Abstract
Objective
To perform a cost study of pharmacist-led medication reviews in patients with an acute hospitalization for adverse drug events.
Method
Emergency department pharmacists performed medication reviews in patients hospitalized after visiting the emergency department for an adverse drug event (ADE). Control patients were hospitalized after an emergency department visit not related to an ADE and received usual care. The costs of the intervention were labour costs of the junior emergency department pharmacist and the cost savings consisted of costs of medication that was stopped or reduced during six months after the intervention. Sensitivity analyses were performed to evaluate different scenarios.
Results
In the intervention group (n = 104) 113 medication changes led to stopping or reducing medication, accounting for averted costs of €22,850. In the control group (n = 112) 39 medication changes led to stopping or reducing medication, accounting for averted costs of €299. The mean labour costs of the intervention were €138 per patient, resulting in saved costs of €61 per patient per six months. Sensitivity analyses showed that if the intervention would be performed by a senior clinical pharmacist, there are no cost savings (€-21), if parts of the intervention would be executed by pharmacy technicians (e.g. administrative tasks), cost savings would be augmented to €87, if outliers in costs associated with medication reduction would be excluded, there are no cost savings (€-35) and if the costs of reduced medication were extrapolated to one year, cost savings would be €260.
Conclusion
In this study, medication reviews by junior emergency department pharmacists in patients hospitalized after an emergency department visit for an ADE lead to a cost reduction over a six month period.
Trial registration
The main study is registered on the ISRCTN registry with trial ID ISRCTN12506329 on 06-03-2022.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The prevalence of visits to the emergency department (ED) caused by adverse drug events (ADEs) and resulting in hospital admission is high, amounting to 5-7% of all acute admissions worldwide [1,2,3,4]. Acute ADE-related hospitalizations are associated with increased length of stay and increased morbidity and mortality [5, 6]. This is not only a medical problem, but also an economic problem as the costs of these ADE-related hospitalizations are high [6,7,8]. Economic evaluations showed that costs increased if the ADE-related hospitalization was preventable [5, 9, 10].
The role of a clinical pharmacist in the ED has been described in different countries [11,12,13,14,15,16]. A part of this role involved recognition of ADEs in the ED, as 40% were not recognized as such by physicians which could result in revisits [17, 18]. Besides contributing to recognition of ADEs, ED pharmacists could also perform medication reviews to reduce drug related problems (DRPs) which could lead to ADEs [16, 18,19,20,21,22,23]. Medication reviews especially contributed to reduction of DRPs involving medication overuse and medication underuse [19, 23]. In the Netherlands, medication reviews are primarily performed within the primary care setting, targeting various patient populations at high risk of DRPs, such as older people with polypharmacy [24]. Medication reviews are not routinely integrated into standard care protocols within hospital settings nor are they routinely conducted for patients with an ADE-related hospitalization [25]. As a pharmacist-led medication review is time-consuming, it remains unclear if the implementation of an ED pharmacist for this purpose can save costs.
Studies looking into cost savings of interventions implemented by pharmacists in the ED showed that annually $1.7–3.1 million can be saved per ED in the United States by implementing an ED pharmacist. Costs can be saved by reductions in adverse drug events, dose optimisations, guideline adherence, and increased use of cost-effective medication therapy [11, 26,27,28,29]. Benefits such as stopping medication or costs such as time the pharmacist spent on administration, were not included. In addition, there is only one study in a European hospital setting which looked into the cost benefit and cost-effectiveness of interventions of ED pharmacists [16, 30].
Therefore, the aim of this study was to perform a cost study of medication reviews performed by junior ED pharmacists in patients with an ADE-related hospitalization after an ED visit.
Methods
Study design
This cost study was carried out using the data of a prospective, multicentre controlled intervention study performed between October 2016 and December 2017 in the Erasmus University Medical Center (EMC) in Rotterdam and in the general teaching hospital OLVG-West in Amsterdam (OLVG), both located in The Netherlands. In this study we examined the effect of medication reviews by an ED pharmacist in patients acutely hospitalized for ADEs. These medication reviews were performed for study purposes only and are not performed in usual care. The study protocol received a waiver from the Medical Ethics Committee as it was outside the scope of the Human Research Act (MEC-2016-346). All included patients provided written informed consent. The methodology of the main study was previously described, and will be briefly summarized in the paragraphs “study population” and “study procedures” below, for an extensive description we refer to the published study results [18].
