Abstract
Background
Cross-sectionally, older age and obesity are associated with increased coronavirus disease-2019 (COVID-19) risk. We assessed the longitudinal associations of baseline and changes in adiposity parameters with COVID-19 incidence in older adults at high cardiovascular risk.
Methods
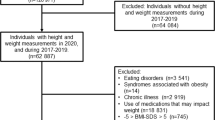
This analysis included 6874 men and women (aged 55–75 years) with overweight/obesity and metabolic syndrome in the PREDIMED-Plus lifestyle intervention trial for cardiovascular risk reduction. Body weight, body-mass-index (BMI), waist circumference, waist-to-height ratio (WHtR), and a body shape index (ABSI) were measured at baseline and annual follow-up visits. COVID-19 was ascertained by an independent Event Committee until 31 December 2021. Cox regression models were fitted to evaluate the risk of COVID-19 incidence based on baseline adiposity parameters measured 5–6 years before the pandemic and their changes at the visit prior to censoring.
Results
At the time of censoring, 653 incident COVID-19 cases occurred. Higher baseline body weight, BMI, waist circumference, and WHtR were associated with increased COVID-19 risk. During the follow-up, every unit increase in body weight (HRadj (95%CI): 1.01 (1.00, 1.03)) and BMI (HRadj: 1.04 (1.003, 1.08)) was associated with increased COVID-19 risk.
Conclusions
In older adults with overweight/obesity, clinically significant weight loss may protect against COVID-19.
Trial registration
This study is registered at the International Standard Randomized Controlled Trial (ISRCT; http://www.isrctn.com/ISRCTN89898870).
Similar content being viewed by others
Background
Coronavirus disease-2019 (COVID-19) is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) infection, which emerged as a global pandemic towards the end of 2019 [1, 2]. SARS-CoV2 had a pandemic potential, unlike the previous zoonotic coronaviruses [3]. The unprecedented impact of the COVID-19 pandemic has had enormous consequences for global health and economy [4]. COVID-19 is known to have an extensive systemic health impact beyond affecting the respiratory system [5], with some of these consequences being persistent [6]. The public health restrictions to “flatten the spread” of the disease until vaccination [7] have also had enormous socio-economic implications [8]. Worryingly, climate change, increasing land use, urbanization, and global connectedness are likely to accelerate the emergence and transmission of novel zoonotic diseases [9]. Thus, it is important and urgent to understand the facilitators and barriers to disease transmission to be better prepared to prevent pandemics like COVID-19 and their catastrophic consequences from recurring.
Various modifiable and non-modifiable risk factors have been associated with higher susceptibility to severe COVID-19 and its complications. Prominently, several cross-sectional examinations from the early phase of the pandemic found that older adults and those with obesity were typically vulnerable to severe infection [10,11,12,13]. Longitudinal associations of adiposity changes are poorly documented in the literature. This evidence is important because while diet-induced weight loss has been shown to improve cardiovascular risks and innate immunity in younger patients with obesity [14], the benefits of weight loss have been debatable in older adults [15]. As the proportion of older adults increases globally [16], understanding long-term associations of adiposity parameters and their changes over time with the risk of zoonotic diseases such as COVID-19 in this target population could contribute to clinical management. Hence, we evaluated the longitudinal associations of adiposity parameters (body weight, body-mass-index (BMI), waist circumference, waist-to-height ratio (WHtR), and a body shape index (ABSI)) and their changes over time prior to incident infections with the risk of developing COVID-19 in older adults with metabolic syndrome. We performed this analysis within the PREvención con DIeta MEDiterránea Plus (PREDIMED-Plus) framework.
Methods
PREDIMED-Plus study
PREDIMED-Plus is a multicenter, randomized controlled trial in Spain assessing the effectiveness of an intensive lifestyle intervention on the primary prevention of cardiovascular diseases in comparison to usual care in 6874 community-dwelling older adults (women and men, aged 55–75 years). A detailed study protocol has been previously published [17, 18] and is available at https://www.predimedplus.com/. In brief, participants were eligible for study enrolment if they were overweight or obese (BMI between 27 and 40 kg/m2), and satisfied a minimum of three criteria for metabolic syndrome [19].
At enrolment, participants were free from cardiovascular disease and active cancer. The PREDIMED-Plus study hypothesizes that an intensive lifestyle intervention that encourages energy reduction with a high-quality Mediterranean dietary pattern and increased physical activity with motivational behavior support will have a larger reduction in the risk of hard cardiovascular events compared to usual care encouraging a Mediterranean diet [18]. The authors postulate that the greater risk reduction would result from the effectiveness of the intensive lifestyle intervention in facilitating long-term weight loss and maintenance, including reductions in waist circumference [18]. The 6-year trial intervention period has recently been completed and the in-person yearly follow-up for 2 years is currently ongoing and scheduled to be completed in 2024. The protocol of PREDIMED-Plus has approvals from the institutional review boards of all participating centers in line with the Declaration of Helsinki (Additional File 1_ SMethods for details). All enrolled participants provided written informed consent. This study is registered with the International Standard Randomized Controlled Trial Registry (ISRCT; http://www.isrctn.com/ISRCTN89898870).
