Abstract
Background
Immune checkpoint inhibitors (ICIs) had modest advances in the treatment of extensive-stage small cell lung cancer (ES-SCLC) in clinical trials, but there is a lack of biomarkers for prognosis in clinical practice.
Methods
We retrospectively collected data from ES-SCLC patients who received ICIs combined chemotherapy from two centers in China, integrated clinical and blood parameters, and constructed risk prognostication for immunochemotherapy. The population was divided into high- and low-risk groups, and the performance of the model was assessed separately in the training and validation cohorts.
Results
Two hundred and twenty and 43 patients were included in the training and validation groups, respectively. The important predictors were screened including body mass index, liver metastases, coefficient variation of red blood cell distribution width, lactate dehydrogenase, albumin, and C-reactive protein. Predicting 1-year overall survival (OS), the AUC values under ROC for the model under training, internal validation, and external validation were 0.760, 0.732, and 0.722, respectively, and the calibration curve and clinical decision curve performed well. Applied the model to divide patients into low-risk and high-risk groups, and the median OS was 23.7 months and 9.1 months, and the median progression-free survival was 8.2 months and 4.8 months, respectively; furthermore, this ability to discriminate survival was also observed in the validation cohort.
Conclusions
We constructed a novel prognostic model for ES-SCLC to predict survival employing baseline tumor burden, nutritional and inflammatory parameters, it is easily measured to screen high-risk patient populations.
Similar content being viewed by others
Background
Lung cancer is the leading cause of cancer death in China and a common type of cancer diagnosis worldwide [1]. Although small cell lung cancer (SCLC) accounts for only 15% of lung cancer cases, it has a high degree of malignancy and is characterized by rapid growth, easy metastasis, and high mortality [2,3,4,5]. Platinum in combination with etoposide has resulted in modest improvements in survival and has been the unshakable standard treatment over the 30 years in patients with extensive stage SCLC (ES-SCLC), with a 2-year survival rate of approximately 7% [6, 7]. Immune checkpoint inhibitors (ICIs) are rapidly progressing, and their synergy in combination with cytotoxic chemotherapy has been validated in some phase III clinical trials [8,9,10,11,12,13,14]. Based on IMpower133 and CASPIAN results, Atezolizumab or Durvalumab in combination with chemotherapy is approved for first-line treatment of ES-SCLC [11,12,13]. However, different types of ICI drugs have vastly different outcomes in clinical trials, Pembrolizumab and Nivolumab have been briefly approved for SCLC patients who have relapsed or metastasized following chemotherapy and have withdrawn their indications following phase III trials [9, 15,16,17]. In fact, only minority ES-SCLC patients could benefit from immunotherapy in clinical trials.
Although immunotherapy made a huger progress than standard chemotherapy, some patients still cannot benefit from it or even develop treatment-related serious adverse reactions and even superprogress, affecting the survival outcome and quality of life [8,9,10, 12,13,14]. Therefore, reliable biomarkers are needed to stratify patient survival risk. Programmed death ligand 1 (PD-L1) expression and tumor mutation burden (TMB) are universally acknowledged as predictive biomarkers for various types of tumors. SCLC has low PD-L1 expression but high TMB; nevertheless, no correlation between the two and treatment response has been found in clinical trials [10, 18]. Currently, a variety of biomarkers based on blood tests have been developed to assess the diagnosis, recurrence, and treatment effects of lung cancer, but studies of these markers have mostly focused on non-small cell lung cancer (NSCLC), and there is insufficient evidence in distinguishing the prognosis of patients treated with SCLC immunotherapy [5, 19, 20]. In addition, the combined effect of multiple indicators on the prediction of disease is often better than a single indicator, and the prognostic model is a good choice. Current studies mostly focus on the collection of clinical data, while ignoring the value of blood parameters.
Therefore, we intend to investigate the prognostic factors affecting ES-SCLC patients receiving immunochemotherapy in clinical practice and combine indicators to develop a model to provide complementary tools for patient stratification.
Methods
Study design and population
This cohort study included the patients diagnosed with ES-SCLC at two institutions in Beijing, China, from June 2019 to June 2023, and reviewed their medical record systems and imaging data. Other inclusion criteria included pathologically or cytologically diagnosed SCLC; age > 18 years; and Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. At the same time, the following patients were excluded: limited-stage SCLC; previous history of immunotherapy; history of other malignancies; active infection; autoimmune diseases or taking prednisone at baseline > 10 mg/day or equivalent; liver, kidney, and blood bone marrow insufficiency.
