Abstract
Introduction
Oral lesions are a common clinical symptom arising from various etiologies and disrupt the patient’s quality of life. However, no definite treatment is not yet possible, due to the constantly changing environment of the mouth. In recent years, herbal treatments have gained popularity among patients and physicians due to their availability, safety, affordability, and antimicrobial properties. This research aims to investigate the therapeutic effects of a nano-emulsion of Plantago major standardized extract (PMSE) on oral ulcers in a Wistar rat model using histomorphometry and stereological parameters.
Materials and Methods
The study involved 72 Wistar rats divided randomly into 24 groups of 3 each: groups A1 to A4 received one dose to 4 doses of 5% PMSE nano emulsion, groups B1 to B4 received one dose to 4 doses of 10% PMSE nano emulsion, and groups C1 to C4 received one dose to 4 doses of 20% PMSE nano emulsion, groups D1 to D4 received one dose to 4 doses of nano-emulsion without PMSE, groups E1 to E4 received one dose to 4 doses of PMSE, and group F served as the control group. An incision measuring 2 mm in diameter was made in the animals’ hard palate using a biopsy punch. A swab containing the necessary material was used to administer the medication orally to the wound. Histological samples were collected on days 2, 4, 6, and 8 and sent to the pathology laboratory for examination. Data analysis was performed using SPSS 26 and setting statistical significance at p < 0.05.
Results
Group A showed a high rate of complete and normal re-epithelialization of the wound at 66.7%, compared to the other groups. Group D had a re-epithelialization rate of 50%, while groups C, E, and F had rates of 7.41% and group B had 7.16%. In terms of inflammation reduction, 23.88% of group A had no inflammation, a higher percentage compared to the other groups. Group B and D had no inflammation in 3.33% of cases, lower than the other groups. The study evaluated frequency of re-epithelialization and inflammation levels in different groups on days 2, 4, 6, and 8 after four doses of the drug with no significant differences found among the groups.
Conclusion
None of the nano emulsions or PMSE enhanced the healing rate of oral ulcers. However, a 5% PMSE nano emulsion displayed an increase in lesion re-epithelialization.
Key message
• Design of PMSE nano emulsion on oral wound healing.
• Select a superior formulation for PMSE nano-emulsion on oral wound healing.
• The composition of 5% PMSE nano emulsion is suggested for healing oral wounds.
Similar content being viewed by others
Introduction
A common reason for a dental visit is oral ulcers, which appear as slightly depressed lesions with well-defined borders, with or without intact epithelium. These lesions usually have a yellowish-white appearance, are acute or chronic, and are caused by various factors [1].
Oral ulcers can occur in any age group or population. Diseases, stress, and anxiety are common causes of oral wounds. In addition, factors such as consuming acidic or spicy foods, using stimulants, chemicals in toothpaste or mouthwash, chewing inside the cheek or biting the tongue, wearing braces, inappropriate prostheses, and certain genetic factors can also contribute to these issues [2].
Symptoms of oral ulcers vary depending on the type and size. Small ulcers, known as minor ulcers, heal within one to two weeks without scarring. Oral ulcers that are large in size and depth take longer to heal, up to six weeks for rough edges to heal [3].
The wound healing process is complex and involves several cellular and biochemical events to restore skin and mucosal integrity. This process includes three stages: inflammation, proliferation (including neoangiogenesis, granulation, and epithelial regeneration), and maturation (including extracellular matrix regeneration) [4,5,6]. The healing process of a wound, which may appear as a necrotic spot in the oral cavity, depends on the rate of epithelial proliferation, inflammation control, and prevention of microbial infection. This process typically lasts between twelve- and 16-days [7].
According to a new global trend, there is an increasing demand for improved patient compliance. This trend has led people to turn to nature-based treatments, specifically medicinal plants. With a wide range of medicinal properties, and diverse biological studies [8,9,10,11], medicinal plants have become an important consideration for advancing global health care.
Extensive studies have reported the use of herbal preparations for treating oral lesions [12,13,14,15,16]. On the other hand, despite the potential advantages of medicinal plants that can act through multiple mechanisms and have fewer side effects than chemical drugs, various challenges such as low stability, poor absorption, and limited bioavailability still exist for them.