The PREDIMED-Plus cohort has scheduled baseline and annual follow-up anthropometric, dietary, and physical activity data providing a cumulative assessment of the exposures before the COVID-19 pandemic. The documentation of sociodemographic and health data in the PREDIMED-Plus study also facilitates adjustments for potential confounders, making the study database well-suited to explore the prospective association of anthropometric parameters and their changes over time with the risk of COVID-19 incidence.
Exposure: adiposity parameters
Adiposity parameters indicating general (body weight, BMI) and central (waist circumference and WHtR) obesity were assessed at baseline and yearly thereafter. This analysis assessed adiposity parameters from two perspectives: (i) values at baseline measured 5–6 years prior to the onset of the pandemic and (ii) changes from baseline over this time period (see below).
Baseline adiposity parameters
Body weight (kg), height (cm), and waist circumference (cm) of participants in light clothing were measured in duplicate by trained personnel at baseline and all annual visits. A mean of the duplicate measures for each visit was calculated. BMI was calculated as weight (kg)/height (m2). WHtR was calculated as a ratio of waist and height measurements in centimeters. A body shape index (ABSI), a measure of body shape independent of body weight and height, was calculated as waist circumference × weight−2/3 × height5/6 [20]. ABSI was multiplied by 1000 to facilitate interpretation [21]. Participants were categorized into tertiles based on baseline body weight, waist circumference, WHtR, and ABSI. Participants’ BMI was categorized into overweight (BMI < 30kg/m2) or obesity (BMI ≥ 30kg/m2).
Changes in adiposity parameters
We calculated changes in adiposity occurring over two time points. Firstly, for the main analysis, “pre-censoring adiposity changes” for incident COVID-19 cases were defined as the difference in adiposity between the available value at the last visit before COVID-19 ascertainment and the baseline value. For non-incident COVID-19 participants, the last available adiposity data on or prior to the date of censoring (31 December 2021) or mortality was used. For a secondary analysis, we calculated adiposity changes from baseline until the last visit on/before 8 March 2020, when community transmission became widespread in Spain [22]. No incident COVID-19 cases were recorded in this cohort before this date. To facilitate clinical interpretation, participants were further categorized into three groups based on the percentage change in adiposity parameters: (i) those who experienced a gain, (ii) those who remained stable or achieved < 5% reduction, and (iii) those who achieved ≥ 5% reduction, relative to the baseline value. This categorization was undertaken since a weight loss of 5% is considered clinically significant [23].
Outcome: COVID-19 incidence
A COVID-19 event was confirmed in a participant as adjudicated by the Clinical Event Ascertainment Committee of the PREDIMED-Plus trial based on medical records that were reviewed annually by physicians blinded to the intervention (see Supplementary Methods, Additional File 1_ SMethods). This analysis used only the first COVID-19 event in a participant adjudicated and confirmed from the start of the pandemic until 31 December 2021.
Ascertainment of covariates
Sociodemographic data (age, sex, education level, marital status), health status, smoking habits, and alcohol consumption were self-reported by the participants at baseline and during the annual follow-up. The baseline data for these variables along with the participants’ recruitment center (location) were obtained for use as covariates.
In the PREDIMED-Plus, lifestyle information including adherence to an energy-reduced Mediterranean diet [24] and physical activity levels [25] was self-reported and documented using validated instruments for this population at all visits. Adherence to the energy-reduced Mediterranean was captured using a 17-item energy-restricted Mediterranean Adherence Screener (er-MEDAS) with a score range of 0–17 [24]. Higher er-MEDAS scores indicated higher adherence to the energy-restricted Mediterranean diet. Physical activity was assessed using the validated REGICOR questionnaire and total leisure-time physical activity-related energy expenditure was estimated in MET·min/week [26]. Baseline er-MEDAS and physical activity level data were obtained from the database for use as covariates.
Since exposure to angiotensin-converting enzyme (ACE) inhibitor drugs is known to affect COVID-19 risk [27], data on prior use of the medication until the pre-censoring visit was sourced from medical records. Data on whether the participants had obtained a first dose of a COVID-19 vaccine before censoring was also obtained from these records.
Statistical analysis
The analysis included all 6874 randomized PREDIMED-Plus participants. A preliminary cross-sectional exploration was undertaken to compare participant characteristics across tertiles of baseline body weight and categories of body weight change at the pre-censoring visit. For this purpose, we used chi-square and Kruskal–Wallis tests, as appropriate. These results are described using median and interquartile range (IQR) or count and percentages for continuous and categorical data, respectively.
Because the exposures in this analysis were collected prior to outcome determination, we conducted a prospective analysis using the Cox proportional regression model, in which time to event for each participant began at randomization and ended at the time of COVID-19 diagnosis or the date of death or last contact on or prior to 31 December 2021, whichever occurred first.
The results of the main Cox proportional regression model using baseline adiposity parameters or their changes from baseline at the pre-censoring visit as the exposure, and COVID-19 status (incident case or not-incident) as the outcome, are presented as Hazards Ratio (HR) with 95% confidence interval (CI). In addition to the crude model without adjustments, two other models were tested. Model 1 was adjusted for baseline age (years), sex (male/female), education (primary or less/secondary/university), marital status (single or divorced/married/widow(er)), and recruitment center (location). Model 2 additionally adjusted for the intervention group, baseline smoking status (never/former/current), Mediterranean diet adherence score (17-point scale), total physical activity (METs.min/week), alcohol intake (g/d as a quadratic term), previous diagnosis of chronic diseases (diabetes, hypertension, hypercholesterolemia (Yes/No), prior use of ACE-inhibitor (Yes/No), and having received at least one dose of COVID-19 vaccine (Yes/No).