Study population
Patients aged 18 years and older were eligible for inclusion if they were hospitalized for more than 24 h after visiting the ED. Exclusion criteria were: no communication possible due to condition, language barrier, cognitive impairment, transfer to other hospital, no pre-admission prescription medication or over the counter (OTC) medication, admission due to problems with anti-cancer treatment, (self) poisoning, psychiatric reasons, intensive care unit (ICU) admission. Also, foreign tourists or homeless patients and patients who provided no informed consent were excluded. Exclusion criteria were based on the feasibility of the intervention. In patients who were admitted multiple times only the first admission was included. Patients were assigned to the intervention group if the reason of ED-visit and admission was possibly ADE-related and to the control group if the reason was unlikely to be ADE-related. The control group received usual care (see below), while the intervention group received the medication reviews by junior ED pharmacists as described under study procedures. The control group would ideally consist of patients with an ADE-related admission following an ED visit, for whom the ED pharmacist would not perform the intervention. However, previous research showed that approximately 40% of ADE-related admissions were not recognized by physicians [17, 18]. For correct identification of all patients with an ADE-related admission, the pharmacist would need to screen all admissions and subsequently randomize the ADE-related ones in medication review by the pharmacist or not. In the latter case, care as usual would be applied. However, this implies that the pharmacist cannot share the ADE-related cause of the admission with the physician, which is not ethical. Therefore, the control group was chosen to be as close to the intervention group as possible, but without an ADE as cause of the admission.
Usual care
Patients in the control group were hospitalized after the ED visit for a non ADE-related problem and received their usual medical and pharmaceutical care. Usual pharmaceutical care consisted of medication reconciliation and computerized medication surveillance on a daily base by clinical pharmacists in both hospitals. Physicians could process medication changes because of DRPs during their normal routine (see Fig. 1).
Intervention
Patients in the intervention group were hospitalized after the ED visit because of an ADE and received the intervention by an ED pharmacist in addition to usual care. The ED pharmacist was a junior clinical pharmacist who followed certified courses and received training by senior clinical pharmacists, who also took care of supervision [18]. The intervention consisted of detection of the ADE-related admissions after consensus with the physician (if not already recognized by the physician), a pharmacist-led medication review according to the STRIP (Systematic Tool to Reduce Inappropriate Prescribing) method including obtaining patient anamnesis to detect DRPs, analysis of medication list (and discussing potential DRPs with the physician), structured patient counselling including teach back methods for any medication changes, and transmission of the recommendations from the medication review to the patient’s general practitioner and community pharmacist after discharge to ensure continuation of care (see Fig. 1) [31]. The pharmacist-led medication review was performed to detect potential DRPs in the categories: medication overuse, medication underuse, contra-indication, and other, resulting in recommended medication changes to physicians to solve these potential DRPs. Patient counselling regarding medication changes took place prior to discharge by using the teach-back method in which the patient was encouraged to repeat the message of the caregiver in order to check whether the patient had understood the explanation [32].
Study procedures
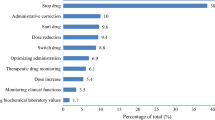
For this cost study, labour costs of the junior ED pharmacist and costs of the reduced medication (i.e. discontinuation of medication or dose reduction) were compared. Medication changes during hospitalisation could be the result of recommendation of the ED pharmacist, or the result of routine care by the physician [18]. The following medication changes were possible: start of new medication, dosage change, stop of medication or switch of medication. All changes had to persist for six months after discharge, which was checked by collecting pharmacy dispensing records. When a medication was stopped during admission, but after discharge a medication for the same indication was started (e.g. simvastatin stopped, but one month later pravastatin started), this was considered to be a switch.
For the cost calculations, only stop of medication and dosage lowering were included. Medication changes resulting in switches (replacing medication A with medication B for the same indication, even if medication B was started anywhere within the six month post-discharge period) were not included in the cost analysis, because we assumed that the costs did not differ significantly (most switches involved generic medication). The costs of medication changes leading to the initiation of new medications or to higher dosages (due to undertreatment with medication) were also excluded from this analysis because these medications or higher dosages should have been prescribed anyway in regular care as described previously [33]. In addition, correcting undertreatment could potentially result in cost savings by preventing ADEs. This would lead to large cost reductions in comparison to the smaller costs for the added medication, but we did not collect information on ADEs and therefore these potential net cost savings were not included.
