Abstract
Background
Antimicrobial stewardship (AMS) aims to improve antibiotic use while reducing resistance and its consequences. There is a paucity of data on the availability of AMS programmes in southern Nigeria. Further, there is no data on Nigerian healthcare professionals’ knowledge of the WHO ‘Access, Watch and Reserve’ (AWaRe) classification of antibiotics. This study sought to assess knowledge of AMS and the AWaRe classification of antibiotics among frontline healthcare professionals in Akwa Ibom State, Nigeria.
Methods
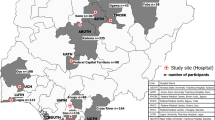
This was a cross-sectional survey of 417 healthcare professionals, comprising medical doctors, pharmacists and nurses, across 17 public hospitals in Akwa Ibom State, Nigeria. A paper-based self-completion questionnaire was used to collect data from the participants during working hours between September and November 2023. Statistical analysis was done using SPSS version 25.0, with p < 0.05 indicating statistical significance.
Results
Four hundred and seventeen out of the 500 healthcare professionals approached agreed to participate, giving an 83.4% response rate. Most of the participants were female (62.1%) and nurses (46.3%). Approximately 57% of participants were familiar with the term antibiotic/antimicrobial stewardship, however, only 46.5% selected the correct description of AMS. Majority (53.0%) did not know if AMS programme was available in their hospitals. 79% of participants did not know about AWaRe classification of antibiotics. Among the 87 (20.9%) who knew, 28.7% correctly identified antibiotics into the AWaRe groups from a given list. Only profession significantly predicted knowledge of AMS and awareness of the AWaRe classification of antibiotics (p < 0.001). Pharmacists were more likely to define AMS correctly than medical doctors (odds ratio [OR] = 2.02, 95% confidence interval [CI] = 1.16–3.52, p = 0.012), whereas nurses were less likely to be aware of the WHO AWaRe classification of antibiotics than medical doctors (OR = 0.36, 95% CI = 0.18–0.72, p = 0.004).
Conclusions
There was a notable knowledge deficit in both AMS and the AWaRe classification of antibiotics among participants in this study. This highlights the need for educational interventions targeted at the different cadres of healthcare professionals on the role of AMS programmes in reducing antimicrobial resistance and its consequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) is ranked fifth among the World Health Organisation’s (WHO) top ten global public health threats [1]. Available reports showed that an estimated 4·95 million deaths in 2019 were associated with AMR, with western sub-Saharan Africa recording highest death rate at 27·3 deaths per 100 000 [2]. Antimicrobial stewardship (AMS) has been promoted at international and national levels as a set of coordinated interventions required to improve antibiotic use and reduce resistance and associated morbidity and mortality [3,4,5,6,7]. To support AMS effort at local, national and global levels, the Antibiotics Working Group for the 21st WHO Model List of Essential Medicines adopted the ‘Access, Watch and Reserve’ (AWaRe) classification of antibiotics in the Essential Medicines List [8]. This classification, which was adopted and endorsed by G20 Health Ministers in October 2018, has since been updated and currently contains 258 antibiotics. The WHO 13th General Programme of Work 2019–2023 recommended that at least 60% of total country-level antibiotic consumption should come from Access group antibiotics [9].
There is a paucity of data on the availability and/or implementation of AMS programmes in African countries. Although a previous systematic review on the implementation of AMS programmes in African countries found no data on established hospital AMS programme in Nigeria, [10] a recent study of 20 hospitals randomly selected from the six geopolitical zones of the country to assess AMS implementation and practice reported that only six (30%) of the 20 hospitals had AMS committees with no regular AMS-related activities [11]. Successful implementation of hospital AMS programmes relies on active participation of healthcare professionals, including medical doctors with expertise in infectious diseases, pharmacists, among others [12, 13]. To this end, the WHO global action plan on AMR emphasised improved awareness and understanding of the link between antibiotic use and development of resistance among healthcare professionals in order to optimise antibiotic use [14]. The objectives of this study therefore were to assess knowledge of AMS and the AWaRe classification of antibiotics among frontline healthcare professionals. Additionally, the study sought to assess the availability and/or implementation of AMS programmes in public hospitals in Akwa Ibom State, Nigeria.