For modeling the linear association between absolute change in adiposity indicators (value at pre-censoring visit/value before 8 March 2020 − baseline value) and COVID-19 incidence, the respective baseline value was used as a covariate.
A simplified supplementary analysis was carried out comparing the HR in those who experienced any amount of weight gain to those who remained weight stable or lost any amount of weight. In this analysis, we used a third model to additionally adjust for the total numbers of leucocytes that have been implicated in inflammation and a positive diagnosis of COVID-19 [28]. Interactions of change in body weight with potential confounding factors (age group < 65 or ≥ 65 years, sex, smoking status, the prevalence of overweight/obesity, prevalence or absence of diabetes, hypercholesterolemia, and ACE inhibitor use) were assessed with a likelihood ratio test. Linear regression modeling stratified by these factors was undertaken and strata-wise HR for COVID-19 risk was inspected graphically.
An additional supplementary analysis using changes in adiposity parameters from baseline until 8 March 2020 (documented date of first community transmission of COVID-19 in Spain) as the exposure was performed using the same models as above to investigate their associations with COVID-19 incidence risk.
Additionally, sensitivity analyses were carried out to verify the results after excluding participants (n = 108) who had deceased prior to the known onset of the COVID-19 pandemic (i.e., death prior to 30 November 2019).
There were no missing data for age at trial entry, sex, education, intervention group, recruitment center, baseline physical activity, anthropometry, and prevalence of chronic conditions. Baseline smoking status and marital status had 0.4% missing data which were replaced with the mode of the variable for the cohort. Baseline alcohol consumption had 0.5% missing data, which was replaced with cohort mean consumption according to sex. Less than 0.1% of baseline Er MEDAS was missing and these were replaced with the cohort mean.
STATA (Version 14.2) was used to perform all analyses with the statistical significance set at 5%. PREDIMED-Plus database updated until 10 March 2023 was used for this analysis. All analyses were conducted with robust estimates of the variance to correct for intra-cluster correlation. Assuming that 10% of PREDIMED-Plus participants were diagnosed with COVID-19, the sample size of the trial provided 80% power to identify a 20% reduction in HR from one level of the exposure category in comparison to the other, assuming similar numbers in each category and with the statistical significance set at p < 0.05.
Results
All 6874 participants randomized to this trial were available for the analysis, with 653 COVID-19-positive cases. Exposures were assessed over a median (IQR) follow-up of 5.8 (5.3–6.6) years which accounted for a total analysis time at risk of 40,497-person-years and an incidence rate of 16.1 (95%CI: 14.9, 17.4) per 1000 person-years.
At baseline, 5046 (73.4%) participants had obesity and the rest 1828 (26.7%) had overweight. Participant characteristics according to tertiles of baseline body weight are presented in Table 1. A heat map showing the correlations between the baseline adiposity indicators, stratified by sex, is presented in Supplementary Figure S1 (Additional File 2_Fig. S1). While BMI, height, and waist circumference showed high degrees of correlation with weight, ABSI, an indicator of body shape (central adiposity), showed lower degrees of correlation with general adiposity indicators such as body weight and BMI that are height dependent.
In preliminary comparisons, participants in the lowest tertile of body weight were older, more likely to be women, non-smokers, had lower levels of education, and more likely to be widowed at baseline. They had lower mean baseline BMI, waist circumference, WHtR, and ABSI than those in the higher body weight tertiles. They were also less likely to have diabetes, but more likely to have hypertension at baseline. They showed higher baseline adherence to the Mediterranean diet and were less likely to have tested COVID-19-positive during the follow-up. Those in the highest tertile of body weight at baseline had a higher total number of leucocytes compared to those in lower tertiles at the most recent visit prior to COVID-19, indicating higher levels of inflammation.
At the pre-censoring visit, 2260 (32.9%) participants had gained weight, 2432 (35.3%) remained weight stable or lost < 5% of their initial body weight, and 2182 (31.7%) lost ≥ 5% of their body weight relative to baseline. Participant characteristics according to body weight change category are presented at the pre-censoring visit in Supplementary Table S1 (Additional File 3_Table S1). Those who gained body weight were more likely to be current smokers at baseline. On the contrary, those achieving significant weight loss were also likely to have higher adiposity indices at baseline and presented a higher prevalence of diabetes and hypertension at baseline. Additionally, they were also more likely to have received at least one dose of the COVID-19 vaccine at the time of censoring. There was no significant difference in the duration spent in the trial at the pre-censoring visit between participants in varying body weight change categories.