Outcomes and data collection
Primary outcome was the costs associated with the intervention by the ED pharmacist. These were composed of the labour costs of the junior ED pharmacist and the costs of reduced medication.
The time the ED pharmacist spent per part of the intervention (i.e. (1) ADE detection (2) patient anamnesis to detect DRPs, (3) analysis of medication list, (4) patient counselling, and (5) other (transport time to patient/administration/transmission of results to general practitioner and community pharmacy) was recorded per patient with a stopwatch. Subsequently, the mean time per part of the intervention and the mean time for the total intervention were determined and used to calculate the labour costs. The Dutch guideline for labour costs of a junior clinical pharmacist was used in the main analysis. [34, 35]. The medication costs consisted of the medication costs that were averted in the six month period, due to discontinued medication or dose reductions of medication, as a result of interventions for a DRP that were implemented during the initial hospital admission and that persisted six months or more after admission. Persistence was established by checking the dispensing records of the community pharmacies of the patients. Medication costs were calculated according to current Dutch prices [36].
The prices of generic medication in the strength fitting the prescribed dose were selected. When medication was prescribed as needed, the mean dose based on the minimum and maximum dose per day was used. We calculated the mean costs of reduced medication per patient in the intervention group as well as in the control group, for the six month time period studied.
Patient characteristics were extracted from the medical records of the hospital information system. All data were processed in Open Clinica (Open Clinica LLC version 2.1, Waltham, USA).
Data analysis
In each group 100 patients were planned to be included. No formal sample size calculation was performed, as the feasibility of the study was dependent on the limited availability of the ED-pharmacists. Differences in patient characteristics between both groups were determined with appropriate tests (t-test, Mann-Whitney U test or Pearson’s Chi-Square test). The cost savings of reduced medication for the intervention group compared to the control group was calculated first, and this was balanced against the labour costs of the intervention.
In a sensitivity analysis four different scenarios were explored; all were fictional scenarios that were not actually studied but were based on certain assumptions. In the first scenario we used labour costs of a senior pharmacist to calculate the costs of the intervention. For this scenario, we assumed that a senior clinical pharmacist requires the same amount of time for the intervention as a junior clinical pharmacist who is trained and experienced in the routine as the duration of the intervention depends on the existing routine and training; in addition we assumed that the junior pharmacist identified the same DRPs as the senior clinical pharmacist would. In the second scenario we used labour costs of a pharmacy technician for three parts of the intervention (i.e. obtaining patient anamnesis to detect DRPs, patient counselling and administrative tasks defined as “other”, combined with labour costs of a junior clinical pharmacist (scenario 2A) and combined with labour costs of a senior clinical pharmacist (scenario 2B) for the two other parts of the intervention (ADE detection and analysis of medication list) to calculate the costs of the total intervention. In the Netherlands, pharmacy technicians routinely perform these tasks and therefore we assume they perform these tasks within the same time span and with equal quality [37]. In the third scenario we excluded the costs of discontinuation of an expensive drug as a potential outlier in the intervention group and used labour costs of a junior pharmacist (scenario 3A), labour costs of a senior pharmacist (scenario 3B) and labour costs of partly a pharmacy technician combined with a junior pharmacist (scenario 3C). In the fourth scenario, we extrapolated the costs of reduced medication to one year in order to compare our results with other studies using the one year horizon and used labour costs of a junior pharmacist (scenario 4A), labour costs of a senior pharmacist (scenario 4B) and labour costs of partly a pharmacy technician combined with a junior pharmacist (scenario 4C). We have made the assumption that the reduction in medication that persisted at six months, would also persist after a year, as we consider it unlikely that medication reduced within the six-month period following the intervention would be resumed within six-twelve months.
Results
Study population
In the main study 104 patients were included in the intervention group and 112 patients in the control group [18]. The median age was 68 [IQR 57–78] and 67 [IQR 54–77] in the intervention group and control group respectively. No significant differences in patient characteristics were observed between both groups (Table 1). We observed no significant difference in the length of hospital stay between the intervention group and control group. Additionally, there was no significant difference in the number of (ADE-related) hospital admissions during the six months before compared to the six months after the intervention (T= -6 months: 57 hospital admissions and 21 ADE-related hospital admissions, T = + 6 months: 54 hospital admissions and 17 ADE-related hospital admissions) [18]. Consequently, these costs were not included in the cost analysis as potential cost savings.