The use of new drug delivery systems such as nano emulsions, niosomes, phytosomes has become increasingly important in improving drug release and concentration in tissues or blood, increasing the bioavailability of drugs, and controlling the drug delivery process [17,18,19].
Nano emulsions are transparent or semi-transparent, thermodynamically unstable systems with droplet sizes below 200 nm. They offer good optical clarity, ease of preparation, increased surface area, and faster drug release [20]. Due to their small particle size, nano emulsions are less prone to destabilizing mechanisms such as sedimentation, flocculation, creaming, and coagulation [21]. These delivery systems have been used for the local and systemic applications of many synthetic and herbal drugs. For example, a study by Hajiliani et al., showed that the nano formulation of plant extracts and natural compounds, while more effective in wound healing, requires a lower dose for complete treatment [22]. Another example is a topical nano emulsion containing vitamins C and E and propolis in oral surgery, which promotes rapid wound healing in the first three days of treatment through anti-inflammatory effects after surgery [23].
The leaves of plantain (Plantago major L.) from the Plantaginaceae family have traditionally been used to heal wounds. In addition to treating various skin diseases, this plant has analgesic and anti-inflammatory effects [24, 25]. Many studies in the literature have reported the wound-healing effect of this plant. For example, the use of a 10% topical gel made from plantain leaves accelerates the healing of diabetic foot ulcers and improves pressure ulcers in patients. This treatment reduces erythema and wound size [26].
In a clinical study, plantain significantly reduced the severity of mucositis, pain intensity, and dry mouth compared to placebo [27]. In another study, the wound-healing effect of the plant in pigs has been reported and attributed to its polyphenol content [28]. In hyperglycemic rats, plantain has been shown to accelerate wound closure. This is attributed to the presence of ursolic acid [29]. Additionally, a gel containing 5% plantain and Aloe vera has been found to accelerate wound closure in rats [30].
Extensive toxicology studies have not been conducted following the topical use of the plant, but it is generally considered to be relatively safe [27]. In terms of oral consumption, the plant’s LC50 and LD50 are reported as 4.74 (µg/mL) and 182.54 (mg/kg) respectively. The average effective dose (ED50)of the plant was determined to be 7.507 mg/kg [31].
In a study involving topical use, a 10% P. major ointment was applied to the dorsal neck of mice after an injury. Monitoring the recovery for up to 21 days post-treatment, exhibited no significant complications or mutations reported in histological studies and mutagenicity tests [32].
Therefore, the main goal of the current study was to evaluate the impact of different concentrations of PMSE nano-emulsions on the re-epithelialization and inflammation of intraoral lesions. The secondary objective was to assess the effectiveness of the most optimal concentration and formulation for PMSE nano-emulsion. The null hypothesis was that different concentrations of PMSE nano-emulsion would not have significant effect on the re-epithelialization and inflammation of intraoral lesions.
Materials and methods
This study was an animal study that utilized histomorphometric and serologic parameters to evaluate the therapeutic impacts of PMSE nano-emulsions on intraoral ulcers in a Wistar rat model.
Preparing the extract and phytochemical tests
Plantain leaves were gathered from Kerman province in July 2022 and authenticated in the Department of Pharmacognosy, Faculty of Pharmacy. A voucher specimen of the plant was kept in the Herbarium Center of the Department (KF1251). The leaves were dried, ground, and sieved through a sieve (35 mesh). The plant’s leaves were evaluated concerning secondary herbal metabolites, including alkaloids, flavonoids, tannins, saponin, and terpenoids [33].
Quality control of the plant
To assess the quality of the collected plantain leaves, experiments were conducted to determine the amount of water, total ash, ash insoluble in acid, and the amount of plant extractable materials. These assessments were performed using the methods outlined previously [34].
Standardization of plantain leaves
After conducting phytochemical tests and confirming the presence of significant amounts of phenolic compounds in the plant, the total content of phenolic compounds was determined and used for standardizing purposes.
The content of phenolic compounds was determined using the Folin-Ciocalteu method. Initially, a gallic acid stock solution (1000 ppm) was prepared. A mixture of 0.1 ml of gallic acid, 0.4 mL of sodium carbonate, 0.5 mL of Folin reagent, and 3 mL of distilled water was incubated at room temperature for 40 min.
