactive protein or fecal calprotectin, which would have strengthen our study design as these markers have better correlation with endoscopic activity in UC. Furthermore, this study did not evaluate the dose-dependent efficacy of SP supplementation; therefore, these relationships remain to be elucidated in future work. Additionally, the intervention period in the current trial was likely not long enough to elicit drastic clinical changes. Consequently, longer interventions utilizing SP supplementation in patients with UC are warranted. Other important components are past knowledge and the duration of UC, which may impact the prognosis and management of this condition and potentially alter the study results since patients learn to self-manage their condition over time. Future work should account for any differences in additional confounding parameters, including past knowledge and length of disease between their experimental groups.
Conclusions
The present study examined the effect of SP supplementation on disease activity, health-related quality of life, serum antioxidant status, and PTX-3 status in patients with UC. Our findings indicate that TAC and stool frequency improved after SP supplementation in this population. In addition, SP supplementation did not change disease activity parameters, PTX-3 levels and ESR. Our findings suggest that SP supplementation may be effective as an adjuvant treatment for managing patients with UC. Therefore, larger trials with longer interventions periods are required to draw more precise conclusions.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due but are available from the corresponding author on reasonable request.
References
Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63(3):629–42.
El-Abhar HS, Hammad LN, Gawad HSA. Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol. 2008;118(3):367–72.
Gophna U, et al. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44(11):4136–41.
Thompson AI, Lees CW. Genetics of ulcerative colitis. Inflamm Bowel Dis. 2011;17(3):831–48.
Hanauer SB. Update on the etiology, pathogenesis and diagnosis of ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2004;1(1):26–31.
Yangyang RY, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: symptoms, extraintestinal manifestations, and disease phenotypes. In: Seminars in pediatric surgery. Elsevier; 2017.
Ishihara S, et al. Irritable bowel syndrome-like symptoms in ulcerative colitis patients in clinical remission: association with residual colonic inflammation. Digestion. 2019;99(1):46–51.
Chaubal A, et al. Anemia in patients with ulcerative colitis in remission: a study from western India. Indian J Gastroenterol. 2017;36(5):361–5.
Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7(4):460–5.
Cioffi M, et al. Laboratory markers in ulcerative colitis: current insights and future advances. World J Gastrointest Pathophysiol. 2015;6(1):13.
Wu Q, et al. Pentraxin 3: a promising therapeutic target for autoimmune diseases. Autoimmun Rev. 2020;19(12):102584.
Kalyon S, Gökden Y, Oyman F. A new biological marker in inflammatory bowel disease: Pentraxin 3. J Surg Med. 2020;4(10):875–8.
Ishida N, et al. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci Rep. 2021;11(1):1–8.
Savchenko AS, et al. Long pentraxin 3 (PTX3) expression and release by neutrophils in vitro and in ulcerative colitis. Pathol Int. 2011;61(5):290–7.
Kato S, et al. Increased expression of long pentraxin PTX3 in inflammatory bowel diseases. Dig Dis Sci. 2008;53(7):1910–6.
Danese S, Fiorino G, Peyrin-Biroulet L. Positioning therapies in ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(6):1280-1290.e1.
Lamb CA, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106.
Naganuma M, et al. Recent trends and future directions for the medical treatment of ulcerative colitis. Clin J Gastroenterol. 2016;9(6):329–36.
Nikkhah-Bodaghi M, et al. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother Res. 2019;33(4):1027–32.
Rastegarpanah M, et al. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin J Integr Med. 2015;21(12):902–6.
Nikkhah-Bodaghi M, et al. Zingiber officinale and oxidative stress in patients with ulcerative colitis: a randomized, placebo-controlled, clinical trial. Complement Ther Med. 2019;43:1–6.
Sadeghi N, et al. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother Res. 2020;34(5):1123–33.
Samsamikor M, et al. Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2016;47(4):304–9.
Amiri M, et al. Efficacy and safety of a standardized extract from Achillea wilhelmsii C. Koch in patients with ulcerative colitis: a randomized double blind placebo-controlled clinical trial. Complement Ther Med. 2019;45:262–8.
Kulshreshtha A, et al. Spirulina in health care management. Curr Pharm Biotechnol. 2008;9(5):400–5.
Yousefi R, Saidpour A, Mottaghi A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: a systematic review. Complement Ther Med. 2018;42.
Wu Q, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90:1817–40.
Mazokopakis EE, et al. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: a prospective study. J Sci Food Agric. 2014;94(3):432–7.
Suliburska J, et al. Effect of Spirulina maxima supplementation on calcium, magnesium, iron, and zinc status in obese patients with treated hypertension. Biol Trace Elem Res. 2016;173(1):1–6.
Neyrinck AM, et al. Spirulina protects against hepatic inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients. 2017;9(6):633.
Bobescu E, et al. Are there any beneficial effects of Spirulina supplementation for metabolic syndrome components in postmenopausal women? Mar Drugs. 2020;18(12):651.
Guazelli CF, et al. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem Biol Interact. 2021;333:109315.