Cost analysis
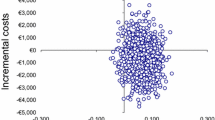
The mean time spent on the intervention was 113 min per patient, resulting in total labour costs for the junior ED pharmacist of €138 per patient (Table 2). In the intervention group, 113 medication changes regarding dose reduction or discontinued medication accounted for averted costs of €22,850 (mean cost/patient: €220, Table 3). In the control group 36 medication changes occurred, accounting for averted costs of €2,299 (mean cost/patient: €21) Table 3). The difference in mean saved costs per patient between intervention and control group was €199. Taking into account the labour costs of €138 per patient the saved costs were €199 - €138= €61 per patient for six months.
Sensitivity analysis
Table 4 shows the outcomes of the four main scenarios explored in the sensitivity analysis. There is no longer a cost saving in case a junior clinical pharmacist would be replaced by a senior clinical pharmacist (€-21 per patient per six months) and cost savings were increased when a pharmacy technician could perform parts of the intervention (€87 per patient per six months for a junior pharmacist and €43 per patient for a senior pharmacist). Excluding outliers in costs of reduced medication led to no cost savings (scenario 3). In scenario 4, the potential cost savings increase from €61 per patient to €260 per patient when the costs of reduced medication are extrapolated to a 12-month period, assuming a junior pharmacist performs the intervention.
Discussion
In this controlled-intervention study, the costs of delivering an intervention by ED pharmacists for patients with an ADE-related hospitalization after an ED visit were assessed. Medication changes which led to a dose reduction or discontinuation of medication and which persisted six months or more after the intervention were included as cost saving. Labour costs of the junior ED-pharmacist were used as costs of the intervention. The intervention saved €61 per patient for six months. Our study showed that cost savings varied between €-21 per patient for six months in case the intervention would be performed by a senior clinical pharmacist and €87 per patient for six months in case the intervention would be performed by a junior clinical pharmacist and a pharmacy technician. Other studies looking into the cost savings of clinical pharmacist-led medication reviews, included various costs (e.g. implementation of medication review tools), and benefits (e.g. ADE-prevention, total health care costs, readmissions) making these studies difficult to compare with ours. The cost savings ranged from €70 to €807 and $107 to $583 per pharmacist’s intervention per year [38,39,40,41,42]. Cost savings were higher compared to our results, which can be explained by the inclusion of ADE prevention as benefit of the intervention [39,40,41,42]. In one study the same medication costs were included as in our study (dose reductions or discontinuing medication), which resulted in cost savings of $107 per patient/year. This study was carried out in 1990 when medication costs were not as high as nowadays, which could explain the difference in cost savings [40]. To our knowledge, this is the first study that described the cost savings of ED pharmacist-led medication reviews in patients with an ADE-related hospitalization after an ED visit. We included direct cost savings of the intervention, namely costs of reduced medication and checked for persistence six months after intervention. Also costs such as administration costs were included as labour costs. By performing a sensitivity analysis, more insight is obtained into the cost savings using different scenarios. The study is performed in two different types of hospitals which contributes to the generalizability of the results.