The absorbance spectrum was then recorded at 200–800 nm, revealing the maximum absorbance wavelength of gallic acid (ℷ max = 765 nm). Various dilutions (50, 100, 200, 300, 400, 500, and 1000 ppm) were prepared to create a calibration curve based on absorbance at ℷ max.
Additionally, different concentrations of the plant extract (250, 500, and 1000 ppm) were analyzed for their absorbance. By referring the calibration curve, the content of the phenolic compounds was quantified in terms of gallic acid equivalents. Each sample was measured three times, and the results were reported as mean ± standard errors [35].
Formulation
The plantain leaves extract was prepared using the sonication method with ethanol 80%. To create the nano-emulsion, surfactants were first prepared using various solutions with weight percentages below the critical micelle concentration (CMC) range.
Once the solutions were ready, they were heated to 50ºC for 15 min to initiate the nucleation process and dissolve the raw materials completely.
Ultrasonic waves were then applied to form micelles with micrometer dimensions, while the mixture was placed in a reflex system. Any unreacted materials were removed by heating the mixture on a stirrer for 72 h.
Next specific concentrations of plantain extract were prepared using liquid-liquid extraction methods. The extract was then loaded into surfactant vectors at 1%, 5% and 10% w/w.
The extract was prepared in 20-ml volumes of micro-surfactants at a 2:1 ratio, heated for 30 min at (Elma-Ultrasonic, Germany) and simultaneously placed in a reflex system (Kimia Aghigh, Iran). To remove the unreacted materials from the surfactant, they were placed on a heater stirrer (Heidolph Co., Germany) for 72 h.
In the next stage, specific concentrations of plantain extract were prepared using the chemical methods of liquid-liquid extraction. Then a synchronized method was used to load plantain extract in surfactant vectors at 1, 5, and 10 w/w% as a default. First, specific concentrations of plantain extract were prepared in 20-mL volumes of micro-surfactants at a 2:1 ratio. The resulting mixture was heated for 30 min at 40ºC, and then cooled to remove the organic solvent phase.
The pH of the solution was monitored using a digital pH meter. Coprecipitation and nucleation conditions were then achieved under alkaline conditions.
Finally, the plantain extract was placed in a sonicator (Samsung, Japan) to offer an extract-containing nano-emulsion. The resulting product was evaluated for its physicochemical properties.
Animal preparation
The study involved 72 Wistar rats divided randomly into 24 groups of 3 each: groups A1 to A4 received one dose to 4 doses of 5% PMSE nano-emulsions), groups B1to B4 received one dose to 4 doses of 10% PMSE nano-emulsions, and groups C1 to C4 received one dose to 4 doses of 20% PMSE, groups D1 to D4 received one dose to 4 doses of nano-emulsion without PMSE, groups E1 to E4 received one dose to 4 doses of PMSE, and group F served as the control group.
The animals were housed separately in cages, with 12 rats per cage, at the Animal Laboratory of the Oral and Dental Research Center at Kerman University of Medical Sciences.
The randomization and data collection processes were carried out blindly by a dental student and a veterinarian in charge of the laboratory. The veterinarian provided training to the dental student on the necessary care for the animals. A pharmaceutical specialist assigned numbers to the medications and the researcher was blinded to the numbering system. The pathologist and statistical consultant involved in the study were also blinded.
All study processes adhered to international guidelines on animal care and were conducted under the supervision of the Ethics Committee of Kerman University of Medical Sciences, with the code IR.KMU.REC.1400.202.
The animal house maintained a temperature range of 23 ± 2 ºC, provided all animals with a soft diet, and ensured proper ventilation, temperature, and moisture control., and a standard light-dark cycle of 12 h each.
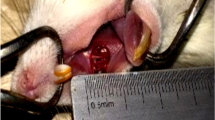
Each animal was secured on a dissection table, with a tweezer attached to a lower incisor tooth, to keep the mouth open. General anesthesia was administered on the first day using 10% ketamine (Bremer Pharma, Germany) at a dose of 50 mg/kg and 2% xylazine (Rompun) (Kelay, Belgium) as a dose of 10 mg/kg as a muscle relaxant via intraperitoneal injection [36]. An incision measuring 2 mm in diameter was made in the animals’ hard palate using a biopsy punch. After clot formation, approximately two drops or the equivalent of 0.1 cc of medication were used for each lesion of 60 animals daily at a specific hour using a swab. The remaining 12 rats (group F) served as control. Ulcers were clinically examined for 8 days before reapplying the medication. The animals were sedated throughout the study. On days 2, 4, 6, and 8, three animals from each group of 12 were euthanized with an overdose of 10% ketamine and 2% xylazine following ethical guidelines. Ulcers were excised with surrounding tissues (Fig. 1) and fixed in 10% formalin. Specimens were processed. Embedded in paraffin, stained with H&E, and evaluated by a blinded pathologist under a light microscope at ×40 (Nikon, Japan). Histopathological criteria for ulcers included tissue inflammation, and samples were scored from 1 to 5 based on epithelialization (Tables 1 and 2) [37]. A CONSORT Flow Diagram illustrating the study protocol is shown in Fig. 2.
