Abstract
Background
An essential component of future-proofing health systems against future pandemics and climate change is strengthening the front lines of care: principally, emergency departments and primary care settings. To achieve this, these settings can adopt learning health system (LHS) principles, integrating data, evidence, and experience to continuously improve care delivery. This rapid review aimed to understand the ways in which LHS principles have been applied to primary care and emergency departments, the extent to which LHS approaches have been adopted in these key settings, and the factors that affect their adoption.
Methods
Three academic databases (Embase, Scopus, and PubMed) were searched for full text articles reporting on LHSs in primary care and/or emergency departments published in the last five years. Articles were included if they had a primary focus on LHSs in primary care settings (general practice, allied health, multidisciplinary primary care, and community-based care) and/or emergency care settings. Data from included articles were catalogued and synthesised according to the modified Institute of Medicine’s five-component framework for LHSs (science and informatics, patient-clinician partnerships, incentives, continuous learning culture, and structure and governance).
Results
Thirty-seven articles were included, 32 of which reported LHSs in primary care settings and seven of which reported LHSs in emergency departments. Science and informatics was the most commonly reported LHS component, followed closely by continuous learning culture and structure and governance. Most articles (n = 30) reported on LHSs that had been adopted, and many of the included articles (n = 17) were descriptive reports of LHS approaches.
Conclusions
Developing LHSs at the front lines of care is essential for future-proofing against current and new threats to health system sustainability, such as pandemic- and climate change-induced events. Limited research has examined the application of LHS concepts to emergency care settings. Implementation science should be utilised to better understand the factors influencing adoption of LHS approaches on the front lines of care, so that all five LHS components can be progressed in these settings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the last three decades, the performance of modern healthcare systems has remained stagnant. Challenges posed by rising healthcare costs, aging populations, and chronic disease burden have not been sustainably addressed, instead being met with top-down change efforts and fragmented healthcare delivery models that constrain system efficiency and progress [1,2,3]. Approximately 60% of care is delivered in line with guidelines, 30% of care is of little value, and 10% of care is harmful to patients [3]. Learning health systems (LHSs) have been recommended as a solution to improve the quality of care delivery, by leveraging big data and exploiting knowledge to create continuous improvement [3, 4].
Since the concept of an LHS was discussed in a seminal 2007 publication by the Institute of Medicine (IoM; now the National Academy of Medicine) [5], research interest in LHS development and application has proliferated [6, 7]. In 2013, the IoM defined four key, inter-related components of an LHS: science and informatics (access, capture, and synthesis of real-time clinical data and care experiences) patient-clinician partnerships (patients, families, and caregivers fully engaged as partners in care), incentives (aligned with continuous improvement and full transparency), and continuous learning culture (leadership-driven collaboration and skill-building) [8, 9]. Work in 2020 by Zurynski et al. added a fifth component: structure and governance, which included policies, regulations, and governance structures aligned with, and in support of, continuous learning and collaboration [9].
To be sustainable, health systems must be accomplished at using data, embedding knowledge into practice, and improving decision making for healthcare professionals and patients at the front lines of care: primary care and emergency departments. Primary care (PC) is typically the first point of contact for people in healthcare systems across the world, and provides both preventative and curative care [10], while emergency departments (EDs) deal with critical, acute medical incidents that require immediate attention [11].
The pressures on these settings are continually in flux; for example, the COVID-19 pandemic brought an increased volume and complexity of patient presentations, staff shortages, and funding limitations, particularly affecting countries with more fragile health systems [12, 13]. Even prior to the pandemic, PC and ED settings in many countries were under considerable pressure. ED visits have been steadily increasing over the last decade in many health systems, a significant proportion of which report ‘inappropriate’ or non-urgent cases, and which could have been managed in PC [11, 14]. Determinants for the rising demand in emergency care include a rapidly ageing population, low availability of PC providers, and financial constraints on communities in economic crises [11].
Despite arguments to deflect cases from EDs to PC settings, they are under substantial stress. There have been global calls to strengthen PC systems to cope with surges in demand associated with the growing burdens of chronic disease [15]. PC plays a critical role in improving health outcomes, health system efficiency and health equity, and there are persuasive arguments that PC is integral to contributing to economic stability and growth [16, 17]. The urgent need for solutions that improve the quality and safety of care in PC and ED settings is heightened when considering the increasing threats exemplified by COVID-19, and of climate change on health system sustainability [18]. Greater frequency and intensity of extreme weather events, such as bushfires and floods, and the harm and diseases associated with them, place additional pressures on health and social care systems, giving rise to new diseases and exacerbating existing illnesses [19, 20]. Care delivery models must adapt and respond to not only acute events, but to the projected increase in volume and complexity of patients, to build resilience against future pandemics and climate change-induced disasters [19, 21].
Future-proofing the front lines of care against current and new threats relies on developing and implementing systems that can improve and learn on-the-go [22]. LHSs can better adapt to constant change, enabling greater support for high quality care rather than just finding acute and reactive solutions to problems [23]. Although LHS concepts have been much discussed in the literature [4], there are few rigorous formative and summative assessments of them [7]. Understanding how far we have progressed in applying LHS approaches to the front lines of care will aid in formulating LHSs 2.0 – LHSs that are prepared for looming health system threats [21].