Some limitations apply to our study as some costs are not included in the cost analysis. First, by including patients with a non-ADE related admission in the control group, potential bias regarding the number of medication changes leading to discontinuation or reduction of medication may be expected. In a post-hoc analysis of the original study [18], we demonstrated that no significant differences in the number of medication changes related to undertreatment or overtreatment existed between patients with an ADE-related admission recognized as such by the physician and patients with a non-recognized ADE-related admission, both in the intervention group. This suggests that a physician is unlikely to make more medication changes when an ADE is present and bias appears to be limited but cannot be ruled out. Second, sensitivity analysis showed that costs were substantially less when an outlier in medication costs was excluded. The outlier concerned tobramycine inhalation powder for treatment of cystic fibrosis, which was discontinued on recommendation of the ED pharmacists, because of lack of effect in this patient. We decided to include this outlier in the main analysis, as it does concern a pharmacist recommendation, but used the sensitivity analyses to demonstrate the effect of exclusion. However, in daily practice pharmacists could address expensive medication justifying the inclusion of this medication into the analysis. Third, the other scenarios were based on assumptions and these sensitivity analyses should therefore be interpreted with caution. Fourth, the time investment in training of the ED pharmacists, the time investment of senior ED pharmacists who supervise junior ED pharmacists who performed the intervention and the time the physicians spent on the intervention were not included as costs. The costs of the training of ED pharmacists are one-time costs and supervision of junior pharmacist is usual care. Also, in our experience the time the physician spent on the intervention was minimal (approximately 2–5 min/patient) and was usual care as well. Finally, we did not include potential benefits such as ADE prevention by starting or optimizing medication and associated healthcare costs. The costs of added medication were not included. ADE-prevention leads to large cost reductions compared to smaller costs for added medication, so overall this would result in more cost savings, making our estimation a conservative one. Future research should ideally include all these costs, cost avoidance and potential cost savings.
Notwithstanding the limitations, we recommend to incorporate this intervention into standard care with some considerations. First, it is recommended to identify which patients with an ADE-related hospitalization are prone to overtreatment with medication and can benefit most from the intervention by reducing medication. Additionally, the development of a tool, for example by utilizing artificial intelligence, for detection of patients with a potentially ADE-related hospitalization can contribute to reducing the costs of the intervention. As indicated by our sensitivity analysis, pharmacy technicians could carry out certain parts of the intervention to minimize costs of the intervention, but additional evidence is necessary regarding the quality and time expenditure of these tasks performed by technicians. As observed in scenario 3 of the sensitivity analysis, the cost savings decrease when expensive medication (outlier) is excluded as reduced medication. This suggests that by focusing on reducing expensive medication, a potentially greater cost saving may be achievable. Given the clinical outcomes of the main study and the aforementioned recommendations for reduction of the intervention costs, the intervention should be integrated into standard care for patients who stand to benefit most from it.
Conclusion
In this study, medication reviews by junior emergency department pharmacists in patients hospitalized after an emergency department visit for an ADE lead to cost reduction.
Data availability
The datasets generated and analyzed during the current study are not publicly available because they contain information that could compromise the privacy of research participants, but are available from the corresponding author upon reasonable request.
Abbreviations
- ADEs:
-
Adverse drug events
- CP:
-
Community pharmacy
- DRP:
-
Drug related problem
- ED:
-
Emergency department
- GP:
-
General practitioner
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
References
Budnitz DS, SN, Kegler SR, Richards CL. Medication use leading to Emergency Department Visits for Adverse Drug Events in older adults. Ann Intern Med. 2007;147:755–65.
Hohl CM, Robitaille C, Lord V, Dankoff J, Colacone A, Pham L, et al. Emergency physician recognition of adverse drug-related events in elder patients presenting to an emergency department. Acad Emerg Med. 2005;12(3):197–205.
Zed PJ, Abu-Laban RB, Balen RM, Loewen PS, Hohl CM, Brubacher JR, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178(12):1563–9.
Leendertse AJ, EACG, Stoker LJ, van den Bemt PMLA, for the HARM Study Group. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. ARCH INTERN MED. 2008;168(17):1890–6.
Rodriguez-Monguio R, JOM, Rovira J. Assessing the economic impact of adverse Drug effects. PharmacoEconomics. 2003;21(9):623–50.
Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37–46.
Ernst FR. GAJ. Drug-related morbidity and mortality updating the cost-of-illness. Model J Am Pharm Assoc. 2001;41:192–9.
Hohl CM, Nosyk B, Kuramoto L, Zed PJ, Brubacher JR, Abu-Laban RB, et al. Outcomes of emergency department patients presenting with adverse drug events. Ann Emerg Med. 2011;58(3):270–9. e4.
Magdelijns FJ, Stassen PM, Stehouwer CD, Pijpers E. Direct health care costs of hospital admissions due to adverse events in the Netherlands. Eur J Public Health. 2014;24(6):1028–33.
Leendertse AJ, Van Den Bemt PM, Poolman JB, Stoker LJ, Egberts AC, Postma MJ. Preventable hospital admissions related to medication (HARM): cost analysis of the HARM study. Value Health. 2011;14(1):34–40.