Methods
Study design, population and setting
This was a cross-sectional survey. Participants were included purposively based on profession, if they were medical doctors, pharmacists or nurses and work in public secondary or tertiary healthcare hospitals in Akwa Ibom State. Participants were drawn from 16 state-run secondary care hospitals and one tertiary care hospital. The Raosoft online sample size calculator formula for unknown population was used to compute sample size of frontline healthcare professionals to recruit for the study. Using a 95% confidence level, 5% margin of error and population size of 20 000 and assuming 50% response distribution, the recommended sample size was 377. To account for non-response and non-completion of questionnaire, the sample size was increased by 10%; therefore a sample size of 415 frontline healthcare professionals was targeted for this study.
Survey instrument and data collection
A 13-item self-administered paper-based questionnaire was used for data collection in this study (Supplementary file). A set of 15 questionnaire items was initially developed from relevant international documents [7,8,9]. To assess content validity, the 15-item questionnaire was sent to two clinical pharmacists who are knowledgeable in questionnaire design and AMS. These experts, who were unconnected to the study, were asked to assess the instrument’s relevance and representativeness of the study objectives. Based on their feedback, two items were dropped: one was unrelated to the study objectives, while the other was redundant. In order to assess face validity, the 13-item survey instrument was administered to 10 professionals (three medical doctors, three pharmacists, and four nurses). Non-response to questionnaire items, time required to complete the questionnaire, and feedback received were used to improve the instrument. The final questionnaire comprised three sections: section A collected demographic information of respondents; section B collected data on knowledge of the term antibiotic/antimicrobial stewardship and the definition/description of AMS. For the description of AMS, six answer options, of which one was correct, were included for participants to select from. Section B also collected data on the availability of AMS in the participating hospitals, while section C contained questions on knowledge of the AWaRe classification of antibiotics. Participants were also asked to identify antibiotics belonging to ‘Access’, ‘Watch’, and ‘Reserve’ groups from a given list of antibiotics. The Cronbach’s alpha coefficient for the survey instrument in this study was 0.67. Data were obtained from the participants during working hours between September and November 2023.
Statistical analysis
Analysis was done using SPSS version 25 (IBM Corp., Amonk, NY). Descriptive statistics was used to present the data. For the item on knowledge of AMS definition, response options were stratified into ‘correct’ and ‘incorrect’. All other options apart from the correct option were coded incorrect. Binary logistic regression was performed to identify the predictors of knowledge of AMS definition and awareness of the WHO AWaRe classification of antibiotics Statistical significance was set at p < 0.05.
Ethical considerations
Ethical approval was obtained from the Health Research Ethics Committees of the Akwa Ibom state Ministry of Health (AKHREC/01/08/23/169; 07/09/2023) and the University of Uyo Teaching hospital (UUTH/AD/S/96/VOLXXI/776; 21/08/2023). Consent to participate in the survey was sought in the questionnaire; participants were required to check a box to consent they agreed to take part in the study.
Results
Four hundred and seventeen out of the 500 healthcare professionals approached agreed to participate, giving an 83.4% response rate.
Participants’ characteristics
A total of 417 frontline healthcare professionals from 17 public hospitals participated in the study, of which majority were female (62.1%, n = 259). 46% (n = 193) of the participants were nurses, while medical doctors and pharmacists made up 25.4% (n = 106) and 28.3% (n = 118), respectively. A summary of the demographic characteristics of participants is provided in Table 1.