Longitudinal associations between baseline adiposity indicators and the risk of COVID-19 incidence are presented in Table 2. All baseline adiposity indicators showed positive longitudinal associations with COVID-19 risk, even when adjusted for potential confounders including sex and vaccination status. When evaluated as tertiles, those in the highest tertile of body weight (HR (95%CI): 1.46 (1.17, 1.83)) and WHtR (HR:1.22 (1.01,1.47)) had significantly higher risks compared to those in the respective lowest tertile. Having obesity versus having overweight at baseline, significantly increased the risk of COVID-19 by an average of 27% (95%CI: 5 to 53%), when fully adjusted. Every additional centimeter in baseline waist circumference was associated with a 1% increase in COVID-19 risk (95%CI: 0.4 to 2% increase) in the fully adjusted model. Body shape indicator (ABSI) was not associated with COVID-19.
Associations between changes in adiposity indicators at the pre-censoring visit and the risk of COVID-19 incidence are presented in Table 3. Every unit reduction in body weight and BMI was significantly associated with lower COVID-19 risk in PREDIMED-Plus participants. However, in the fully adjusted model, only having ≥ 5% reduction in body weight over the follow-up decreased COVID-19 risk on average by 19% (95%CI: 0.04 to 33% reduction) compared to gaining body weight. Losing < 5% of body weight did not appear to have a significant association with the risk of contracting the disease compared to gaining weight (also see Supplementary Table S2- Additional File 4_Table S2). Accordingly, every unit increase in BMI was associated with an increased COVID-19 risk (HR (95%CI): 1.04 (1.003 to 1.08)). Changes in waist circumference and WHtR were not associated with COVID-19 risk. Compared to ABSI measure gains, reductions ≥ 5% were significantly associated with a higher incidence of COVID-19 when fully adjusted (32%, 95%CI: 2 to 72% increase). Supplementary Figure S2 (Additional File 5_Fig. S2) indicates a trend for a higher incidence rate of COVID-19 in participants who had increases in body weight and body mass index compared to those who maintained or had reductions in these measures. This trend was reversed for ABSI and non-prominent for girth measures.
The interaction between potential factors of interest and pre-censoring visit body weight change and strata-wise HR (95% CI) for COVID-19 per kg increase in body weight is shown in Fig. 1. No significant interactions of body weight change were observed with any of the factors tested in their association with COVID-19 risk.
Supplementary analyses that used changes in adiposity parameters that occurred prior to community transmission of COVID-19 in Spain as the exposure did not alter the directionality of the results (Supplementary Table S3, Additional File 6_Table S3). The exclusion of participants who had deceased prior to the emergence of COVID-19 (n = 108) for the sensitivity analysis also did not alter the results for associations of COVID-19 risk with baseline adiposity indicators (Supplementary Table S4, Additional File 7_Table S4) or in their changes at the pre-censoring visit (Supplementary Table S5, Additional File 8_Table S5).
Discussion
We prospectively investigated, the association of baseline adiposity indices and their changes over time with the risk of a SARS-CoV2 infection in the PREDIMED-Plus cohort of older adults with overweight/obesity and metabolic syndrome. Expectedly, several baseline adiposity parameters measured 5–6 years prior to the pandemic were positively associated with COVID-19 risk. In addition, while decreases in body weight and BMI during this period were associated with decreased risk of COVID-19, even when adjusted for baseline values, reductions in girth measures were not associated with significant protective effect against COVID-19.
Previously, several cross-sectional examinations from the early phase of the pandemic found that older adults and those with excessive body weight were typically more vulnerable to severe infection [10,11,12,13]. Our study extends these findings by showing that several adiposity parameters in older adults measured 5–6 years prior to the pandemic were also significantly longitudinally associated with increased COVID-19 risk. These associations can be explained by obesity-related metabolic alterations that increase inflammation and result in poor immune response to viruses, including in SARS-CoV2 infections [13, 29]. This suggestion is further supported by a positive association of the total number of leucocytes observed with baseline body weight and body weight gain. It is of interest to note the higher level of total lymphocytes prior to COVID-19 in those who gained body weight (Supplementary Table S1, Additional File 3_Table S1) given a trend for higher lymphocyte level reported in COVID-19-positive patients [28].
Given the health consequences of excess body weight, it is universally recommended that those with overweight/obesity lose weight. However, there is scant information on the effect of the body weight trajectory of individuals in the period prior to COVID-19 on the disease risk, specifically in older adults. The findings from the current analysis suggest the importance of body weight loss in older adults with overweight and obesity, for improving resistance to viral infections such as COVID-19. These results are specifically useful given the expected increase in the emergence and transmission of zoonotic diseases [9] and the globally aging population [16]. The findings importantly show the utility of easily accessible and practical measures such as body weight and BMI in follow-up evaluation among older adults.