Since the data were not normally distributed, the non-parametric tests including Fisher’s exact test, Kruskal-Wallis test, and Mann-Whitney test were used to compare animals and epithelialization scores between the groups. The data were analyzed using PSS 26 at a significance level of p < 0.05.
Results
Extraction, quality control and standardization of P. major
The plant extraction procedures yielded an extract of 11.3% w/w. Phytochemical analyses confirmed the presence of alkaloids, flavonoids, tannins, and terpenoids in the plant.
Based on the results of plant quality control tests, the plant moisture content, total ash, acid-insoluble ash, and the percentage of extractable materials were determined to be 4.61%, 8.67%, 1.96% and 9.53% respectively.
The total phenolic compounds in the plant were quantified using a gallic acid calibration curve, resulting in a measurement of 6.14 ± 0.86 mg gallic acid equivalent /100 mg dried leaves.
The results of re-epithelialization activity
According to the results of the present study, the rate of complete, normal re-epithelialization of ulcers in group A was 66.7%, which was higher compared to the other study groups. Group D had a rate of 50%, while groups C, E, and F had rates of 41.7%. group B had the lowest rate of 16.7%. Furthermore, groups B, D, and F exhibited a complete re-epithelialization rate of 41.7%, while groups A and C had a rate of 33.3% with irregular ulcer borders.
Group A demonstrated a higher re-epithelialization than other groups (Table 3; Fig. 3). The application of a PMSE nano-emulsion did not show a statistically significant effect on re-epithelialization score compared to the control group (P = 0.178).
Moreover, inflammation compression between the study groups showed no inflammation in 88.3% of the cases, which was higher than in other groups. In addition, in groups B and E, no inflammation was observed in 33.3% of the cases, which was lower than in the other groups (Table 4; Fig. 4). The application of a PMSE nano-emulsions did not show a statistically significant effect on inflammation score and compared to the control group (P = 0.862).
According to Fisher’s exact test, no significant difference was observed in epithelialization and inflammation between the groups on days 2, 4, 6, and 8. Figures 5 and 6 present the histopathologic views of ulcers treated with 5% and 10% PMSE nano-emulsions.
Discussion
The results of the present study indicated that a 5% PMSE nano emulsion increased the rate of complete re-epithelialization in 66.7% of cases and reduced inflammation in 88.3% of intraoral wound cases more than other groups. The 10% and 20% nano emulsions showed less effect on re-epithelialization and inflammation.
The wound-healing effect of plantain has been reported in various sources [26, 28, 30, 38]. However, in this study, the group E that received plantain extract alone did not show significant improvement in terms of re-epithelialization and reduction of inflammation. This lack of improvement may be due to technical errors in sampling, such as sampling from an area with inflammation or a diagonal cut.
On the other hand, climate is one of the factors that affects the active ingredients of plants and their biological effects [39]. Therefore, the plantain sample used in the current study, collected from Kerman, may differ from other research samples in terms of active ingredients due of different climate conditions. This necessitates more comprehensive phytochemical studies.
Several mechanisms are involved in promoting wound-healing. Natural remedies can aid in this process by reducing scar formation. Metabolites with antimicrobial and antioxidant effects help prevent infection at the scar site, accelerate blood coagulation, and promote wound healing [40]. Reactive oxygen species (ROS) cause cell damage and play a crucial role in initiating the natural wound-healing response. To stimulate effective wound healing, it is important to protect tissues from infection and activate cell survival signals [41, 42].