Zuo K, et al. Purification and antioxidant and anti-inflammatory activity of extracellular polysaccharopeptide from sanghuang mushroom, Sanghuangporus lonicericola. J Sci Food Agric. 2021;101(3):1009–20.
Tzachor A, et al. Photosynthetically controlled Spirulina, but not solar Spirulina, inhibits TNF-α secretion: potential implications for COVID-19-related cytokine storm therapy. Mar Biotechnol. 2021;23(1):149–55.
Szulinska M, et al. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: a randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2017;21(10):2473–81.
Kordi MR, Attarzade Hosseini SR, Davaloo T. Aerobic exercises and supplement Spirulina reduce inflammation in diabetic men. J Jahrom Univer Med Sci. 2018;16(4):10–8.
Morsy MA, et al. Protective effect of Spirulina platensis extract against dextran-sulfate-sodium-induced ulcerative colitis in rats. Nutrients. 2019;11(10):2309.
El-Boghdady NA, Kamel MA, El-Shamy RM. Omeprazole and Spirulina platensis ameliorate Steatohepatitis in experimental nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2020;18(9):426–34.
Windari HAS, et al. Antioxidant activity of Spirulina platensis and sea cucumber Stichopus hermanii in Streptozotocin-induced diabetic rats. Trop Life Sci Res. 2019;30(2).
Gómez-Téllez A, et al. Effects of a low-dose Spirulina/turmeric supplement on Cardiometabolic and antioxidant serum markers of patients with abdominal obesity. Front Nutr. 2020;7.
Nasirian F, et al. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabet Metab Syndr Obes: Targets Ther. 2018;11:375.
Martínez-Sámano J, et al. Spirulina maxima decreases endothelial damage and oxidative stress indicators in patients with systemic arterial hypertension: results from exploratory controlled clinical trial. Mar Drugs. 2018;16(12):496.
Jowett S, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38(2):164–71.
Zeinalian R, et al. The effects of Spirulina platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial. BMC Complement Altern Med. 2017;17(1):1–8.
Mazokopakis EE, et al. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: a prospective pilot study. Ann Gastroenterol: Q Publ Hellenic Soc Gastroenterol. 2014;27(4):387.
Rezaei N, et al. The protective effects of honey and spirulina platensis on acetic acid-induced ulcerative colitis in rats. Iran Red Crescent Med J. 2018;20(4):8.
Dai Y-C, et al. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis: a randomized controlled trial. Medicine. 2017;96(16):e6651.
Naganuma M, et al. Efficacy of indigo Naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154(4):935–47.
Committee, I.R., Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. http://www.ipaq.ki.se/scoring.pdf, 2005.
Walmsley R, et al. A simple clinical colitis activity index. Gut. 1998;43(1):29–32.
Jowett SL, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96(10):2921–8.
Bijari B, Soltani B. Validation of the Persian version of inflammatory bowel disease questionnaire in patients who referred to clinics and hospitals of Birjand university of medical sciences, Iran. Ann Colorectal Res. 2017;5(1-2).
Faul F, et al. G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Langmead L, et al. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment Pharmacol Ther. 2004;19(7):739–47.
Kamali M, et al. Efficacy of the Punica granatum peels aqueous extract for symptom management in ulcerative colitis patients. A randomized, placebo-controlled, clinical trial. Complement Ther Clin Pract. 2015;21(3):141–6.
Park WS, et al. Two classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules. 2018;23(8):2065.
Coskun ZK, et al. The study of biochemical and histopathological effects of spirulina in rats with TNBS-induced colitis. Bratisl Lek Listy. 2011;112(5):235–43.
Abdel-Daim MM, et al. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol. 2015;37(2):126–39.
Ismail M, et al. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. Biomed Res Int. 2015;2015.
Yousefi R, Saidpour A, Mottaghi A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: a systematic review. Complement Ther Med. 2019;42:137–44.
Grover P, et al. C-Phycocyanin-a novel protein from Spirulina platensis- in vivo toxicity, antioxidant and immunomodulatory studies. Saudi J Biol Sci. 2021;28(3):1853–9.
Schafer FQ, et al. Comparing β-carotene, vitamin E and nitric oxide as membrane antioxidants. Biol Chem. 2002;383(3–4):671–81.
Rasmussen B, et al. Predictors of health-related quality of life in patients with moderate to severely active ulcerative colitis receiving biological therapy. Scand J Gastroenterol. 2020;55(6):656–63.
van der Waal MB, et al. Probiotics for improving quality of life in ulcerative colitis: exploring the patient perspective. PharmaNutrition. 2019;7:100139.
Amiriani T, et al. Effect of Lactocare® synbiotic on disease severity in ulcerative colitis: a randomized placebo-controlled double-blind clinical trial. Middle East J Dig Dis. 2020;12(1):27.
Shirazi KM, et al. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: a randomized, double-blind controlled clinical trial. Clin Res Hepatol Gastroenterol. 2020;45.
Dhillon P, Singh K. Therapeutic applications of probiotics in ulcerative colitis: an updated review. PharmaNutrition. 2020;13:100194.
Dang X, et al. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL# 3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846.