Morgan SR, Acquisto NM, Coralic Z, Basalyga V, Campbell M, Kelly JJ, et al. Clinical pharmacy services in the emergency department. Am J Emerg Med. 2018;36(10):1727–32.
Dryden L, Dewhurst NF. Integration of a clinical pharmacist into a Canadian, urban emergency department: a prospective observational study. Int J Pharm Pract. 2019;27(2):175–9.
Roulet L, Asseray N, Ballereau F. Establishing a pharmacy presence in the emergency department: opportunities and challenges in the French setting. Int J Clin Pharm. 2014;36(3):471–5.
Proper JS, Wong A, Plath AE, Grant KA, Just DW, Dulhunty JM. Impact of clinical pharmacists in the emergency department of an Australian public hospital: a before and after study. Emerg Med Australas. 2015;27(3):232–8.
Greenwood D, Tully MP, Martin S, Steinke D. The description and definition of Emergency Department Pharmacist Practitioners in the United Kingdom (the ENDPAPER study). Int J Clin Pharm. 2019;41(2):434–44.
Nymoen LD, Flatebø TE, Moger TA, Øie E, Molden E, Viktil KK. Impact of systematic medication review in emergency department on patients’ post-discharge outcomes-A randomized controlled clinical trial. PLoS ONE. 2022;17(9):e0274907.
Roulet LBF, Hardouin J, Chiffoleau A, Moret L, Potel G, Asseray N. Assessment of adverse drug event recognition by emergency physicians in a French teaching hospital. Emerg Med J. 2013;30:63–7.
Rahman RN, Nikolik B, de Ridder MAJ, Hoek AE, Janssen MJA, Schuit SCE, et al. The effect of emergency department pharmacists on drug overuse and drug underuse in patients with an ADE-related hospitalisation: a controlled intervention study. BMC Health Serv Res. 2022;22(1):1363.
Celikkayalar E, Puustinen J, Palmgren J, Airaksinen M. Collaborative Medication Reviews to identify Inappropriate Prescribing in Pre-admission medications at Emergency Department Short-Term Ward. Integr Pharm Res Pract. 2021;10:23–32.
Brown JN, Barnes CL, Beasley B, Cisneros R, Pound M, Herring C. Effect of pharmacists on medication errors in an emergency department. Am J Health Syst Pharm. 2008;65(4):330–3.
Abu-Ramaileh AM, Shane R, Churchill W, Steffenhagen A, Patka J, Rothschild JM. Evaluating and classifying pharmacists’ quality interventions in the emergency department. Am J Health Syst Pharm. 2011;68(23):2271–5.
Sin B, Lau K, Tong R, Ruiz J, Sarosky K, DiGregorio R, et al. The feasibility and Impact of Prospective Medication Review in the Emergency Department. J Pharm Pract. 2018;31(1):22–8.
Hohl CM, Zed PJ, Brubacher JR, Abu-Laban RB, Loewen PS, Purssell RA. Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann Emerg Med. 2010;55(6):493–502. e4.
Huiskes VJ, Burger DM, van den Ende CH, van den Bemt BJ. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract. 2017;18(1):5.
Dautzenberg L, Bretagne L, Koek HL, Tsokani S, Zevgiti S, Rodondi N, et al. Medication review interventions to reduce hospital readmissions in older people. J Am Geriatr Soc. 2021;69(6):1646–58.
Megan A, Rech MA, AW, Smetana KS, Gurnani PK, Van Berkel Patel MA, Peppard WJ, Hammond DA, Flannery AH. PHarmacist avoidance or reductions in medical costs in patients presenting the EMergency Department: PHARM-EM Study. Crit Care Explorations. 2021;3(4).
Aldridge VE, Park HK, Bounthavong M, Morreale AP. Implementing a comprehensive, 24-hour emergency department pharmacy program. Am J Health Syst Pharm. 2009;66(21):1943–7.
Lada P, Delgado G Jr. Documentation of pharmacists’ interventions in an emergency department and associated cost avoidance. Am J Health Syst Pharm. 2007;64(1):63–8.
Hammond DA, Gurnani PK, Flannery AH, Smetana KS, Westrick JC, Lat I, et al. Scoping Review of Interventions Associated with cost avoidance able to be performed in the Intensive Care Unit and Emergency Department. Pharmacotherapy. 2019;39(3):215–31.