Antioxidants prevent damage caused by free radicals to other vital molecules such as proteins or DNA. They activate a cascade of reactions that convert ROS into more stable molecules, and reduce the non-toxic levels of ROS to protect tissues [43]. Antioxidants are kept in the wound tissues and help improve the healing process.
On the other hand, inflammation is important for preventing microbial infection and removing dead cells. However, increasing the intensity and duration of inflammation can lead to scars, fibrosis and slow down wound healing duction of anti-inflammatory mediators becomes more crucial in the later stages of wound- healing.
Anti-inflammatory agents could help accelerate this stage [44]. In the natural process of wound healing, the immune system responds rapidly to eliminate invading pathogens. This includes gram-positive microbes in the early stages of wound development and gram-negative organisms in the later stages of infection, thus preventing infection.
Without a proper immune response, the destruction of granulation tissue, growth factors, and extracellular matrix components can occur, disrupting the natural healing process. The use of antimicrobial compounds can help reduce the challenges of wound- healing [45].
Plantain is rich in antioxidant compounds, especially phenolic ones, which are believed to be a key factor in its wound-healing properties [46]. Additionally, antimicrobial activity of this plant has been reported. Apart from its antioxidant and antibacterial activity, plantain also possesses the ability to shield cells from damage caused by inflammatory mediators [47].
Ghanadian et al. reported that plantain extract has anti-inflammatory, antioxidative, and antibacterial properties due to its ingredients, including flavonoids such as hispidin and baicalin. The plantain prevents the release of prostaglandins and histamine to avoid their destructive impacts on tissues and increases nitric oxide production [26], inducing cell proliferation in vitro, which might be a crucial stage in the wound healing procedure [48].
Various compounds in the leaves of the plant may help in healing wounds. For example, the antibacterial activity of plantamajoside and acteoside [49] or the anti-inflammatory effect of its iridoides and terpenoids such as plantamajoside, baicalein, aucubin, and ursolic acid [50] can be observed. The pectic polysaccharides of the plant have immune-stimulating activity [25], and many of the phenolic compounds of the plant, especially its flavonoids and caffeic acid derivatives (plantamajoside and acteoside), are associated with antioxidant activity and inhibition of free radicals in wound closure and rapid healing [51].
Plant leaf wax, which contains long-chain saturated primary alcohols, has the ability to heal surface wounds [25]. The high concentration of mucilaginous carbohydrates in the plant acts as an immunostimulant and antioxidant and promotes wound closure [52].
The exact activity of these known substances in combination with many unknown compounds of the plant is not definitively known, and the healing effect of the plant may be more complex than the mechanism mentioned.
The comparison between nano emulsion with and without extract shows that the increased effectiveness of herbal nano emulsion is probably due to the optimal delivery of plantain by nano emulsions. It appears that 5% PMSE nano emulsion penetrates into the wound properly. The lower effectiveness of 10% and 20% of the nano emulsions might be due to less drug release and penetration into the wound.
On the other hand, perhaps one of the reasons we could not observe a significant difference between experimental groups is due to the location of oral lesions compared to skin ulcers, which have less controlled conditions. In oral lesions, the drug may be washed away by saliva and the duration of medicine contact will be reduced.
Sucweenadhi et al. (2021) reported that in the preparation of plantain nanoparticles at concentrations of 2% and 3%, agglutination occurs in plant particles and prevents the formation of nanoparticles, but not at the concentration of 0.25%, where no agglutination occurs. Therefore, the nano emulsion exhibits superior effects at lower concentrations [53]. This may be due to improved drug delivery to the target tissue.
Fidoski et al., reported that a combination of vitamin C-containing nano-emulsion and propolis helps the direct absorption of the medication due to its nanostructure and creates a protective bioactive wall on the ulcer, which prevents the oral environment moisture from reaching the ulcer, positively affecting wound healing [23].
On the other hand, the hermetic phenomenon is a common occurrence in the dose-response of herbal medicines. In this phenomenon, there is no logical relationship between drug dose and response. One contributing factor to this phenomenon is the activation of plant components at high doses, resulting in unpredictable responses due to pharmacodynamic interactions (or pharmacokinetics in living organisms). This phenomenon plays a positive or negative role in inducing the desired effect in the plant [54].
In the current study, it was challenging to measure the size of the wound during the study period due to the small size and rapid healing in the palate of the rats. Therefore, it is recommended to create larger wounds in more prominent anatomical points or utilize larger animals in future studies. Additionally, conducting more studies with higher concentrations of this medicinal extract, larger sample sizes, and human studies with longer follow-up periods are necessary to more accurately evaluate the effect of this extract on wound healing.