The study complied with the principles of the Declaration of Helsinki and local laws and regulations. The study was approved by the Ethics Committees of Beijing Chest Hospital, Capital Medical University, and Beijing Friendship Hospital, Capital Medical University before data collection began (YJS-2022–19). Given its retrospective nature, two institutional committees waived informed consent for the study.
Treatment regimen and follow-up
Immunotherapy used in this study included PD-L1 inhibitors and Programmed death 1 (PD-1) inhibitors which were approved by FDA and NMPA. Chemotherapy is mainly carboplatin or cisplatin combined with etoposide (EP/EC). ICIs may be continued as maintenance therapy in patients with responding or stable disease following immunotherapy. Combined treatment cycles were no more than 6 cycles and at least 2 cycles generally once every 3–4 weeks. Patients were followed until death or until the cut-off date for follow-up (01 December 2023). Response to therapy is usually assessed every 6–8 weeks, and tumor progression is viewed based on chest and abdominal computed tomography (CT) scans and brain magnetic resonance imaging (MRI). In addition, patients were followed up by clinic or regular telephone calls for survival after the end of treatment.
Data collection and study endpoints
Demographic and clinicopathological information such as gender, age, smoking history, body mass index (BMI), ECOG PS, T stage, N stage, distant metastasis site, treatment regimen, therapeutic effect, and follow-up data, and baseline laboratory indicators blood routine, biochemistry, coagulation, and tumor markers, etc., were collected. For parameters where missing values emerged during variable collection, we did not choose to remove them directly but instead supplemented the data using multiple imputations (Additional file 1: Fig. S1). The primary study endpoint was overall survival (OS), defined as the time from the start of treatment to death from any cause. Meanwhile, time to disease progression was recorded and progression-free survival (PFS) was calculated. Cases for which no outcome events were observed as of the last follow-up were defined as censored data, and their survival time was the interval between the start of immunotherapy and the last follow-up. Patients’ best response to treatment after the start of immunochemotherapy was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, with objective response rate (ORR) defined as the proportion of patients with complete response (CR) and partial response (PR), while disease control rate (DCR) added the proportion of patients with stable disease (SD) on the basis of ORR.
Model development and evaluation
The patient cohort receiving immunochemotherapy at Beijing Chest Hospital, Capital Medical University served as the training set for this study to construct the model, while the study population of Beijing Friendship Hospital, Capital Medical University was the external validation set. In addition, some chemotherapy cohorts alone were selected to evaluate the model performance. With survival status and time as outcome measures, all clinical variables and test measures were screened using the least absolute shrinkage and selection algorithm (Lasso), and then the model was fitted by multivariate Cox regression, while fully considering expert opinion to determine the final prognostic variables, constructing the model and drawing nomograms.
Predicting 1-year OS, receiver operating curve (ROC), calibration curve, and decision curve analysis (DCA) were plotted to assess the discrimination, calibration, and clinical benefit of the model in construction and validation groups, respectively. The time-dependent area under the ROC curve (AUC) in the modeling group was calculated to quantitatively describe how the discriminative ability of the model changed over follow-up time. In addition, fivefold cross-validation was used to assess the internal stability of the model, splitting the original data into 5 subsets, 1 subset as the validation set, and the remaining 4 subsets as the training set to obtain 5 AUC values, re-splitting the data to repeat the process 200 times, and calculating the mean AUC values at 6, 12, and 18 months. Finally, X-tile software was used to stratify the nomogram scores, divide the patient population into high and low-risk groups, and compare OS, PFS, and each variable. Figure 1A shows the design idea of the study.