The inclusion of ABSI is a unique feature of this analysis that facilitates evaluating baseline or changes in central obesity independent of weight and height parameters (Supplementary Figure S1, Additional File 2_Fig. S1). While waist circumference also evaluates central obesity, it correlates with weight and height and therefore may not be specific for body shape [20]. We found that baseline ABSI was not a predictor of COVID-19 risk in this cohort. This is counter-intuitive given the superiority of ABSI to conventional adiposity parameters such as body weight, BMI, or waist circumference in predicting several mortality in American and European populations [20, 21]. However, both these previous studies included healthy adults aged over 18 years. Given the inclusion criteria of the PREDIMED-PLUS trial, our participants were older and had overweight/obesity and metabolic syndrome. Central obesity which is a hallmark of metabolic syndrome was present in > 99% of PREDIMED-participants. It is possible that older age and predominance of central adiposity diminish the discriminatory ability of baseline ABSI in our participants as demonstrated previously in a cohort of older adults in China [30]. Additionally, larger reductions in ABSI were associated with increased COVID-19 risk in this cohort, although this association was no longer significant in the sensitivity analysis that was restricted to assess changes in adiposity that occurred prior to community transmission of COVID-19. This suggests the potential for residual confounding that could affect the association between changes in ABSI and COVID-19 risk (Supplementary Table S3, Additional File 6_Table S3). Also, baseline but not changes in girth measures were associated with the disease risk unlike body weight and BMI. This could be because body weight and height are less prone to errors as compared to girth measurements making estimates of change less precise [31].
In this context of our study findings, the recent highlight on the need to monitor and investigate weight loss in older adults aged over 65 years by the ASPREE trial is informative [32]. This trial reported that a weight loss of 5% or more and reductions in waist circumference were associated with increased mortality [32]. The authors of the ASPREE trial explained that weight loss commonly precedes a diagnosis of chronic diseases in older adults and that it is associated with a reduced appetite and food intake. The authors have further elaborated on the complex pathways through which appetite suppression in the early stages of chronic disease development is associated with increased inflammation and decreases in muscle mass, muscle strength, and frailty. However, it is important to distinguish two elements of interest between the PREDIMED-Plus study and the ASPREE trial [32], as the health implication of weight loss/gain may be related to age and baseline weight [33]. PREDIMED-Plus participants on average were younger than the ASPREE participants. Secondly, while the ASPREE trial included participants with and without overweight and obesity, the PREDIMED-Plus study used to perform the current analysis is a trial encouraging weight loss and only included older adults with overweight/obesity and metabolic syndrome. While the opportunities for involuntary weight loss are greater in the ASPREE trial, given the nature of the trial, PREDIMED-Plus participants are more likely to have experienced voluntary weight loss. Future evaluations on the health benefits of voluntary weight loss, including reductions in girth in older adults with overweight/obesity, are necessary to tailor recommendations for this age group.
This is one of the first studies to investigate in older adults with overweight/obesity and metabolic syndrome the longitudinal association of baseline anthropometric measures and their changes in 5–6 years prior to the COVID-19 pandemic with the risk of infection. The strength of the analysis lies in its large sample size and documentation of exposures and several confounder variables repeatedly with standardized techniques for a considerable duration before the pandemic. This facilitates the evaluation of the longitudinal association of baseline and changes in adiposity indices with COVID-19 risk while adjusting for several potential confounders, including participant location. Additionally, the duration of follow-up across categories of weight loss is comparable, negating the time-dependent effects of the intervention. Moreover, COVID-19 event adjudication was performed by an independent committee removing any potential bias in the ascertainment of cases. We have also used several adiposity indices including body weight, BMI, waist circumference, and WHtR to define the exposure. This is specifically useful to describe features of general and central obesity and identify differences, if any, in their association with COVID-19 risk.
We also acknowledge the following limitations to this analysis. First, the small number of COVID-19 cases could have lowered the power of the study to identify associations of small magnitude. However, the incidence rate of COVID-19 in this study was similar to the national data reported for the time [34]. Next, since PREDIMED-Plus is currently ongoing, we do not have access to data on the incidence of cancer, diabetes, cardiovascular disease, or illness requiring surgical treatment. The cohort does not have precise measures quantifying body fat%, visceral adiposity, or subcutaneous adiposity. However, we believe while measurements using precision techniques such as dual-energy X-ray absorptiometry (DEXA) are of interest in high-resource research settings, they are not practical for use in primary health care or community settings. Also, weight loss normally accompanies aging [32]. Thus, we cannot ascribe all changes observed in body weight and shape to be voluntary in nature as a consequence of improved lifestyle habits. However, in the sensitivity analysis that confirmed the main findings, we excluded participants who had deceased prior to the onset of the COVID-19 pandemic. This may have offset the above limitation, at least in part, by excluding participants who experienced extreme body weight change due to severe illness. Thirdly, we cannot discount the misclassification of some cases as few participants may have had asymptomatic infections that went undiagnosed. However, we believe this to have been highly unlikely as we scrutinized all medical records during 2020 and 2021 when stringent public health strategies for COVID-19 testing were in place in Spain. Additionally, the supplementary analysis that investigated pre-pandemic anthropometric changes to exclude any reverse causality associated with asymptomatic cases confirmed the original findings. We cannot discount residual confounding given the observational nature of this analysis. Finally, this analysis uses participants included in a clinical trial and therefore caution is necessary while generalizing these results to all older adults or in younger age groups.
Conclusions
In older adults with overweight/obesity, higher body weight, BMI, waist circumference, and WHtR at baseline, measured 5–6 years prior to the pandemic, were associated with increased COVID-19 risk. Also, every unit reduction in body weight and BMI over this period was associated with decreased COVID-19 risk. Achieving ≥ 5% reductions in body weight loss compared to having gains in these measures was associated with lower COVID-19 incidence even when adjusted for baseline adiposity. In older adults with prior overweight/obesity, achieving clinically significant weight loss may be important to optimize immunity against COVID-19 and potentially other similar infections.