Limitations
In order to draw better and more accurate conclusions from this study, it is necessary to perform biochemical analysis of inflammation, and collagen, as well as measure anti-inflammatory and pro-inflammatory cytokines, and immunohistochemical markers. These studies will surely be conducted in future studies.
One of the challenges in investigating the wound-healing effects on oral lesions is the location of the lesions created and the high likelihood of the drug being washed away immediately. Despite the strategies employed, this risk is significant and remains a major factor in achieving the desired result.
Conclusion
Many studies have been conducted on the wound-healing effect of the plant, but it has not been investigated in the treatment of oral ulcers. Based on the results of the present study, it is recommended that the 5% plant nano emulsion be considered as a suitable alternative to traditional medicaments for oral ulcers. This recommendation is supported by its cost-effectiveness, ease of preparation, and positive impact on the wounds in animal models. Clinical trials are needed to confirm these findings and establish the product’s efficacy for routine use.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Greenberg MS. Ulcerative, vesicular, and bullous lesions. Burket’s Oral Medicine Diagnosis & Treatment 2003.
Pannerselvam B, Jothinathan MKD, Rajenderan M, Perumal P, Thangavelu KP, Kim HJ, Singh V, Rangarajulu SK. An in vitro study on the burn wound healing activity of cotton fabrics incorporated with phytosynthesized silver nanoparticles in male Wistar albino rats. Eur J Pharm Sci. 2017;100:187–96.
Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1–2):241–51.
Yang Y, Wang F, Yin D, Fang Z, Huang L. Astragulus polysaccharide-loaded fibrous mats promote the restoration of microcirculation in/around skin wounds to accelerate wound healing in a diabetic rat model. Colloids Surf B. 2015;136:111–8.
Ranjbar-Mohammadi M, Rabbani S, Bahrami SH, Joghataei M, Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater Sci Engineering: C. 2016;69:1183–91.
Vargas ET, do, Vale Baracho N, De Brito J, De Queiroz A. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta biomaterialia 2010, 6(3):1069–1078.
Vélez I, Tamara LA, Mintz S. Management of oral mucositis induced by chemotherapy and radiotherapy: an update. Quintessence Int. 2004;35(2):129–36.
Noudeh GD, Sharififar F, Noodeh rD, Sakhtianch R. Antitumor and antibacterial activity of four fractions from Heracleum Persicum Desf. And Cinnamomum zeylanicum Blume. J Med Plants Res. 2010;4:2176–80.
Sharififar F, Mirtajadini M, Azampour MJ, Zamani E. Essential oil and methanolic extract of Zataria multiflora Boiss with anticholinesterase effect. Pak J Biol Sci. 2012;15(1):49–53.
Son MJ, Kim YE, Song YI, Kim YH. Herbal medicines for treating acute otitis media: a systematic review of randomised controlled trials. Complement Ther Med. 2017;35:133–9.
Khan MA, Kassianos AJ, Hoy WE, Alam AK, Healy HG, Gobe GC. Promoting plant-based therapies for chronic kidney disease. J Evid Based Integr Med. 2022;27:2515690X221079688.
Prayoga DK, Aulifa DL, Budiman A, Levita J. Plants with anti-ulcer activity and mechanism: a review of preclinical and clinical studies. Drug Des Devel Ther. 2024;18:193–213.
Hamedi S, Sadeghpour O, Shamsardekani MR, Amin G, Hajighasemali D, Feyzabadi Z. The most common herbs to cure the most common oral disease: Stomatitis Recurrent Aphthous Ulcer (RAU). Iran Red Crescent Med J. 2016;18(2):e21694.
Eubank PLC, Abreu LG, Violante IP, Volpato LER. Medicinal plants used for the treatment of mucositis induced by oncotherapy: a systematic review. Support Care Cancer. 2021;29(11):6981–93.
Srivastava A, Gc S, Pathak S, Ingle E, Kumari A, Shivakumar S, Pg NK, Singh AK. Evidence-based effectiveness of herbal treatment modality for recurrent aphthous ulcers - a systematic review and meta-analysis. Natl J Maxillofac Surg. 2021;12(3):303–10.