Objective
This rapid review aimed to understand the breadth and range of LHS approaches used in PC and ED settings, the extent to which LHS approaches have been adopted in these key settings, and the barriers and facilitators associated with their adoption.
Methods
Review protocol and registration
The rapid review was conducted in accordance with the Cochrane Library guidelines for rapid reviews [24] and followed a registered protocol on PROSPERO (CRD42023416536).
Search strategy
Comprehensive search strategies were developed to capture LHS concepts as applied to PC and ED settings. Three databases (Embase, Scopus, and PubMed) were searched on 14th March 2023 (see Supplementary Material 1 for Embase search strings). Searches were limited to publications written in English and published from 1st January 2018 to 14th March 2023, to focus on contemporary LHS research from the last five years.
Article selection
References were downloaded from databases into Endnote where duplicates were identified and removed. Titles and abstracts were screened within Endnote by two team members (GD, SS) according to inclusion and exclusion criteria (Table 1). PC settings were classified as the first service sought by a patient outside of a hospital or specialist service, including diagnostic and treatment services and long-term care, health promotion, and prevention services [25]. In accordance with Cochrane Library rapid review guidelines [24], 20% of references were independently screened by two reviewers to establish reliability of screening decisions, with the interrater reliability assessed to be sufficiently high (κ ≥ 0.80).
After title and abstract screening, the full texts of articles deemed potentially relevant were reviewed by three team members (GD, SS, LP), who initially independently screened 20% of articles, assessing for eligibility based on the inclusion and exclusion criteria (Table 1). Each team member then independently screened a third of the remaining 80% of articles, according to Cochrane guidelines [24]. For title, abstract, and full text screening, disagreements were resolved through discussion, and consultation with the broader team (GF, LAE, CLS, JB) as needed.
Data extraction and synthesis
Data were extracted, organised and synthesised into key categories using a purpose-designed Excel data extraction sheet which was developed a priori by three team members (SS, GD, LP), piloted with a small subset (10%) of articles, and iteratively refined until the final version was reached [25]. Following the pilot of the data extraction sheet, the same three team members extracted data from the remaining 90% of articles, meeting regularly to ensure alignment in information extracted, discuss discrepancies, and reach consensus on data categorisation.
Categories of data extraction included the five components of the Zurynski et al. [9] LHS framework (science and informatics, patient-clinician partnerships, incentives, continuous learning culture, and structure and governance), article author, publication year, country of publication, study type, setting, LHS definitions (according to the Zurynski et al. [9] definition categories), barriers and facilitators associated with the adoption of LHSs (where applicable), and whether frameworks and/or models were used to guide exploration of barriers and facilitators. Extracted findings were organised and synthesised to identify patterns and explore relationships in the data [26]. Data were also quantified to analyse the extent and distribution of articles across the data extraction categories, and these numerical analyses were tabulated and summarised.
Quality assessment
As the included articles were broad in their publication design, three appraisal tools were used: 1) Mixed-Methods Appraisal Tool (MMAT) [27]; 2) Scale for the quality Assessment of Narrative Reviews (SANRA) [28]; and 3) Joanna Briggs Institute (JBI) Text and Opinion [29]. Each article was assessed by one of three team members (SS, GD, LP), and where there were any uncertainties, a second team member was consulted to discuss and reach consensus on quality ratings. If an article could not be comprehensively assessed using any of these tools (e.g., field reports describing methodologies), the authorship team met to make a judgement on its quality. This process involved the team discussing potential bias in the design, conduct, and analysis of studies, and whether each criterion was adequately addressed by the authors of the article in question.
Results
Search results
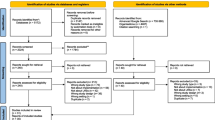
Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. Database searches yielded 388 references. After removing duplicates, 221 articles were screened by title and abstract against inclusion criteria. Of these, 147 did not meet eligibility criteria, leaving 74 for full-text review. Full text review yielded 37 articles eligible for inclusion (see reasons for exclusion in Fig. 1).
Characteristics of included articles
This rapid review included 37 articles that examined LHS components within PC (n = 32, 86%) and/or ED (n = 7, 19%) settings. Table 2 and Supplementary Material 2 display the characteristics of included articles. Most articles were published in North America (n = 25; 68%), followed by Europe (n = 7; 19%), and Africa (n = 3; 8%). The remaining two articles were published in Australia and Asia. Twenty articles (54%) reported on empirical findings from implementation work that progressed health systems toward an LHS [30, 31]. Seventeen articles (46%) were non-empirical and were largely focused on describing the process by which LHSs were adopted [32, 33], discussing the implications and reach of previous work [34, 35], or providing recommendations for future LHSs [36, 37]. See Supplementary Material 3 for the LHS components captured in each article.
Various definitions were cited in the included articles to define or describe components of an LHS. The IoM definition was the most consistently used, cited in nine articles (24%) [38,39,40,41,42,43,44,45,46]. Three articles (8%) used the Agency for Healthcare Research and Quality (AHRQ) definition of an LHS [47,48,49], and 8 articles (27%) referred to other reports of LHSs to define the concept [30, 50,51,52,53], including that of Foley and colleagues [31], and Menear and colleagues [54, 55]. Seventeen articles (46%) did not include a definition of an LHS [32, 34, 56,57,58,59,60].