Availability of data and materials
The study protocol of PREDIMDED Plus including its statistical analysis plan for the main study has been published earlier [35]. The protocol can also be downloaded from https://www.predimedplus.com/. The datasets generated and analyzed during the current study are not publicly available due to data regulations and ethical reasons. However, collaboration for data analyses can be requested by sending a letter to the PREDIMED-Plus Steering Committee (predimed_plus_scommittee@googlegroups.com). The request will then be passed to all the members of the PREDIMED-Plus Steering Committee for deliberation.
Abbreviations
- ABSI:
-
A body shape index
- ACE:
-
Angiotensin-converting enzyme
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease-2019
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- SARS-CoV2:
-
Severe acute respiratory syndrome coronavirus-2
- WHtR:
-
Waist-to-height ratio
References
Guo P, Benito Ballesteros A, Yeung SP, Liu R, Saha A, Curtis L, et al. COVCOG 1: factors predicting physical, neurological and cognitive symptoms in long COVID in a community sample. A first publication from the COVID and cognition study. Front Aging Neurosci. 2022;14:804922–804922.
Guo P, Benito Ballesteros A, Yeung SP, Liu R, Saha A, Curtis L, et al. COVCOG 2: cognitive and memory deficits in long COVID: a second publication from the COVID and cognition study. Front Aging Neurosci. 2022;14:804937–804937.
Mackenzie JS, Smith DW. COVID-19—a novel zoonotic disease: a review of the disease, the virus, and public health measures. Asia Pac J Public Health. 2020;32(4):145–53.
WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int. Accessed 24 Feb 2023.
Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, et al. Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system. Hypertension. 2020;76(5):1350–67.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144.
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93.
Kaye AD, Okeagu CN, Pham AD, Silva RA, Hurley JJ, Arron BL, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: international perspectives. Best Pract Res Clin Anaesthesiol. 2021;35(3):293–306.
Holmes EC. COVID-19—lessons for zoonotic disease. Science. 2022;375(6585):1114–5.
Channappanavar R, Perlman S. Age-related susceptibility to coronavirus infections: role of impaired and dysregulated host immunity. J Clin Invest. 2020;130(12):6204–13.
Wee AKH. COVID-19’s toll on the elderly and those with diabetes mellitus – is vitamin B12 deficiency an accomplice? Med Hypotheses. 2021;146:110374.
Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging. 2020;12(10):9959–81.
Alberca RW, de Oliveira L M, Branco ACCC, Pereira NZ, Sato MN. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr. 2021;61(13):2262–76.
Lorenzo PM, Sajoux I, Izquierdo AG, Gomez-Arbelaez D, Zulet MA, Abete I, et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin Nutr. 2022;41(7):1566–77.
Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165(9):1035–40.
Kaplan MA, Inguanzo MM. The social, economic, and public health consequences of global population aging: implications for social work practice and public policy. J Soc Work Glob Community. 2017;2(1):1.
Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, Basora J, Fitó M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus Trial. Diabetes Care. 2018;42(5):777–88.
Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque J, et al. Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol. 2019;48(2):387–388o.
Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366(9491):1059–62.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504.
Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, et al. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10(1):14541.
Minder R. Spain becomes latest epicenter of coronavirus after a faltering response. The New York Times. 2020 Mar 13. https://www.nytimes.com/2020/03/13/world/europe/spain-coronavirus-emergency.html. Accessed 21 Apr 2023.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. J Am Coll Cardiol. 2014;63(25_Part_B):2985–3023.
Schröder H, Zomeño MD, Martínez-González MA, Salas-Salvadó J, Corella D, Vioque J, et al. Validity of the energy-restricted Mediterranean diet adherence screener. Clin Nutr. 2021;40(8):4971–9.
Molina L, Sarmiento M, Peñafiel J, Donaire D, Garcia-Aymerich J, Gomez M, et al. Validation of the Regicor Short Physical Activity questionnaire for the adult population. PLoS ONE. 2017;12(1):e0168148.
Rosique-Esteban N, Díaz-López A, Martínez-González MA, Corella D, Goday A, Martínez JA, et al. Leisure-time physical activity, sedentary behaviors, sleep, and cardiometabolic risk factors at baseline in the PREDIMED-PLUS intervention trial: a cross-sectional analysis. PLoS ONE. 2017;12(3):e0172253.
Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Köse M, Tükek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID-19. Anatol J Cardiol. 2020;24(1):21–9.
Usul E, Şan İ, Bekgöz B, Şahin A. Role of hematological parameters in COVID-19 patients in the emergency room. Biomark Med. 2020;14(13):1207–15.
MartínezUrbistondo M, Mora Vargas A, ExpósitoPalomo E, Aparicio de Miguel M, CastejónDíaz R, Daimiel L, et al. Evolution of patients infected with SARS-CoV-2 according to previous metabolic status. Nutr Hosp. 2021;38(5):1068–74.
Tsou MT, Chang YC, Hsu CP, Kuo YC, Yun CH, Huang WH, et al. Visceral adiposity index outperforms conventional anthropometric assessments as predictor of diabetes mellitus in elderly Chinese: a population-based study. Nutr Metab. 2021;18(1):87.