Li CL, Huang HL, Wang WC, Hua H. Efficacy and safety of topical herbal medicine treatment on recurrent aphthous stomatitis: a systemic review. Drug Des Devel Ther. 2016;10:107–15.
Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M. Proliferation and in Vitro Wound Healing effects of the microniosomes containing Narcissus tazetta L. Bulb extract on primary human fibroblasts (HDFs). Daru. 2018;26(1):31–42.
Raeiszadeh M, Pardakhty A, Sharififar F, Mehrabani M, Nejat-Mehrab-Kermani H, Mehrabani M. Phytoniosome: a Novel Drug Delivery for Myrtle Extract. Iran J Pharm Res. 2018;17(3):804–17.
Puglia C, Lauro MR, Tirendi GG, Fassari GE, Carbone C, Bonina F, Puglisi G. Modern drug delivery strategies applied to natural active compounds. Expert Opin Drug Deliv. 2017;14(6):755–68.
Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28(5):978–85.
Koroleva MY, Yurtov EV. Nanoemulsions: the properties, methods of preparation and promising applications. Rus Chem Rev. 2012;81(1):21.
Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomed 2018:5023–43.
Fidoski J, Benedetti A, Kirkov A, Iliev A, Stamatoski A, Baftijari D. Nano-Emulsion complex (propolis and vitamin C) promotes wound healing in the oral mucosa. Oral Maxillofacial Pathol J 2020, 11(1).
Farid A, Sheibani M, Shojaii A, Noori M, Motevalian M. Evaluation of anti-inflammatory effects of leaf and seed extracts of Plantago major on acetic acid-induced ulcerative colitis in rats. J Ethnopharmacol. 2022;298:115595.
Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol. 2000;71(1–2):1–21.
Ghanadian M, Soltani R, Homayouni A, Khorvash F, Jouabadi SM, Abdollahzadeh M. The effect of Plantago major hydroalcoholic extract on the healing of diabetic foot and pressure ulcers: a randomized open-label controlled clinical trial. Int J Low Extrem Wounds 2022:15347346211070723.
Najafian Y, Hamedi SS, Farshchi MK, Feyzabadi Z. Plantago major in traditional Persian Medicine and modern phytotherapy: a narrative review. Electron Physician. 2018;10(2):6390–9.
Baldea I, Lung I, Opris O, Stegarescu A, Kacso I, Soran ML. Antioxidant, anti-inflammatory effects and ability to stimulate Wound Healing of a common-plantain extract in Alginate Gel formulations. Gels 2023, 9(11).
Kartini K, Wati N, Gustav R, Wahyuni R, Anggada YF, Hidayani R, Raharjo A, Islamie R, Putra SED. Wound healing effects of Plantago major extract and its chemical compounds in hyperglycemic rats. Food Bioscience. 2021;41:100937.
Ashkani-Esfahani S, Khoshneviszadeh M, Noorafshan A, Miri R, Rafiee S, Hemyari K, Kardeh S, Hosseinabadi OK, Fani D, Faridi E. The healing effect of Plantago major and Aloe vera mixture in excisional full thickness skin wounds: stereological study. World J Plast Surg. 2019;8(1):51.
Logarto Parra A, Silva Yhebra R, Guerra Sardinas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8(5):395–400.
Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, Frade MAC. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37.
Sarhadynejad Z, Sharififar F, Pardakhty A, Nematollahi MH, Sattaie-Mokhtari S, Mandegary A. Pharmacological safety evaluation of a traditional herbal medicine Zereshk-e-Saghir and assessment of its hepatoprotective effects on carbon tetrachloride induced hepatic damage in rats. J Ethnopharmacol. 2016;190:387–95.
Zahabi ZF, Sharififar F, Almani PGN, Salari S. Antifungal activities of different fractions of Salvia Rhytidea Benth as a valuable medicinal plant against different Candida species in Kerman province (Southeast of Iran). Gene Rep 2020, 19.
Khazaeli P, Goldoozian R, Sharififar F. An evaluation of extracts of five traditional medicinal plants from Iran on the inhibition of mushroom tyrosinase activity and scavenging of free radicals. Int J Cosmet Sci. 2009;31(5):375–81.
Green C, Knight J, Precious S, Simpkin S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab Anim. 1981;15(2):163–70.