Astó E, et al. The efficacy of probiotics, prebiotic inulin-type fructans, and synbiotics in human ulcerative colitis: a systematic review and meta-analysis. Nutrients. 2019;11(2):293.
Hu J, et al. Dose effects of orally administered Spirulina suspension on colonic microbiota in healthy mice. Front Cell Infect Microbiol. 2019;9:243.
Finamore A, et al. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxidative Med Cell Longev. 2017;2017.
Parada JL, et al. Lactic acid bacteria growth promoters from Spirulina platensis. Int J Food Microbiol. 1998;45(3):225–8.
Beheshtipour H, et al. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Compr Rev Food Sci Food Saf. 2013;12(2):144–54.
Kaka R, et al. The effect of nonlinear resistance training with supplementation of Spirulina on serum leptin and ghrelin in obese women. J Appl Health Stud Sport Physiol. 2019;6(1):69–77.
Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin–implications for inflammatory bowel disease. Mol Nutr Food Res. 2008;52(8):855–66.
Ghomraoui FA, et al. Plasma ghrelin and leptin in patients with inflammatory bowel disease and its association with nutritional status. Saudi J Gastroenterol: Off J Saudi Gastroenterol Assoc. 2017;23(3):199.
Jung JY, et al. Circulating ghrelin levels and obestatin/ghrelin ratio as a marker of activity in ulcerative colitis. Intest Res. 2015;13(1):68–73.
Biesiada G, et al. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J Physiol Pharmacol. 2012;63(5):471–81.
Fujimoto M, et al. Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig Liver Dis. 2012;44(9):767–74.
Heo M-G, Choung S-Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018;9(9):4906–15.
Akbarpour M, Samari Z. The effect of aerobic training and Spirulina supplementation on Resistin and C-reactive protein in women with type 2 diabetes with overweight. KAUMS J (FEYZ). 2020;24(5):576–84.
Mahmoud YI, et al. Spirulina inhibits hepatocellular carcinoma through activating p53 and apoptosis and suppressing oxidative stress and angiogenesis. Life Sci. 2021;265:118827.
Elbialy ZI, et al. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed Pharmacother. 2021;137:111349.
Mateescu RB, et al. Vascular endothelial growth factor-key mediator of angiogenesis and promising therapeutical target in ulcerative colitis. Romanian J Morphol Embryol. 2017;58(4):1339–45.
Aksoy EK, et al. Vascular endothelial growth factor, endostatin levels and clinical features among patients with ulcerative colitis and irritable bowel syndrome and among healthy controls: a cross-sectional analytical study. Sao Paulo Med J. 2018;136(6):543–50.
Sandor Z, et al. Altered angiogenic balance in ulcerative colitis: a key to impaired healing? Biochem Biophys Res Commun. 2006;350(1):147–50.
Aldina R, Haryati SW. Effect of Spirulina platensis extract on vascular endothelial growth factor (VEGF) expression in corneal inflammation in rat (Rattus novergicus) strain wistar. EurAsian J BioSci. 2019;13(2):823–9.
Zeinalian R, et al. The effects of Spirulina platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial. BMC Complement Altern Med. 2017;17(1):225.
Mehdinezhad N, et al. Effect of spirulina and chlorella alone and combined on the healing process of diabetic wounds: an experimental model of diabetic rats. J Diab Metab Disord. 2021;20.
Ercan G, Yigitturk G, Erbas O. Therapeutic effect of adenosine on experimentally induced acute ulcerative colitis model in rats. Acta Cir Bras. 2019;34(12).
Rubin DT, et al. Budesonide multimatrix is efficacious for mesalamine-refractory, mild to moderate ulcerative colitis: a randomised, placebo-controlled trial. J Crohn’s Colitis. 2017;11(7):785–91.
Acknowledgements
We would like to thank all participants who kindly contributed to the study.
Funding
The current work is a part of a PhD thesis supported by a grant (No.398533) from the Vice-Chancellor for Research at Isfahan University of Medical Sciences in Iran.
Author information
Authors and Affiliations
Contributions
“SM, MZ and ME designed this study. SM, MZ and ME contributed to the conduct of the trial. SM and NC-h performed the statistical analysis and interpreted the results. SM wrote the initial manuscript. AW and RB critically revised the manuscript and contributed to the subsequent drafts of the manuscript. PA and Mahsa Zarpoosh were contributes to revise. All authors approved the final version of the manuscript”.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All study protocols were carried out according to the Declaration of Helsinki. All patients provided written informed consent before participation in the trial. The study protocol was approved by the ethical committee at the Isfahan University of Medical Sciences (code: IR.MUI.RESEARCH.REC.1398.436, approval date 23/10/2019) and registered online at http://www.IRCT.ir (code: IRCT20191204045612N1, register date 05/04/2021).
Consent for publication
All authors have agreed to submit the manuscript to BMC Complementary Medicine and Therapies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moradi, S., Bagheri, R., Amirian, P. et al. Effects of Spirulina supplementation in patients with ulcerative colitis: a double-blind, placebo-controlled randomized trial. BMC Complement Med Ther 24, 109 (2024). https://doi.org/10.1186/s12906-024-04400-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04400-w