Learning health system components across settings
Tables 2 and Supplementary Material 2 describe the LHS components across PC and ED settings that were extracted from the included studies. Of the 37 included articles, 32 (86%) explored LHS components or models within PC settings, including general practice (n = 14; 38%), community health (n = 13; 35%), multidisciplinary care (n = 9; 24%), and allied health (n = 5; 14%). Multidisciplinary settings included care-at-home models [34, 61], integrated care organisations or clinics [30, 54], and multidisciplinary community health services [43, 51]. Seven articles (19%) explored LHS components or models within the ED setting, and two articles (5%) explored LHSs in both the PC and ED setting.
In investigations of PC, most articles described LHSs that had been adopted, at least at pilot stage (n = 28; 76%), and four of these articles reported on the adoption of all five LHS components [30, 38, 48, 62]. Four PC-based articles (11%) recommended initiatives or innovations to facilitate LHS progression, and one of these articles recommended an LHS model that contained all five components [37].
Barriers to adopting LHSs in PC were reported in 18 articles (49%). Overarching barriers that prevented LHS progression in PC included difficulties scheduling the participation of clinicians and other intervention participants [32, 45,46,47,48, 50, 57] and limited resources (e.g., funding, staffing) to support interventions [32, 35, 44, 47, 55]. Facilitators of adopting LHS in PC were reported in 19 articles (51%). General facilitators included stakeholder buy-in [32, 34, 45, 46, 57] and forward planning [43, 46,47,48, 51, 55, 57] for interventions. Barriers and facilitators which applied to the adoption of specific LHS components in PC are described in the sections below. Two articles utilised a framework (Consolidated Framework for Implementation Research; CFIR) to explore barriers and facilitators associated with LHS explorations [47, 48].
Of the articles investigating ED settings, most reported on LHS components that had been adopted (n = 6; 16%), and one article (3%) made recommendations for the development and adoption of LHS components into ED. There were no articles that reported on an LHS model in an ED setting that contained all parts of the five-component framework. Barriers and facilitators associated with implementing LHS components in ED were reported in 3 articles (8%), all of which were specific to the LHS interventions conducted.
Science and informatics
Science and informatics was reported on in 34 articles (92%). In PC, systems and processes that leverage electronic health records (EHRs) were a common focus, aimed at improving medication safety [51], improving concordance with best practice guidelines [38, 39], facilitating quality improvement [35, 63], identifying high-risk patients [30], and streamlining clinical work [38, 39]. Web-based tools were also leveraged to improve evidence-based knowledge transfer between stakeholders [35, 63], and to build knowledge repositories to inform learning and practice [64].
Common barriers to embedding science and informatics interventions into PC included the complexity of data infrastructure [61], data standardisation and management issues within EHRs [34, 61], professional resistance to implementation [51], and underdeveloped relationships with information technology teams [34]. Facilitators included leadership buy-in for resource allocation [34], trusting relationships between healthcare professionals involved in implementation [51], and making iterative changes to process [51].
In ED settings, there was also a strong focus on utilising EHRs to improve decision-making accuracy based on patient data [65], guideline adherence [56], and critical care during COVID-19 [33, 49]. Similar to PC settings, there were several barriers to EHR use including unreliable or missing data, and a lack of information on social determinants of health (e.g., homelessness), both of which were crucial for decision-making [65, 66]. Facilitators of embedding science and informatics tools into EDs included local implementation adapted to contextual factors, and the involvement of clinicians and community stakeholders in the continual development of interventions [49, 65].
Continuous learning culture
Continuous learning culture was reported in 31 articles (84%). Within PC, continuous learning most frequently centred on the creation of teams or learning collaboratives to facilitate communication, disseminate implementation results, share learnings [53] and build leadership to drive implementation [32, 35, 40]. Barriers to creating a continuous learning culture in PC included time constraints for clinicians [46], communication issues between healthcare professionals [37, 42], a lack of understanding of the respective roles and responsibilities of team members [50, 59, 62], and challenges with technology use [61]. Facilitators included regular meetings to ensure alignment and reduce the risk of misunderstanding [46], ‘huddles’ to share learnings [48], and mentorship to generalise findings between contexts [32] and to upskill leaders [61].
In ED settings, continuous learning and improvement focused primarily on leveraging real-time data collection platforms to improve clinical decision-making [36, 65]. One study reported a lack of insight into barriers to implementing these platforms, potentially due to the limited number of high-quality interventions in ED that examine implementation amidst time pressures and information overload [65]. Learning communities for knowledge sharing were also explored within the ED and were facilitated by strong relationships among clinicians and between professional organisations [51].
Structure and governance
Structure and governance was reported on in 28 articles (76%). Within PC, structure and governance primarily involved the creation of committees for oversight of intervention development and implementation [45, 55], many of which included patients and community members [48]. Structures to engage and involve local and national leadership (e.g., through partnerships) were both adopted and recommended in future models to facilitate quality improvement interventions [35, 37, 41, 50]. Protocols to guide the coordination and implementation of LHS interventions were an additional focus [48]. In ED settings, partnership structures were also leveraged to strengthen research and collaboration [33, 41, 63], including leadership to scale implementation [49], and planned protocols to guide intervention delivery [43].
Barriers to implementing structure and governance were mostly reported in PC settings, and included lengthy time periods waiting for ethics approval [46], difficulties securing funding [46], and varying barriers and policies across implementation contexts [37, 63]. Facilitators included formal leadership training and mentorship models [32, 61], formal strategic planning [57], and strong ongoing relationships with stakeholders [33].