Rosenstein ED, Kushner LJ, Kramer N, Kazandjian G. Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukot Essent Fatty Acids. 2003;68(3):213–8.
Amini M, Kherad M, Mehrabani D, Azarpira N, Panjehshahin M, Tanideh N. Effect of Plantago major on burn wound healing in rat. J Appl Anim Res. 2010;37(1):53–6.
Pant P, Pandey S, Dall’Acqua S. The influence of environmental conditions on secondary metabolites in Medicinal plants: a Literature Review. Chem Biodivers. 2021;18(11):e2100345.
Vitale S, Colanero S, Placidi M, Di Emidio G, Tatone C, Amicarelli F, D’Alessandro AM. Phytochemistry and Biological Activity of Medicinal plants in Wound Healing: an overview of current research. Molecules 2022, 27(11).
Scialo F, Fernandez-Ayala DJ, Sanz A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: potential roles in Health and Disease. Front Physiol. 2017;8:428.
Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired Wound Healing: a systematic review. Antioxid (Basel) 2018, 7(8).
Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89–96.
Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic Wound Healing. Int J Mol Sci 2017, 18(7).
Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol. 2010;28(5):519–26.
Yokozawa T, Dong E, Liu ZW, Shimizu M. Antioxidative activity of flavones and flavonols in vitro. Phytotherapy Research: Int J Devoted Med Sci Res Plants Plant Prod. 1997;11(6):446–9.
Zubair M, Widén C, Renvert S, Rumpunen K. Water and ethanol extracts of Plantago major leaves show anti-inflammatory activity on oral epithelial cells. J Traditional Complement Med. 2019;9(3):169–71.
Velasco-Lezama R, Tapia-Aguilar R, Roman-Ramos R, Vega-Avila E, Perez-Gutierrez MS. Effect of Plantago major on cell proliferation in vitro. J Ethnopharmacol. 2006;103(1):36–42.
Zhakipbekov K, Turgumbayeva A, Issayeva R, Kipchakbayeva A, Kadyrbayeva G, Tleubayeva M, Akhayeva T, Tastambek K, Sainova G, Serikbayeva E et al. Antimicrobial and other Biomedical properties of extracts from Plantago major, Plantaginaceae. Pharmaceuticals (Basel) 2023, 16(8).
Adom MB, Taher M, Mutalabisin MF, Amri MS, Abdul Kudos MB, Wan Sulaiman MWA, Sengupta P, Susanti D. Chemical constituents and medical benefits of Plantago major. Biomed Pharmacother. 2017;96:348–60.
Amakura Y, Yoshimura A, Yoshimura M, Yoshida T. Isolation and characterization of phenolic antioxidants from Plantago herb. Molecules. 2012;17(5):5459–66.
Wadhwa J, Nair A, Kumria R. Potential of plant mucilages in pharmaceuticals and therapy. Curr Drug Deliv. 2013;10(2):198–207.
Sukweenadhi J, Setiawan KI, Avanti C, Kartini K, Rupa EJ, Yang D-C. Scale-up of green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S Afr J Chem Eng. 2021;38(1):1–8.
Jalal A, de Oliveira Junior JC, Ribeiro JS, Fernandes GC, Mariano GG, Trindade VDR. Dos Reis AR: Hormesis in plants: physiological and biochemical responses. Ecotoxicol Environ Saf. 2021;207:111225.
Acknowledgements
The authors would like to express their gratitude to the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences.
Funding
The current study was carried out based on research project number 99001142approved and financed by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
F.JM and F.F wrote the main manuscript text andAJ Methodology, Writing, MR, MT Methodology, SK Data curation, F.SHprepared Figs. 1, 2, 3, 4 and 5. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This investigation was carried out in accordance with the revised animals Act 1986 in the UK used for scientific procedure, and confirm the study complied with the ARRIVE guidelines. Animal selection, management, surgical protocol, and preparation followed a routine protocol approved by the Institutional Animal Care and Ethics Committee of the Kerman University of Medical Sciences under the code IR.KMU.REC.1400.202.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jahanimoghadam, F., Javidan, A., Ranjbar, M. et al. The healing effect of nano emulsified Plantago major L extract on oral wounds in a wistar rat model. BMC Complement Med Ther 24, 327 (2024). https://doi.org/10.1186/s12906-024-04621-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04621-z