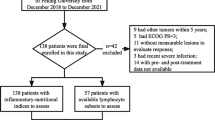
A Flow chart of the study design. Patients’ baseline clinical and hematological data were collected first, and the model was constructed after Lasso-Cox screening variables. Models were evaluated using multiple metrics and assessed using internal and external validation data. Finally, X-tile software divided the patient population into low-risk and high-risk group and observed their survival differences. B Patient enrollment flow chart. Patients with extensive stage small cell lung cancer were included, and patients receiving immune checkpoint inhibitors in combination with chemotherapy at both centers entered the training set and the validation set after screening according to the inclusion and exclusion criteria, respectively, as well as into cohorts of patients receiving chemotherapy alone, and the patient population was divided into high-risk and low-risk groups. OS, overall survival; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; ROC, receiver operating curve; CC, calibration curve; DCA, decision curve analysis; IV, internal verification; EV, external validation; KM, Kaplan–Meier; RF, risk factor plot; ES-SCLC, extensive-stage small cell lung cancer; IO, immunotherapy; Che, chemotherapy
Statistical analysis
Data analysis was performed using R software (version 4.3.1, 2023–06-16), and flow diagrams were drawn by BioRender. Quantitative data were expressed as mean plus or minus standard deviation or median (interquartile range (IQR)) according to the type of data distribution; qualitative data were described by frequency (percentage). Quantitative and qualitative data were compared between groups using Mann–Whitney U test and chi-square or Fisher’s exact test, respectively. Survival data can be plotted in Kaplan–Meier curves, described by median survival and 95% confidence intervals (CIs), and compared by the Log-Rank test. Correlations were initially explored by calculating the Spearman correlation matrix between variables, and the variance inflation factor (VIF) was used to evaluate whether the regression model was collinear. P values < 0.05 were considered statistically significant.
Results
Patient baseline characteristics
Figure 1B shows the screening process of the patients, and finally, 220, 43 and 38 patients were included in the training, validation and chemotherapy alone groups, respectively. In the training cohort, the majority were male (n = 173, 78.6%) and had ECOG PS 0–1 (n = 206, 93.6%); the median age of patients was 64.0 [IQR, 58.0–69.0] years and the median BMI was 24.3 [IQR, 21.8–27.1] kg/m2; and the number of patients with distant metastases to bone, liver, brain, and adrenal glands was 78 (35.5%), 67 (30.5%), 67 (30.5%), and 50 (22.7%), respectively. While immunochemotherapy was first-line in 165 (75.0%) patients, the rest were second line and above. Regarding smoking history, 167 (75.9%) patients had ever smoked or were smoking, 5 (2.3%) patients had never smoked, while the rest had an ominous smoking status. Analysis of the association between smoking and gender found that women were more likely to be non-smokers or have an unknown smoking history (P < 0.001) (Additional file 1: Table S1). No significant differences in baseline clinical data were found between the validation cohort and the training cohort, except for intrathoracic metastases, first-line therapy, and type of immunology agent (Table 1), and blood test parameters in both cohorts are listed in Additional file 1: Table S2.
Immunotherapy efficacy
As of the last follow-up, 132 patients in the training cohort had progressed and 74 patients had died. According to RECIST criteria, 107 (48.6%) patients had objective responses (1 CR and 106 PR) and 194 (88.2%) had disease control. Median OS and PFS in the training group were 19.1 (95% CI, 17.5–28) months and 7.7 (95% CI, 6.8–8.9) months, respectively. While median OS and PFS in the validation cohort were only 10.2 months and 5.1 months, ORR and DCR were 34.9 and 79.1%, respectively (Table 1).
Variables and survival analysis
Taking into account the clinical correlation between treatment lines and ECOG PS with patient survival, we plotted KM curves against their survival differences (Additional file 1: Fig. S2). First-line and above first-line immunotherapy had median OS of 21.1 and 18.1 months (P = 0.57) and median PFS of 7.8 and 7.6 months (P = 0.75), respectively; ECOG-PS0/1 and ECOG-PS2 patients had median OS of 20.6 and 18.4 months (P = 0.58) and median PFS of 7.8 and 6.5 months (P = 0.7), respectively. No survival differences were observed for these factors by KM curves.