Knowledge of antimicrobial stewardship, antimicrobial stewardship availability and AWaRe classification among healthcare professionals in participating hospitals
Over half (56.8%, n = 237) of the healthcare professionals indicated they had heard the term antibiotic/antimicrobial stewardship. Within the professional cohorts, more than half, 57% (n = 108) of the nurses indicated they have never heard the term. More than half (53.0%, n = 221) of the healthcare professionals indicated they did not know if AMS programme was available in their hospitals. Among those who indicated that the programme was available in their hospitals, more than half (57.4%) did not select which core element(s) applied to their hospitals.
Regarding the definition/description of AMS, less than half (46.5%, n = 194) of the participants selected the correct description of AMS.
Of the 417 participants, majority (79.1%, n = 330) indicated they have not heard the term ‘Access, Watch, Reserve’ classification of antibiotics. Within each professional group, less than half knew about the AWaRe classification of antibiotics. A summary of the knowledge of AMS, AMS availability and the AWaRe classification is shown in Table 2.
Knowledge of the AWaRe classification of antibiotics among healthcare professionals
Only 87 (20.9%) health professionals indicated they knew about the AWaRe classification, and attempted questions on the AWaRe classification. Among the 87 who responded ‘yes”, only 25 (28.7%) correctly identified all nine antibiotics from a given list into “Access’, ‘Watch’ and ‘Reserve’. A summary of knowledge of AWaRe classification details and identification of antibiotics belonging to Access, Watch and Reserve is as shown in Table 3.
Effects of participants’ characteristics on knowledge of antimicrobial stewardship
Table 4 presents the results of binary logistic regression to determine the effects of gender, age, profession and length of practice on the likelihood that participants correctly define AMS. Of the variables assessed, only profession significantly (p < 0.001) predicted the model. Pharmacists were more likely to define AMS correctly than medical doctors (odds ratio [OR] = 2.02, 95% confidence interval [CI] = 1.16–3.52, p = 0.012).
Effects of participants’ characteristics on knowledge of the AWaRe classification of antibiotics
Results of binary logistic regression to assess the impact of gender, age, profession and length of practice on the likelihood that participants were aware of the WHO AWaRe classification of antibiotics revealed that only profession significantly (p < 0.001) predicted the model. Nurses were less likely to be aware of the WHO AWaRe classification of antibiotics than medical doctors (OR = 0.36, 95% CI = 0.18–0.72, p = 0.004) (Table 5).
Discussion
A number of studies have investigated the knowledge, attitude and perceptions of healthcare professionals (medical doctors, pharmacists and nurses) towards AMR and the effectiveness of AMS programmes in reducing AMR. Majority of the studies reported that healthcare professionals generally agree on the global and national burden of AMR, the association between antibiotic use in humans and agriculture and the development of resistance, and that AMS can reduce resistance [15,16,17,18]. Our findings show that more than half of the participants were familiar with the term ‘antibiotic/antimicrobial stewardship’, however, a few of the healthcare professionals selected the correct definition/description of AMS. More than half of the participants did not know if AMS programmes were available in their hospitals, especially among nurses, as well as which core components of AMS applied to their practice setting. A study of knowledge and practices of healthcare professionals towards AMS found that the majority of participants had poor knowledge of AMS, with pharmacists having better knowledge of AMS compared to nurses [19]. Although nurses have vital roles in hospital AMS, [20,21,22] and various models of nurses’ engagement in AMS have been described, [23] majority of the nurses in our study were not aware of AMS nor the correct description of the term. This finding is consistent with a previous study which found that more than half of the nurses who participated in a study to assess knowledge and attitudes were not familiar with AMS, although about 95% of the nurses believed they had a role in AMS interventions [24]. There is a likelihood that nurses’ knowledge of AMS may be setting- and/or location-specific. A previous study of nurses’ attitudes toward AMS found that approximately half of the nurses reported familiarity and knowledge of the term AMS [25]. Furthermore, a study of comparative self-assessment of knowledge on antimicrobials, AMR and AMS between medical doctors, pharmacists and nurses reported that a greater percentage of nurses had higher confidence level on knowledge of all three topics compared to pharmacists and doctors who had less confidence level [26].