Løvsletten O, Jacobsen BK, Grimsgaard S, Njølstad I, Wilsgaard T, Løchen ML, et al. Prevalence of general and abdominal obesity in 2015–2016 and 8-year longitudinal weight and waist circumference changes in adults and elderly: the Tromsø Study. BMJ Open. 2020;10(11):e038465.
Hussain SM, Newman AB, Beilin LJ, Tonkin AM, Woods RL, Neumann JT, et al. Associations of change in body size with all-cause and cause-specific mortality among healthy older adults. JAMA Netw Open. 2023;6(4):e237482.
Park SY, Wilkens LR, Maskarinec G, Haiman CA, Kolonel LN, Marchand LL. Weight change in older adults and mortality: the multiethnic cohort study. Int J Obes (Lond). 2018;42(2):205–12.
Pinedo E. One in 10 Spaniards have had coronavirus, antibody study shows. Reuters. 15 Dec 2020. https://www.reuters.com/article/health-coronavirus-spain-study-idUSKBN28P23M. Accessed 2 May 2023.
Sayón-Orea C, Razquin C, Bulló M, Corella D, Fitó M, Romaguera D, et al. Effect of a nutritional and behavioral intervention on energy-reduced Mediterranean diet adherence among patients with metabolic syndrome. JAMA. 2019;322(15):1486–99.
Acknowledgements
The authors wish to thank the PREDIMED-Plus participants and staff for their engagement, as well as the primary care centers involved in the study. We also thank the Cerca Programme of the Generalitat de Catalunya, the CIBEROBN, CIBERESP, and CIBERDEM initiatives of Instituto de Salud Carlos III in Spain and INSA-Ma María de Maeztu Unit of Excellence (grant CEX2021-001234-M funded by MICIN/AEI/FEDER, UE).
Funding
This work was supported by a project grant from the Fundación Francisco Soria Melguizo. The project also received support from the Community of Madrid and the European Union, through the European Regional Development Fund (ERDF)-REACT-EU resources of the Madrid Operational Program 2014–2020, in the action line of R + D + i projects in response to COVID-19, FACINGLCOVID-CM. The PREDIMED-Plus trial was supported by the official Spanish Institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS), which is co-funded by the European Regional Development Fund. This study has been funded by Instituto de Salud Carlos III (ISCIII) through six coordinated FIS projects leaded by JS-S and JVi, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158; the Especial Action Project entitled: “Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus grant to JS-S” and co-funded by the European Union. It is also supported by the European Research Council (Advanced Research Grant 2014–2019; agreement #340918) granted to MÁM-G; the Recercaixa (number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, PI0137/2018); the PROMETEO/2017/017, PROMETEO 21/2021 grants from the Generalitat Valenciana; and the SEMERGEN grant. S.G.S is a recipient of the Maria Zambrano Fellowship with funding support from the Ministry of Universities and the Recovery, Transformation and Resilience Plan, Spain. The Fellowship is “Funded by the European Union – NextGenerationEU”. S.K.N. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR, MFE-171207). JS-S was partially supported by ICREA under the ICREA Academia program. We thank CERCA Programme/Generalitat de Catalunya for institutional support. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors (SS, JFGG, IPG, JJG, MAM, DC, JAM, AMA, JW, JV, DR, JL1, RE, FTJ, JL2, JLS, AB, JAT, WMS, XP, PM, JV, CV, LD, ER, FF, SKN, OG, ET, EMA, OC, AG, LT, EG, MAZ, NGR, RC, NC, LT, GAM, JVS, SC, SM, PJP, AO, RP, MDZ, AC, MD, NB, MF, JSS) made substantial contributions to the study concept or the data analysis or interpretation; SS and JSS drafted the manuscript; all authors revised it critically for the important intellectual concept; SS, MF, and JSS agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the study was conducted according to the guidelines of the Declaration of Helsinki. The study was approved by the Institutional Review Boards of all participating centers as follows (Facultad de Medicina de Málaga: CEI Provincial de Málaga-Servicio Andaluz de Salud:O01_feb_PR2—Predimedplus nodo 1, Centro Salud San Pablo de Sevilla: CEI de los Hospitales Universitarios Virgen Macarena y Virgen del Rocío-Servicio Andaluz de Salud: PI13/00673, Nutrición y Farmacia, Univ. De Navarra: CEIC Universidad de Navarra: 053/2013, Hospital Son Espases de Mallorca: CEI de las Illes Balears—Conselleria de Salut Direcció General de Salut Publica i Consum: IB 2242/14 PI, Hospital Clinic de Barcelona: CEIC del Hospital Clínic de Barcelona: HCB/2016/0287, IMIM, Barcelona: CEIC Parc de Salut Mar y IDIAP Jordi Gol: PI13/120, Universitat Rovira i Virigili: CEIC del Hospital Universitari Sant Joan de Reus y IDIAB Jordi Gol:13–07-25/7proj2, Universida de Granda: CEI de la Provincia de Granada- Servicio Andaluz de Salud: MAB/BGP/pg, Fundación Jiménez Díaz: CEIC de la Fundacion Jiménez Díaz: EC 26–14/IIS-FJD, Facultad de Mecina, Univ. De