Model development and evaluation
First, we used Lasso regression to screen variables associated with patient survival, including 62 variables, containing all variables in Table 2 and Additional file 1: Table S2 except smoking history, with different colors representing different variables in Fig. 2A, which narrowed to 0 as regularization parameters increased; Fig. 2B indicates that the likelihood deviation value became smaller during cross-validation. At the lowest deviation, 11 variables were selected, including BMI, liver metastases, eosinophils (EOS), coefficient variation of red blood cell distribution width (RDW-CV), platelet volume (PCT), alanine aminotransferase (ALT), albumin (ALB), lactate dehydrogenase (LDH), C-reactive protein (CRP), neuron-specific enolase (NSE), and D-dimer (Additional file 1: Fig. S3). Combined inclusion of these variables and line of therapy as well as ECOG-PS in multivariate Cox regression revealed that BMI, liver metastasis, RDW-CV, ALB, and CRP were independent predictors of OS in patients (Additional file 1: Table S3). Given the good performance of LDH in univariate analysis, as well as the endorsement of clinical experts, we included this variable. In addition, we presented OS and PFS from traditional univariate Cox regression analysis in Additional file 1: Table S4, and the parameters of the final model are provided in Additional file 1: Table S5. The Spearman correlation analysis showed low to moderate correlations between liver metastasis and LDH (r = 0.33, P < 0.001), RDW-CV and ALB (r = − 0.03, P < 0.05), LDH and ALB (r = − 0.12, P < 0.05), as well as CRP (r = 0.30, P < 0.01), ALB and CRP (r = − 0.39, P < 0.001) (Fig. 2C), and VIF values of BMI, liver metastasis, RDW-CV, ALB, LDH and CRP were 1.03, 1.23, 1.12, 1.23, 1.19, and 1.17, respectively, indicating no collinearity between each variable and other independent variables (Fig. 2D). Patients without liver metastases had significantly longer OS (21.1 months vs 11.5 months, p = 0.002) and PFS (7.8 months vs 6.1 months, p = 0.008) than patients with liver metastases (Fig. 2E,F). A nomogram was constructed with the above six variables to predict the survival probability of patients at 6, 12, and 18 months (Fig. 2G).
A Lasso coefficient path diagram, abscissa is log λ, ordinate is coefficient value of variable, 62 variables are represented by line segments of different colors, and variables gradually shrink to 0 with the increase of regularization parameters. B Lasso regression analysis cross-validation curve, abscissa is log λ, ordinate is likelihood deviation value under different indicators. The left dashed line corresponds to the smallest deviation (lambda.min), the dashed line on the right represents one standard error of lambda.min (lambda.1se), and its upper abscissa is the number of variables selected. C The correlation heatmap, the value in the figure and the size of the circle represent the r value, that is, the degree of correlation, blue represents a positive correlation, red represents a negative correlation, *** represents P < 0.001, ** represents P < 0.01, while * represents P < 0.05. D Variance inflation factor results are presented graphically, with abscissa for variable names and ordinate for VIF values to assess the degree of collinearity between different variables. E KM curves of presence or absence of liver metastases and overall survival in patients. F KM curves of presence or absence of liver metastases and progression-free survival in patients. G Nomograms including six variables to predict overall survival at 6, 12, and 18 months. BMI, body mass index; Liver-M, liver metastases; RDW-CV, red cell distribution width—coefficient of variation; LDH, Lactate Dehydrogenase; ALB, albumin; CRP, C-reactive protein; OS, overall survival
Figure 3A calculates time-dependent AUCs based on survival outcomes at different moments, and overall, AUCs decrease as prediction time increases. The AUC of the training set at 6, 12, and 18 months was 0.828 (95% CI, 0.741–0.915), 0.760 (95% CI, 0.668–0.852), and 0.722 (95% CI, 0.617–0.828), respectively, and ROC, calibration curve, and DCA all showed good discrimination, calibration, and clinical utility of the model (Fig. 3B–F). The fivefold internal cross-validation showed that the mean AUC of the model for predicting survival at 6, 12, and 18 months was 0.804, 0.732, and 0.689, respectively (Fig. 3G). In addition, the model performed well in the external validation cohort with AUC values of 0.644 (95% CI, 0.317–0.970), 0.722 (95% CI, 0.554–0.890), and 0.719 (95% CI, 0.547–0.891) at 6, 12, and 18 months, respectively (Fig. 3H–L).