Patient-clinician partnerships
Patient-clinician partnerships was examined in 17 articles (46%). In PC, patient-clinician partnerships mostly related to the inclusion of patients in governance structures, such as advisory committees, to identify patient and community priorities during intervention planning and implementation [43, 45]. Patient experiences and preferences were assessed primarily to inform LHS exploration or development [50, 53, 59, 62], rather than to measure patient satisfaction or preferences for care pathways. Barriers to involving patients in LHS interventions included issues recruiting and receiving feedback from patients [62] and lack of adequate communication processes between patients and implementation teams [48]. Facilitators included planning better communication strategies [48] and tailoring appointment lengths for greater patient contact [47, 48].
In the ED setting, only one article focused on patient engagement and empowerment in implementing a joint PC-ED based intervention [63]. Person-centredness was most frequently about ensuring patients and families received timely information and support [50, 59, 62], rather than involving patients and communities in LHS interventions. No articles reported on implementation factors associated with patient-clinician partnerships in ED.
Incentives
Incentives was assessed in 17 articles (46%). In PC, incentives were most commonly directed toward prioritising high value rather than high volume care [38, 52, 54], followed by data sharing and transparency [40, 50, 62]. Achieving stakeholder buy-in was also emphasised [32, 46] as well as the use of incentives to encourage training for healthcare professionals [42]. Barriers to successfully utilising incentives included inconsistently applied financial incentives among clinicians and other staff [38] and challenges to ascertaining stakeholder preferences in data availability [62]. Facilitators identified included ongoing discussions about appropriate incentive systems [38] and better planning with stakeholders around the availability of data from LHS interventions [62].
In ED, incentives was advocated in one article, for the purpose of supporting quality improvement in medication safety [49]. Quality incentives could be facilitated by clinician certification requirements, leadership endorsement, and opportunities to join learning collaboratives [49].
Quality assessment
Eighteen studies were appraised using the MMAT. Of these, 10 (56%) were qualitative, four (22%) were quantitative descriptive studies, three (17%) were mixed methods, one was a quantitative randomized controlled trial, and one was a quantitative non-randomized study. One study was appraised using the JBI critical appraisal checklist for text and opinion papers. Two studies were appraised using the SANRA tool. All included studies were deemed to be of high-quality following appraisal. Thirteen studies were unable to be appraised with existing tools but were included in the review as they were deemed by the authorship team to show low risk of bias. Details of quality appraisal can be found in Supplementary Material 4.
Discussion
This review explored how, and the extent to which, LHS principles have been applied to the front lines of care in support of health system preparedness, and the barriers and facilitators to doing so. Although there has been considerable progress with adoption of LHSs within the last five years, this research has been largely focused on PC settings. PC encompasses a broad array of services and settings, and multidisciplinary care has been highlighted as crucial for improved patient outcomes amidst growing disease burden and ageing populations [67, 68]. Indeed, many articles included in the current review reported multidisciplinary interventions [30] and other alternative care models to standard general practice [54], which appear to be increasingly popular. LHSs in EDs are beginning to emerge, but the focus on acute and urgent care in these settings, compounded by time and space constraints, can challenge attempts to improve health system efficiency and quality [69].
Science and informatics was the most frequently assessed LHS component across PC and ED settings, echoing previous work on LHS schematic frameworks [9]. Cycles of data collection, analysis, and feedback and the use of evidence-based methods were consistent themes across the included articles, with the ultimate aims of improving system performance and/or care outcomes [51, 63]. This improved system performance will have the added effect of reducing the carbon footprint of the PC and ED settings, an important component of future proofing the front lines of care [70, 71]. The complexities of data management tended to require extensive resourcing across PC and ED settings [41, 65], and were frequently enabled by strong relationships [50], regular communication [49], and governance teams [54]. These factors should be considered when planning to prepare PC and ED for future challenges.
Evidently, exploration of the ‘human’ aspects of LHSs [9] has increased overall in the last five years, through greater focus on learning communities, human interactions with technology, and patient involvement. Incentives and patient-clinician partnerships, however, were assessed almost 50 percent less frequently than the other three LHS components, despite being crucial for achieving the ‘buy-in’ needed to make technical LHS processes possible in both PC [32] and EDs [49]. Incentives create necessary willingness and motivation for individuals (e.g., healthcare professionals, managers, patients) to participate in interventions, which is important for ensuring the integrity of data obtained [46]. Furthermore, patients are increasingly viewed as key actors in healthcare, and LHS efforts should utilise the numerous frameworks for patient involvement that have been developed [72]. This is particularly relevant in the context of preparing the front lines of care for future pandemics and climate change, both of which will directly impact patients globally. These patients have a right to be involved in future-proofing the system which will ultimately protect their wellbeing in a changing world.
Although many barriers and facilitators to LHS progress were reported in the articles reviewed, few utilised implementation frameworks to systematically understand the factors influencing LHS adoption [47, 48]. Implementation science models and frameworks aim to improve the uptake of evidence into practice, and thus align closely with the goals of LHSs [7, 73]. For example, implementation science has been successfully utilised in PC to identify factors affecting LHS adoption, and inform implementation strategies tailored to local settings [48]. In ED settings, implementation science has only recently begun to gain traction [74], but has thus far not been utilised for LHS approaches. Implementation science tools should be exploited when planning LHS approaches to better understand how incentives, partnerships, governance, informatics, and learning can be embedded into healthcare organisations and workplaces [7, 73].