Of the variables assessed, only profession significantly predicted knowledge regarding the definition of AMS. Pharmacists were twice as likely to define AMS correctly as medical doctors. In contrast to our finding, profession was not a significant predictor of AMS knowledge in the study of Sefah et al [19]. This difference in knowledge between these professions observed in our study may be because, as medicine experts, pharmacists are more aware and conscious of the association between antibiotic use and the development of resistance. The differences in knowledge could also be due to differences in undergraduate curriculum and training, as well as professional focus between pharmacists and medical doctors [27, 28]. Prior research involving final-year medical and pharmacy students revealed that a greater proportion of pharmacy students than medical students had received formal instruction in antimicrobial stewardship [29].
The knowledge gap reported in this study and others [12, 19, 24, 26] highlights the need for educational interventions such as, meetings, academic detailing, distribution of educational materials and educational outreaches [30] targeted at different cadres of healthcare professionals. Education is one of the enabling interventions of AMS which has been reported to improve compliance with antibiotic policies alone and in combination with restrictive interventions [30].
The goal of the AWaRe classification of antibiotics is to reduce AMR. The ‘Watch’ and ’Reserve’ groups of antibiotics are to be prioritised as key targets of stewardship programmes and monitoring to preserve their effectiveness [8, 9]. In this study, an overwhelming majority of participants had no idea of the AWaRe classification. Amongst the medical doctors who participated in the study, only about a quarter knew about the AWaRe classification. This finding is of concern because of the risk of overprescribing antibiotics from the ‘Watch’ and “Reserve’ groups, which ought to be used for specific infectious syndromes and in highly specific patients and settings, [8] respectively. The current recommendation is that at least 60% of antibiotic use should come from the ‘Access’ group antibiotics [9]. Pharmacists play important roles in AMS, including prospective monitoring of antibiotic use and providing feedback and education on rational prescribing [31].
Our study revealed that only profession significantly predicted awareness of the WHO AWaRe classification of antibiotics. Although there was no significant difference in the level of awareness of the WHO AWaRe classification between pharmacists and medical doctors, nurses were less likely to be aware of this classification than medical doctors. Older guidelines for hospital AMS, [31, 32] however, emphasised the roles of medical doctors and pharmacists in successful implementation rather than nurses’ roles. Nevertheless, the majority of the participants had no knowledge of the AWaRe classification and may therefore be unable to provide education and feedback that can promote the prescribing of the ‘Access’ group antibiotics. Among the few frontline healthcare professionals who knew about the AWaRe classification, less than one-third correctly identified nine antibiotics included in the survey instrument into ‘Access’, ‘Watch’ and ‘Reserve’ groups. The authors are unaware of studies that assessed knowledge of the AWaRe classification of antibiotics among healthcare professionals with which to compare this study findings. Nevertheless, the knowledge gap identified echoes the need for improved awareness of AMR and judicious use of antibiotics in the Watch’ and ‘Reserve’ groups through effective communication, education and training of frontline healthcare professionals.
Limitations
While this study recruited a little above the target sample size, a major limitation is that it was a single-state study, thus findings may not be generalised to other states and practice settings. Furthermore, due to the cross-sectional design of the study, causality cannot be ascertained. As is common with survey research, participant bias, which arises when participants’ responses are deliberately or unintentionally different from their intended responses, is another limitation; selection bias due to the use of the non-probability sampling method in the recruitment of study participants and the possibility of social desirability bias among the participants may have affected the findings of the study.