Miarons M, Marín S, Amenós I, Campins L, Rovira M, Daza M. Pharmaceutical interventions in the emergency department: cost-effectiveness and cost-benefit analysis. Eur J Hosp Pharm. 2021;28(3):133–8.
Drenth-van Maanen AC, Leendertse AJ, Jansen PAF, Knol W, Keijsers C, Meulendijk MC, et al. The systematic Tool to reduce Inappropriate Prescribing (STRIP): combining implicit and explicit prescribing tools to improve appropriate prescribing. J Eval Clin Pract. 2018;24(2):317–22.
Ha Dinh TT, Bonner A, Clark R, Ramsbotham J, Hines S. The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: a systematic review. JBI Database Syst Rev Implement Rep. 2016;14(1):210–47.
Gallagher JBS, Woods NB, Lynch D, McCarthy S. Cost-outcome description of clinical pharmacist intervention in a university teaching hospital. BMC Health Serv Res. 2014;14(177).
Centra NFvUM. Cao universitair medische centra 2015–2017 NL 2017. https://www.nfu.nl/sites/default/files/2020-07/Cao_umc_2015-2017_NL_incl._aanvullend_deelakkoord.pdf
Ziekenhuizen NVv. CAO Ziekenhuizen 2019–2021, 2017. https://cao-ziekenhuizen.nl/downloads
Nederland Z. Farmacotherapeutisch Kompas. https://www.farmacotherapeutischkompas.nl/
van den Bemt PM, van der Schrieck-de Loos EM, van der Linden C, Theeuwes AM, Pol AG, Dutch CBOWHOHSG. Effect of medication reconciliation on unintentional medication discrepancies in acute hospital admissions of elderly adults: a multicenter study. J Am Geriatr Soc. 2013;61(8):1262–8.
Gallagher J, O’Sullivan D, McCarthy S, Gillespie P, Woods N, O’Mahony D, et al. Structured pharmacist review of medication in older hospitalised patients: a cost-effectiveness analysis. Drugs Aging. 2016;33(4):285–94.
Chinthammit CEPAEP, Boesen K, Martin R, Taylor AM, Warholak T. Cost-effectiveness of Comprehensive Medication Reviews Versus Noncomprehensive Medication Review Interventions and subsequent successful medication changes in a Medicare Part D Population. J Managed Care Specialty Pharm. 2015;21(5):381–9.
PHILLIPS S.L C-LSM. Impact of a pharmacist on medication discontinuation in a hospital-based geriatric clinic. Am J Hosp Pharm. 1990;47:1075–9.
Poremba M, Champa K, Reichert E. Evaluating reduction in medical costs associated with pharmacists’ presence in the emergency department using a novel cost avoidance framework. Am J Health Syst Pharm. 2022.
Dietrich SK, Bushong BT, Schneider-Smith EA, Mixon MA. Emergency medicine pharmacist interventions reducing exposure to costs (EMPIRE-C). Am J Emerg Med. 2022;54:178–83.
Funding
The study was funded by the Innovation Fund of Dutch Healthcare Insurance Companies in the form of a non-conditional grant. This funder had no role in the collection, analysis, or interpretation of data, or preparation, review, or approval of the manuscript for publication.
Author information
Authors and Affiliations
Contributions
PvdB, FKC, RR, SP designed the study. PvdB, FKC, MJ obtained research funding. SS and AH facilitated recruitment of participating patients. RR and BN performed the trial and collected data. PvdB, FKC and MJ supervised the conduct of the trial and data collection. RR analysed the data. SP supervised analysing the data. RR drafted the manuscript, and all authors contributed to its editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is registered on the ISRCTN registry with trial ID ISRCTN12506329 on 06-03-2022. The Medical Ethics Committee (METC) Erasmus MC Rotterdam approved the study and decided that it was outside the scope of the Human Research Act (registration number: MEC-2016-346 on 14-06-2016). Written informed consent was contained from all subjects. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rahman, R.N., Polinder, S., Nikolik, B. et al. Medication reviews by emergency department pharmacists in patients hospitalised for an adverse drug event: a cost study. BMC Health Serv Res 24, 975 (2024). https://doi.org/10.1186/s12913-024-11346-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11346-9