Navarra: CEIC Universidad de Navarra: 053/2013, Hospital Txagorritxu, Vitoria: CEIC Euskadi: PI2014044, Facultad de Mecina de Valencia: CEIC Corporativo de Atención Primaria de la Comunitat Valenciana: 2011–005398-22, Universidad de las Palmas de Gran Canaria: CEI Humana de la Universidad de las Palmas de Gran Canaria: CEIH-2013–07, Hospital Universitari de Bellvitge: CEIC del Hospital de Bellvitge: PR240/13, Universida de Córdoba: CEI de Cordoba-Junta de Salud: 3078, IMDEA, Madrid: CEI de la Fundación IMDEA Alimentación: PI-012, Hospital Clínico de Madrid:CEIC Hospital Clínico San Carlos de Madrid-Piloto-CEIC Servicio Madrileño de salud-General:30/15, Hospital de Málaga: CEI Provincial de Málaga-Servicio Andaluz de Salud, Universitat de les Illes Baleares: CEI de las Illes Balears—Conselleria de Salut Direcció General de Salut Publica i Consum: IB 2251/14 PI, Endocrinología, Hospital Clínic de Barcelona: CEIC del Hospital Clínic de Barcelona: HCB/2017/0351, Universidad Miguel Hernandez de Alicante: CEIC del Hospital General Universitario de Alicante: CEIC PI2017/02, Universidad de Jaén: CEIC de la Investigación Biomédica de Andalucía (CCEIBA), Universidad de León: CEI de la Universidad de León: ÉTICA-ULE-014–2015). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
Fernando Fernandez-Aranda acknowledges consulting fees from Novo Nordisk and Wiley as EIC for the European Eat Dis Rev. He has also received honoraria for lectures and support for travel/meetings from Novo Nordisk. He discloses his honorary participation in the Data Safety Monitoring Board or Advisory Board of Sustain-Consortium-Germany. Emilio Ros has received grants from the California Walnut Commission. He has also received consulting fees from Alexion. He had received honoraria for lectures and travel/meeting grants from Spanish Atherosclerosis Society. Ramon Estruch has received research grants from Instituto de Salud Carlos III, Madrid, Spain; Patrimonio Comunal Olivarero, Spain; Borges, SA, Spain; California Walnut Commission, USA; European Commission, Brussells, Belgium; National Institute of Health, Bethesda, USA; and Fundación Bosch i Gimpera, Barcelona. He has received consulting fees from Cerveza y Salud, Madrid, Spain, and Dallant Laboratories, Spain. He has received honoraria for presentations from Brewers of Europe, Belgium; Pernaud Richart, Mexico; and Wine and Culinary International Forum and Grand-Fountain Laboratories, Spain. He has received support for travel or meetings from Karolinska Institute, Menarini Laboratories, Sweden, Fundación Iberoamericana de Nutrición Italian Pavilion, EXPO DUBAI 2020, Cretan Lifestyle, European Parliament, Brussels, Belgium, and the Pontifical Academy of Sciences. He has chaired or was part of the committee that organized Beer and Health Innitiative, Brussels, Belgium; Fundación Dieta Mediterránea, Barcelona, Spain; and FIVIN, Spain. Sangeetha Shyam received consulting fees from Abbot Laboratories Sdn Bhd. Stephanie K. Nishi is a volunteer member of Plant-Based Canada, a non-profit organization. Jordi Salas-Salvadó reported receiving nonfinancial support from Patrimonio Comunal Olivarero, the California Walnut Commission, Almond Board of California, La Morella Nuts, Pistachio Growers and Borges S.A; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and grants and personal fees from Instituto Danone; and serving in the Board of Danone Institute International. The other authors declare that they have competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SMethods – [Supplementary Methods].
Additional file 2:
Fig. S1. [Fig S1- Correlations between the baseline adiposity indicators, stratified by sex].
Additional file 3:
Table S1. [Table S1-Participant characteristics according to body weight change at the pre-COVID-19 visit].
Additional file 4:
Table S2. [Table S2- Changes in body weight and risk of COVID-19 (HR & 95%CI)- supplementary analysis (simplified)].
Additional file 5:
Fig. S2. [Fig S2 - Incidence rate of COVID-19 by anthropometric change category].
Additional file 6:
Table S3. [Table S3: Changes in adiposity parameters prior to 8th March 2020 and risk of COVID-19 (HR & 95%CI)-Supplementary analysis].
Additional file 7:
Table S4. [Table S4: Baseline adiposity indicators and the risk of COVID (HR & 95%CI) (Sensitivity analysis)].
Additional file 8:
Table S5. [Table S5: Changes in adiposity indicators and risk of COVID-19 (HR & 95%CI) (Sensitivity analysis)].
Additional file 9.
STROBE_Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shyam, S., García-Gavilán, J.F., Paz-Graniel, I. et al. Association of adiposity and its changes over time with COVID-19 risk in older adults with overweight/obesity and metabolic syndrome: a longitudinal evaluation in the PREDIMED-Plus cohort. BMC Med 21, 390 (2023). https://doi.org/10.1186/s12916-023-03079-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03079-z