Conclusions
Overall, there was a notable knowledge deficit in both AMS and the AWaRe classification of antibiotics among healthcare professionals who participated in this study. Educational programmes should be developed for different professional groups to enhance competency and proficiency in AMS to ensure judicious use of antibiotics with a high potential for selection of resistance.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMR:
-
Antimicrobial resistance
- AMS:
-
Antimicrobial stewardship
- AWaRe:
-
Access, Watch, and Reserve
- CI:
-
Confidence interval
- G20:
-
Group of 20
- OR:
-
Odds ratio
- SPSS:
-
Statistical Product and Service Solutions
- WHO:
-
World Health Organisation
References
Ten threats to global health in 2019. World Health Organisation. 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed January 10, 2024.
Murray C, Ikuta K, Sharara F, Swetschinski L, Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;12(10325):629–55.
Dyar OJ, Huttner B, Schouten J, Pulcini C. What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23(11):793–8.
Policy guidance on integrated antimicrobial stewardship activities. World Health Organisation. 2021. https://iris.who.int/bitstream/handle/10665/341432/9789240025530-eng.pdf?sequence=1. Accessed January 10, 2024.
Start smart then focus: antimicrobial stewardship toolkit for inpatient care settings. UK Health Security Agency. 2023. https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus/start-smart-then-focus-antimicrobial-stewardship-toolkit-for-inpatient-care-settings. Accessed January 10, 2024.
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51.
Core elements of hospital antibiotic stewardship programs. Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/antibioticuse/healthcare/pdfs/hospitalcore-elements-H.pdf. Accessed January 10, 2024.
Model List of Essential Medicines, 23rd List. World Health Organisation. 2023. https://iris.who.int/bitstream/handle/10665/371090/WHO-MHP-HPS-EML-2023.02-eng.pdf?sequence=1. Accessed January 10, 2024.
2021 AWaRe classification. World Health Organisation. 2021. https://www.who.int/publications/i/item/2021-aware-classification. Accessed January 10, 2024.
Akpan M, Isemin N, Udoh A, Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. 2020;22:317–24.
Iregbu K, Nwajiobi-Princewill P, Medugu N, Umeokonkwo C, Uwaezuoke N, Peter Y, et al. Antimicrobial stewardship implementation in Nigerian hospitals: gaps and challenges. Afr J Clin Exper Microbiol. 2021;22:60–6.
Fishman N. Policy statement on amtimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33:322–7.
Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. National Institute of Health and Care Excellence. 2015. https://www.nice.org.uk/guidance/ng15/resources/antimicrobial-stewardship-systems-and-processes-for-effective-antimicrobial-medicine-use-pdf-1837273110469. Accessed January 17, 2024.
Global action plan on antimicrobial resistance. World Health Organisation. 2015. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 Accessed January 17, 2024.
Tegagn GT, Yadesa TM, Ahmed Y. Knowledge, attitudes and practices of healthcare professionals towards antimicrobial stewardship and their predictors in Fitche Hospital. J Bioanal Biomed. 2017;9:091–7.
Labi A, Obeng-Nkrumah N, Bjerrum S, Adu Aryee NA, Ofori-Adjei YA, Yawson AE, Newman MJ. Physicians’ knowledge, attitudes, and perceptions concerning antibiotic resistance: a survey in a Ghanaian tertiary care hospital. BMC Health Serv Res. 2018;18:126.
Al-Halawa D, Abu Seir R, Qasrawi R. Antibiotic resistance knowledge, attitudes, and practices among pharmacists: a cross-sectional study in West Bank, Palestine. J Environ Public Health. 2019. https://doi.org/10.1155/2023/2294048.
Tembo N, Mudenda S, Banda M, Chileshe M, Matafwali S. Knowledge, attitudes and practices on antimicrobial resistance among pharmacy personnel and nurses at a tertiary hospital in Ndola, Zambia: implications for antimicrobial stewardship programmes. JAC Antimicrob Resist. 2022. https://doi.org/10.1093/jacamr/dlac107.