Strengths and limitations
This review was the first to identify the breadth and range of LHS approaches that have been developed and adopted to improve care at the front lines. The findings highlighted various methods and conceptualisations of LHS components in PC and ED, and pointed to areas in which further research is warranted.
Although our search strategies were designed to broadly encompass LHS approaches, it is possible that the articles reviewed most frequently reported on effective interventions rather than those considered less effective, but no less useful, approaches to LHS development and implementation. Furthermore, most articles reviewed were published in high-income countries. It is possible that searches unlimited by language, and designed to capture challenges relating to LHS development, might have revealed more articles reporting from lower-income countries.
Conclusions
The creation of LHSs at the front lines of care is essential for future-proofing against the challenges and risks facing health systems. While there has been greater attention placed on the ‘human aspects’ of LHSs, science and informatics remains the most frequently assessed component, and incentives and patient-clinician partnerships have been least examined. Implementation science should be utilised to better understand the factors influencing LHS adoption, so that increasingly stronger and more adaptable LHSs can be created – LHSs that are prepared for the pressures of future pandemics and climate change.
Availability of data and materials
Data supporting these research findings are available in supplementary material and further inquiries can be directed to the corresponding author.
Abbreviations
- IoM:
-
Institute of Medicine
- LHS:
-
Learning health system
- PC:
-
Primary care
- ED:
-
Emergency department
- EHR:
-
Electronic health record
References
Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6(11):e1196–252.
Braithwaite J. Changing how we think about healthcare improvement. BMJ. 2018;361:361.
Braithwaite J, Glasziou P, Westbrook J. The three numbers you need to know about healthcare: the 60–30–10 challenge. BMC Med. 2020;18:1–8. https://doi.org/10.1186/s12916-020-01563-4.
Pomare C, Mahmoud Z, Vedovi A, Ellis LA, Knaggs G, Smith CL, et al. Learning health systems: a review of key topic areas and bibliometric trends. Learn Health Syst. 2022;6(1):e10265. https://doi.org/10.1002/lrh2.10265.
Olsen L, Aisner D, McGinnis JM. In: Olsen L, Aisner D, McGinnis JM, editors. The learning healthcare system: workshop summary. Washington, DC: Institute of Medicine of the National Academies; 2007.
Smith M, Halvorson G, Kaplan G. What’s needed is a health care system that learns: recommendations from an IOM report. JAMA. 2012;308(16):1637–8. https://doi.org/10.1001/jama.2012.13664.
Ellis LA, Sarkies M, Churruca K, Dammery G, Meulenbroeks I, Smith CL, et al. The science of learning health systems: scoping review of empirical research. JMIR Med Inform. 2022;10(2):e34907. https://doi.org/10.2196/34907.
McGinnis JM, Stuckhardt L, Saunders R, Smith M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, editors. Best care at lower cost: the path to continuously learning health care in America. Washington, DC: Institute of Medicine of the National Academies; 2013.
Zurynski Y, Smith CL, Vedovi A, Ellis LA, Knaggs G, Meulenbroeks I, et al. Mapping the learning health system: a scoping review of current evidence. In: A white paper. Sydney: Australian Institute of Health Innovation and the NHMRC Partnership Centre for Health System Sustainability, Macquarie University; 2020. Report No.: 1741384761.
Schäfer WL, Boerma WG, Schellevis FG, Groenewegen PP. GP practices as a one‐stop shop: how do patients perceive the quality of care? A cross‐sectional study in thirty‐four countries. Health Serv Res. 2018;53(4):2047–63. https://doi.org/10.1111/1475-6773.12754.
Berchet C. Emergency care services: trends, drivers and interventions to manage the demand. Paris: OECD Publishing; 2015.
Qidwai W. Primary health care in pandemics: barriers, challenges and opportunities. World Fam Med J. 2021;19(8):1–153. https://doi.org/10.5742/MEWFM.2021.94090.
Mazurik L, Javidan AP, Higginson I, Judkins S, Petrie D, Graham CA, et al. Early lessons from COVID-19 that may reduce future emergency department crowding. EMA. 2020;32(6):1077–9. https://doi.org/10.1111/1742-6723.13612.
Santana IR, Mason A, Gutacker N, Kasteridis P, Santos R, Rice N. Need, demand, supply in health care: working definitions, and their implications for defining access. Health Econ Policy Law. 2023;18(1):1–13. https://doi.org/10.1017/S1744133121000293.
Scarpetta S, Pearson M, Colombo F, Guanais F, Berchet C, Van Den Berg M, et al. Strengthening the frontline: how primary health care helps health systems adapt during the COVID 19 pandemic. Paris: Organisation for Economic Co-operation and Development; 2021.
World Health Organization. Building the economic case for primary health care: a scoping review. Geneva: World Health Organization; 2018.
Schoen C, Osborn R, Huynh PT, Doty M, Peugh J, Zapert K. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff. 2006;25(Suppl1):W555–71. https://doi.org/10.1377/hlthaff.25.w555.
Watts N, Amann M, Ayeb-Karlsson S, Belesova K, Bouley T, Boykoff M, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet. 2018;391(10120):581–630. https://doi.org/10.1016/S0140-6736(17)32464-9.