A Time-dependent AUC in the training set. B Calibration curve for predicting 12-month survival in the training set versus actual 12-month survival in the training set. C DCA curve for predicting 12-month survival in the training set. D,E ROC curve for predicting 6-month, 12-month, and 18-month survival in the training set. G Box plots for AUC values predicted by fivefold internal validation for 6-month, 12-month, and 18-month survival in the training set. H Calibration curve for predicting 12-month survival in the validation set versus actual 12-month survival in the validation set. I DCA curve for predicting 12-month survival in the validation set. J–L ROC curve for predicting 6-month, 12-month, and 18-month survival in the validation set. AUC, area under the curve
Risk stratification
The nomogram total score for each patient was calculated using the weights of the individual variables, and the optimal cutoff value of 66.5 was selected according to the X-tile software (Additional file 1: Fig. S4). Thus, the patient cohort was divided into two risk groups, 80 and 13 people at high risk in the training and validation groups, respectively, and 140 and 30 in the low-risk group, respectively. Risk factor linkage map consists of three parts: risk score scatter diagram, patient survival and death scatter diagram, and index level heat map. The risk score in Fig. 4A was calculated using the formula: Risk Score = − 0.285 − 0.0342*BMI + 0.8189*Liver-M + 0.1481*RDW-CV + 0.0003*LDH-0.0357*ALB + 0.0149*CRP, ranked from small to large, with a total nomogram score of 66.5 corresponding to a risk score of 0.16, which was divided into low-risk and high-risk groups according to this cut-off value; the survival data of patients are described in Fig. 4B, with blue dots representing patient survival, red dots representing death, and ordinate as survival time; Z-score standardization was performed for each variable in Fig. 4C, the lower the BMI and ALB levels, the higher the RDW-CV, LDH and CRP levels, and the presence of liver metastasis, and the higher the score, the higher the risk of death. In the training cohort, median OS was 9.1 months (95% CI, 8.3–18.1 months) and median PFS was 4.8 months (95% CI, 3.9–6.8 months) in the high-risk group, 23.7 months (95% CI, 20.6-not reached (NR) months) and 8.2 months (95% CI, 7.7–12.5 months) in the low-risk group. When the high-risk group was compared to the low-risk group, their KM curves separated significantly (p < 0.0001) (Fig. 4D,E). Again, this statistical difference was observed in both OS (7.9 months vs 16.2 months, p = 0.008) and PFS (4.0 months vs 6.2 months, p = 0.037) in the validation cohort (Fig. 4F,G). In addition, box plots showed that significant differences in distribution were observed between the two groups for all variables (Additional file 1: Fig. S5).
A Risk score scatter diagram, risk scores are arranged from small to large, and the low-risk and high-risk groups are represented by blue and red dots, respectively. B Patient survival scatter diagram, blue dots represent patient survival, red dots represent death, and ordinate is survival time. C Variable level heat map, and the included variables are the six indicators of the final model. D,E KM curves of high and low risk groups and overall survival and progression-free survival in the training set. F,G KM curves of high and low risk groups and overall survival and progression-free survival in the validation set. BMI, body mass index; Liver-M, liver metastases; RDW-CV, red cell distribution width—coefficient of variation; LDH, lactate dehydrogenase; ALB, albumin; CRP, C-reactive protein; OS, overall survival; PFS, progression-free survival
In addition, we assessed the predictive ability of blood markers combined with some indicators for 1-year survival of patients (Fig. 5), and the AUCs of neutrophil–lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), and platelet-lymphocyte ratio (PLR) were 0.531, 0.492, and 0.505, respectively; the AUCs of systemic immune-inflammatory index (SII), prognostic nutritional index (PNI), and derived NLR (dNLR) were 0.549, 0.554, and 0.519, respectively; and the AUCs of lung immune prognostic index (LIPI), advanced lung inflammatory index (ALI), and modified Glasgow prognostic score (mGPS) were 0.612, 0.509, and 0.596, respectively, and the AUC of our model was as high as 0.760, and the discriminatory ability was much higher than the above markers.
A–C ROC curves for the prediction of 12-month survival of patients by multiple blood combined indicators and the model, and compared according to AUC values. AUC, area under the curve; NLR, neutrophil–lymphocyte ratio; LMR, lymphocyte-monocyte ratio; PLR, platelet-lymphocyte ratio; SII, systemic immune-inflammatory index; PNI, prognostic nutritional index; dNLR, derived neutrophil–lymphocyte ratio; LIPI, lung immune prognostic index; ALI, advanced lung inflammatory index; mGPS, modified Glasgow prognostic score
In addition, we screened 38 ES-SCLC patients who received chemotherapy alone in our center, whose baseline characteristics and univariate Cox regression results are shown in Supplementary Table 6, and the results showed that liver metastasis and serum LDH levels were associated with patient survival and progression. Additional file 1: Fig. S6A-B shows the OS and PFS differences in patients with or without liver metastases, respectively. Meanwhile, the performance of this model was assessed with an AUC value of 0.661 and poor discrimination, calibration, and clinical applicability (Additional file 1: Fig. S6C-E). According to the model defined high-risk and low-risk populations, there was a trend towards differences in OS (10.2 months vs 19.2 months, P = 0.067) and PFS (5.5 months vs 8.6 months, P = 0.24), but statistical differences were not reached (Additional file 1: Fig. S6F-G).