Sefah I, Chetty S, Yamoah P, Meyer JC, Chigome A, Godman B, Bangalee V. A Multicenter cross-sectional survey of knowledge, attitude, and practices of healthcare professionals towards antimicrobial stewardship in Ghana: findings and implications. Antibiotics. 2023;12:1497.
Carter E, Greendyke WG, Furuya E, Srinivasan A, Shelley A, Bothra A, et al. Exploring the nurses’ role in antibiotic stewardship: a multisite qualitative study of nurses and infection preventionists. Am J Infect Control. 2018;46:492–7.
Huizen P, Kuhn L, Russo PL, Connell CJ. The nurses’ role in antimicrobial stewardship: a scoping review. Int J Nurs Stud. 2021;113:103772.
Davey K, Aveyard H. Nurses’ perceptions of their role in antimicrobial stewardship within the hospital environment. An integrative literature review. J Clin Nurs. 2022;31:3011–20.
Castro-Sánchez E, Gilchrist M, Ahmad R, Courtenay M, Bosanquet J, Holmes AH. Nurse roles in antimicrobial stewardship: lessons from public sectors models of acute care service delivery in the United Kingdom. Antimicrob Resist Infect Control. 2019;8:162.
Merrill K, Hanson SF, Sumner S, Vento T, Veillette J, Webb B. Antimicrobial stewardship: staff nurse knowledge and attitudes. Am J Infect Control. 2019;47:1219–24.
Ju J, Han K, Ryu J, Cho H. Nurses’ attitudes toward antimicrobial stewardship in South Korea. J Hosp Infect. 2022;129:162–70.
Balliram R, Sibanda W, Essack SY. The knowledge, attitudes and practices of doctors, pharmacists and nurses on antimicrobials, antimicrobial resistance and antimicrobial stewardship in South Africa. S Afr J Infect Dis. 202110.4102/ sajid.v36i1.262.
Keijsers CJPW, Brouwers JRBJ, Wildt DJ, Custers EJF, Cate OT, Hazen ACM, et al. A comparison of medical and pharmacy students’ knowledge and skills of pharmacology and pharmacotherapy. Br J Clin Pharmacol. 2014;78(4):781–8.
Harrington AR, Warholak TL, Hines LE, Taylor AM, Sherill D, Malone DC. Healthcare professional students’ knowledge of drug-drug interactions. Am J Pharm Educ. 2011;75:199. https://doi.org/10.5688/ajpe7510199.
Ubaka CM, Schellack N, Nwomeh B, Goff DA. Antimicrobial resistance and stewardship knowledge and perception among medical and pharmacy students in Nigeria. Open Forum Infect Dis. 2019. https://doi.org/10.1093/ofid/ofz360.1703.
Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD003543.pub4.
MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–56.
Dellit T, Owens R, McGowan J, Gerding D, Weinstein R, Burke J, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77.
Acknowledgements
The authors are grateful to all healthcare professionals who participated in the study.
Funding
No funds, grants, or other support was received for conducting this study.
Author information
Authors and Affiliations
Contributions
MA conceptualised the study. All authors contributed to methods design and ethics processes. Data collection: SM; Data analysis: MA, IJ & SM; Writing of original draft: MA. All authors reviewed, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval to conduct the study was obtained from the Health Research Ethics Committees of the Akwa Ibom State Ministry of Health (AKHREC/01/08/23/169; 07/09/2023) and the University of Uyo Teaching hospital (UUTH/AD/S/96/VOLXXI/776; 21/08/2023). Informed consent to participate in the survey was sought in the questionnaire; participants were required to check a box to consent they agreed to take part in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Akpan, M.R., Jackson, I.L., Eshiet, U.I. et al. Knowledge of antimicrobial stewardship and the Access, Watch and Reserve (AWaRe) classification of antibiotics among frontline healthcare professionals in Akwa Ibom State, Nigeria: a cross-sectional study. BMC Health Serv Res 24, 1014 (2024). https://doi.org/10.1186/s12913-024-11428-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11428-8