Vardoulakis S, Matthews V, Bailie RS, Hu W, Salvador-Carulla L, Barratt AL, et al. Building resilience to Australian flood disasters in the face of climate change. MJA. 2022;217(7):342. https://doi.org/10.5694/mja2.51595.
Salas RN, Jha AK. Climate change threatens the achievement of effective universal healthcare. BMJ. 2019;366. https://doi.org/10.1136/bmj.l5302.
Braithwaite J, Dammery G, Spanos S, Smith CL, Ellis LA, Churruca K, Fisher G, Zurynski Y. Learning health systems 2.0: future-proofing healthcare against pandemics and climate change. A White Paper. Sydney: Australian Institute of Health Innovation, Macquarie University; 2023. ISBN: 9781741385014. https://www.mq.edu.au/__data/assets/pdf_file/0006/1254147/Learning-health-systems-2.0-AIHI-2023.pdf.
Friedman C, Rubin J, Brown J, Buntin M, Corn M, Etheredge L, et al. Toward a science of learning systems: a research agenda for the high-functioning Learning Health System. JAMIA. 2015;22(1):43–50. https://doi.org/10.1136/amiajnl-2014-002977.
Menear M, Blanchette MA, Demers-Payette O, Roy D. A framework for value-creating learning health systems. Health Res Policy Syst. 2019;17(1):1–13. https://doi.org/10.1186/s12961-019-0477-3.
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. https://doi.org/10.1016/j.jclinepi.2020.10.007.
Department of Health and Aged Care. About primary care. Commonwealth of Australia; 2023. Available from: https://www.health.gov.au/topics/primary-care/about. Accessed 2 June 2023.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC methods programme. Institute for Health Research, Lancaster University; 2006. https://doi.org/10.13140/2.1.1018.4643.
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285–91. https://doi.org/10.3233/EFI-180221.
Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4(1):1–7. https://doi.org/10.1186/s41073-019-0064-8.
McArthur A, Klugarova J, Yan H, Florescu S. Chapter 4: Systematic reviews of text and opinion. In: Aromataris E, Munn Z, editors. JBI Reviewer's Manual. JBI. 2017. Available from https://reviewersmanual.joannabriggs.org/. https://doi.org/10.46658/JBIRM-17-04.
Brannon E, Wang T, Lapedis J, Valenstein P, Klinkman M, Bunting E, et al. Towards a learning health system to reduce emergency department visits at a population level. AMIA Annu Symp Proc. 2018;2018:295–304. PMID: 30815068.
Groenhof TKJ, Lely AT, Haitjema S, Nathoe HM, Kortekaas MF, Asselbergs FW, et al. Evaluating a cardiovascular disease risk management care continuum within a learning healthcare system: a prospective cohort study. BJGP Open. 2020;4(5):1–10. https://doi.org/10.3399/bjgpopen20X101109.
Cornick R, Wattrus C, Eastman T, Ras CJ, Awotiwon A, Anderson L, et al. Crossing borders: the PACK experience of spreading a complex health system intervention across low-income and middle-income countries. BMJ Glob Health. 2018;3. https://doi.org/10.1136/bmjgh-2018-001088.
Hunt RC, Struminger BB, Redd JT, Herrmann J, Jolly BT, Arora S, et al. Virtual peer-to-peer learning to enhance and accelerate the health system response to COVID-19: the HHS ASPR Project ECHO COVID-19 clinical rounds initiative. Ann Emerg Med. 2021;78(2):223–8. https://doi.org/10.1016/j.annemergmed.2021.03.035.
Campbell CM, Murphy DR, Taffet GE, Major AB, Ritchie CS, Leff B, et al. Implementing health care quality measures in electronic health records: a conceptual model. J Am Geriatr Soc. 2021;69(4):1079–85. https://doi.org/10.1111/jgs.17033.
Awoonor-Williams JK, Phillips JF, Aboba M, Vadrevu L, Azasi E, Tiah JAY, et al. Supporting the utilization of community-based primary health care implementation research in Ghana. Health Policy Plan. 2022;37(3):420–7. https://doi.org/10.1093/heapol/czab156.
Branch-Elliman W, Sundermann AJ, Wiens J, Shenoy ES. The future of automated infection detection: innovation to transform practice (Part III/III). ASHE. 2023;3(1). https://doi.org/10.1017/ash.2022.333.
Li X, Krumholz HM, Yip W, Cheng KK, De Maeseneer J, Meng Q, et al. Quality of primary health care in China: challenges and recommendations. Lancet. 2020;395(10239):1802–12. https://doi.org/10.1016/S0140-6736(20)30122-7.
Dammery G, Ellis LA, Churruca K, Mahadeva J, Lopez F, Carrigan A, et al. The journey to a learning health system in primary care: a qualitative case study utilising an embedded research approach. BMC Prim Care. 2023;24(1):22. https://doi.org/10.1186/s12875-022-01955-w.
Delvaux N, Aertgeerts B, Van Bussel JCH, Goderis G, Vaes B, Vermandere M. Health data for research through a nationwide privacy-proof system in belgium: design and implementation. JMIR Med Inform. 2018;6(4). https://doi.org/10.2196/11428.
Khanna N, Klyushnenkova EN, Kaysin A, Stewart DL. Utilizing the learning health system adaptation to guide family medicine practice to COVID-19 response. J Prim Care Community Health. 2020;11:2150132720966409. https://doi.org/10.1177/2150132720966409.
McCreary EK, Bariola JR, Minnier TE, Wadas RJ, Shovel JA, Albin D, et al. The comparative effectiveness of COVID-19 monoclonal antibodies: a learning health system randomized clinical trial. Contemp Clin Trials. 2022;119:106822. https://doi.org/10.1016/j.cct.2022.106822.
McGuire MJ. Building learning health care systems in primary care. Qual Manag Health Care. 2019;28(4):252–3. https://doi.org/10.1097/QMH.0000000000000230.
Myers RE, DiCarlo M, Romney M, Fleisher L, Sifri R, Soleiman J, et al. Using a health system learning community strategy to address cancer disparities. Learn Health Syst. 2018;2(4). https://doi.org/10.1002/lrh2.10067.
Nash DM, Brown JB, Thorpe C, Rayner J, Zwarenstein M. The alliance for healthier communities as a learning health system for primary care: a qualitative analysis in Ontario, Canada. J Eval Clin Pract. 2022;28(6):1106–12. https://doi.org/10.1111/jep.13692.
Nash DM, Rayner J, Bhatti S, Zagar L, Zwarenstein M. The Alliance for Healthier Communities' journey to a learning health system in primary care. Learn Health Syst. 2023;7(1). https://doi.org/10.1002/lrh2.10321.
Thandi M, Wong ST, Aponte-Hao S, Grandy M, Mangin D, Singer A, et al. Strategies for working across Canadian practice-based research and learning networks (PBRLNs) in primary care: focus on frailty. BMC Fam Pract. 2021;22(1):220. https://doi.org/10.1186/s12875-021-01573-y.
Pestka DL, Paterson NL, Brummel AR, Norman JA, White KM. Barriers and facilitators to implementing pharmacist-provided comprehensive medication management in primary care transformation. Am J Health Syst Pharm. 2022;79(15):1255–65. https://doi.org/10.1093/ajhp/zxac104.
Pestka DL, White KM, Deroche KK, Benson BJ, Beebe TJ. “Trying to fly the plane while we were building it”. Applying a learning health systems approach to evaluate early-stage barriers and facilitators to implementing primary care transformation: a qualitative study. BMJ Open. 2022;12(1):e053209. https://doi.org/10.1136/bmjopen-2021-053209.
Vandenberg AE, Kegler M, Hastings N, Hwang U, Wu D, Stevens MB, et al. Sequential implementation of the EQUIPPED geriatric medication safety program as a learning health system. Int J Qual Health Care. 2020;32(7):470–6. https://doi.org/10.1093/intqhc/mzaa077.
Golden RE, Klap R, Carney DV, Yano EM, Hamilton AB, Taylor SL, et al. Promoting learning health system feedback loops: experience with a VA practice-based research network card study. Healthcare. 2021;8(Supplement 1):100484. https://doi.org/10.1016/j.hjdsi.2020.100484.
Jeffries M, Keers RN, Phipps DL, Williams R, Brown B, Avery AJ, et al. Developing a learning health system: insights from a qualitative process evaluation of a pharmacist-led electronic audit and feedback intervention to improve medication safety in primary care. PLoS One. 2018;13(10):e0205419. https://doi.org/10.1371/journal.pone.0205419.
Palin V, Tempest E, Mistry C, van Staa TP. Developing the infrastructure to support the optimisation of antibiotic prescribing using the learning healthcare system to improve healthcare services in the provision of primary care in England. BMJ Health Care Inform. 2020;27(1). https://doi.org/10.1136/bmjhci-2020-100147.
Porat T, Marshall IJ, Sadler E, Vadillo MA, McKevitt C, Wolfe CDA, et al. Collaborative design of a decision aid for stroke survivors with multimorbidity: a qualitative study in the UK engaging key stakeholders. BMJ Open. 2019;9(8):e030385. https://doi.org/10.1136/bmjopen-2019-030385.
Burdick TE, Moran DS, Oliver BJ, Eilertsen A, Raymond J, Hort S, et al. Transitional care management quality improvement methods that reduced readmissions in a rural, primary care system. J Am Board Fam Med. 2022;35(3):537–47. https://doi.org/10.3122/jabfm.2022.03.190435.
Van Rensburg AJ, Petersen I, Awotiwon A, Bachmann MO, Curran R, Murdoch J, et al. Applying learning health systems thinking in codeveloping integrated tuberculosis interventions in the contexts of COVID-19. BMJ Glob Health. 2022;7(10). https://doi.org/10.1136/bmjgh-2022-009567.
Myers SR, Abbadessa MKF, Gaines S, Lavelle J, Ercolani JM, Shotwell C, et al. Repurposing video review infrastructure for clinical resuscitation care in the age of COVID-19. Ann Emerg Med. 2021;77(1):110–6. https://doi.org/10.1016/j.annemergmed.2020.08.030.
Nelson K, Reddy A, Stockdale SE, Rose D, Fihn S, Rosland AM, et al. The primary care analytics team: integrating research and clinical care within the veterans health administration office of primary care. Healthcare. 2021;8(Supplement 1):100491. https://doi.org/10.1016/j.hjdsi.2020.100491.
Neprash HT, Vock DM, Hanson A, Elert B, Short S, Karaca-Mandic P, et al. Effect of integrating access to a prescription drug monitoring program within the electronic health record on the frequency of queries by primary care cinicians: a cluster randomized clinical trial. JAMA Health Forum. 2022;3(6): e221852. https://doi.org/10.1001/jamahealthforum.2022.1852.
Safaeinili N, Brown-Johnson C, Shaw JG, Mahoney M, Winget M. CFIR simplified: pragmatic application of and adaptations to the Consolidated Framework for Implementation Research (CFIR) for evaluation of a patient-centered care transformation within a learning health system. Learn Health Syst. 2020;4(1). https://doi.org/10.1002/lrh2.10201.
Yigzaw KY, Chomutare T, Wynn R, Berntsen GKR, Bellika JG. A privacy-preserving audit and feedback system for the antibiotic prescribing of general practitioners: survey study. JMIR Form Res. 2022;6(7). https://doi.org/10.2196/31650.
Abraham TH, Stewart GL, Solimeo SL. The importance of soft skills development in a hard data world: learning from interviews with healthcare leaders. BMC Med Educ. 2021;21(1):147. https://doi.org/10.1186/s12909-021-02567-1.
Hek K, Rolfes L, van Puijenbroek EP, Flinterman LE, Vorstenbosch S, van Dijk L, et al. Electronic health record-triggered research infrastructure combining real-world electronic health record data and patient-reported outcomes to detect benefits, risks, and impact of medication: development study. JMIR Med Inform. 2022;10(3). https://doi.org/10.2196/33250.
Archambault PM, Rivard J, Smith PY, Sinha S, Morin M, LeBlanc A, et al. Learning Integrated health system to mobilize context-adapted knowledge with a wiki platform to improve the transitions of frail seniors from hospitals and emergency departments to the community (LEARNING WISDOM): protocol for a mixed-methods implementation study. JMIR Res Protoc. 2020;9(8). https://doi.org/10.2196/17363.
Deruiter WK, Barker M, Rahimi A, Ivanova A, Zawertailo L, Melamed OC, et al. Smoking cessation training and treatment: options for cancer centres. Curr Oncol. 2022;29(4):2252–62. https://doi.org/10.3390/curroncol29040183.
Jones BE, Collingridge DS, Vines CG, Post H, Holmen J, Allen TL, et al. CDS in a learning health care system: Identifying physicians’ reasons for rejection of best-practice recommendations in pneumonia through computerized clinical decision support. Appl Clin Inform. 2019;10(1):1–9. https://doi.org/10.1055/s-0038-1676587.
McCreary EK, Bariola JR, Wadas RJ, Shovel JA, Wisniewski MK, Adam M, et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022:E226920. https://doi.org/10.1001/jamanetworkopen.2022.6920.
Saint-Pierre C, Herskovic V, Sepúlveda M. Multidisciplinary collaboration in primary care: a systematic review. Fam Pract. 2018;35(2):132–41. https://doi.org/10.1093/fampra/cmx085.
Mitchell GK, Tieman JJ, Shelby-James TM. Multidisciplinary care planning and teamwork in primary care. MJA. 2008;188:S61–4. https://doi.org/10.5694/j.1326-5377.2008.tb01747.x.
Graff L, Stevens C, Spaite D, Foody J. Measuring and improving quality in emergency medicine. Acad Emerg Med. 2002;9(11):1091–107.
Nicolet J, Mueller Y, Paruta P, Boucher J, Senn N. What is the carbon footprint of primary care practices? A retrospective life-cycle analysis in Switzerland. Environ Health. 2022;21(1):1-10.https://doi.org/10.1186/s12940-021-00814-y.
Vali M, Salimifard K, Chaussalet T. Carbon footprints in emergency departments: a simulation-optimization analysis. Oper Res Simul Health Care. 2021:193–207. https://doi.org/10.1007/978-3-030-45223-0_9.
Braithwaite J, Churruca K, Wells L, Rapport F, Lawson T, Arro P, et al. Partnering with patients for change and improvement: an Australian perspective. In: In: Patient engagement: how patient-provider partnerships transform healthcare organizations. 2019. p. 169–98.
Braithwaite J, Churruca K, Long JC, Ellis LA, Herkes J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med. 2018;16:1–14. https://doi.org/10.1186/s12916-018-1057-z.
Southerland LT, Hunold KM, Van Fossen J, Caterino JM, Gulker P, Stephens JA, et al. An implementation science approach to geriatric screening in an emergency department. J Am Geriatr Soc. 2022;70(1):178–87. https://doi.org/10.1111/jgs.17481.
Acknowledgements
Not applicable.
Funding
JB is funded and supported by an NHMRC Leadership Investigator Award (1176620), multiple other grants, and the NHMRC Partnership Centre for Health System Sustainability (9100002).
Author information
Authors and Affiliations
Contributions
Funding and Supervision: JB. Conception and design: SS, GD, LAE, GF, CLS, JB; acquisition and analysis: SS, GD, LP; interpretation of data: SS, GD, LP; writing original draft: SS, GD, LP; substantive revision of manuscript: all authors. All authors have read and approved the manuscript, and agree to be personally accountable for their contribution.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Spanos, S., Dammery, G., Pagano, L. et al. Learning health systems on the front lines to strengthen care against future pandemics and climate change: a rapid review. BMC Health Serv Res 24, 829 (2024). https://doi.org/10.1186/s12913-024-11295-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11295-3