Integrating the training and validation cohorts, we divided the patient population into high-risk and low-risk groups and compared survival with patients receiving chemotherapy alone. Median OS was 10.2, 19.2, 9.1, and 20.6 months, and median PFS was 5.5, 8.6, 4.6, and 7.9 months in high-risk (Che/high risk), low-risk (Che/low risk), high-risk (IO + Che/high risk), and low-risk (IO + Che/low risk) populations, respectively. Compared with chemotherapy alone in the low-risk population, the hazard ratios for survival and progression of chemotherapy alone in the high-risk population were 0.37 (95% CI 0.13–1.08, P = 0.069) and 0.58 (95% CI 0.23–1.47, P = 0.254), respectively; the hazard ratios for survival and progression of chemotherapy alone in the low-risk population were 1.13 (95% CI 0.45–2.86, P = 0.791) and 1.24 (95% CI 0.53–2.86, P = 0.619), respectively; and the hazard ratios for survival and progression of chemotherapy alone in the high-risk population were 0.34 (95% CI 0.14–0.86, P = 0.022) and 0.55 (95% CI 0.24–1.26, P = 0.158), respectively (Additional file 1: Fig. S7A-B). When treatment and risk groups were tested for interaction effects, the P values for OS and PFS were 0.736 and 0.590, respectively (Additional file 1: Fig. S7C-D). Regardless of the regimen, the low-risk group had a longer survival time compared with the high-risk group, and the interaction effect did not support the predictive value of this model, so our model could identify the low-risk population and thus identify subgroups of patients with a good prognosis.
Discussion
Chemotherapy combined with PD-1 or PD-L1 inhibitors has immune-activating effects on ES-SCLC, and improved patient survival has been demonstrated in multiple studies [8, 12,13,14], but not all patients could achieve sustained therapeutic effects. This study focuses on immunochemotherapy in clinical practice to evaluate the efficacy and safety of immunotherapy in ES-SCLC patients, and aims at screening variables with prognostic value and construct models to distinguish patient populations. This model, which exploited readily available clinical factors, could effectively distinguish between high- and low-risk populations and is validated in external cohorts and chemotherapy alone cohorts. The training cohort in this study had a higher median OS and PFS than the validation cohort, which may be associated with more intrathoracic metastases and more patients in the second line and above in the validation cohort. Our results reveal the reproducibility of ES-SCLC ICIs combined with chemotherapy in the real world, and this encouraging result provides confidence for the development of biomarkers.
SCLC is highly associated with smoking, and previous studies have suggested that 95–98% of SCLC have a history of smoking. However, due to the retrospective study design, there were patients with an unknown smoking history in this study, and never or unknown smoking history occurred mostly in women, which may be associated with Asian regions as well as secondhand smoke exposure. Given the large difference in sample size between the two groups with or without smoking history in practice, we did not impute this data and removed this variable from the data analysis.
Survival differences in ES-SCLC patients remained significant even with the introduction of immunotherapy, highlighting the importance of prognostic marker identification. However, there are currently insufficient studies based on blood parameters in baseline, and our study found that BMI, liver metastasis, RDW-CV, LDH, ALB, and CRP were associated with patient survival. Liver metastasis is a risk factor for lung cancer and acts as an independent prognostic factor in NSCLC treated with nivolumab or pembrolizumab [21,22,23], which may be associated with mechanisms of hepatic immune tolerance, with decreased CD8 + T-cell infiltration at the invasive tumor margin [24]. Similarly, the presence of liver metastases makes ES-SCLC immunochemotherapy lack a synergistic effect, which was confirmed in our study [25, 26].
Chronic inflammation in the host is a characteristic of tumor cells, closely associated with patient survival, and may influence tumor immune responses [27, 28]. Elevated serum CRP levels are associated with IL-6-driven inflammation and may lead to cachexia and sarcopenia [23, 29]. CRP was found to be an independent factor of OS in platinum-etoposide-atezolizumab-treated ES-SCLC [30]. In addition, ALB, as a nutrition-related indicator, assesses the prognosis of cancer patients [29, 31]. The mGPS combined with inflammation and nutritional status can predict NSCLC treatment outcomes and can be used as an inflammatory marker for neoadjuvant immunotherapy for resettable NSCLC, immune consolidation after chemoradiotherapy for locally advanced NSCLC, and immunochemotherapy for advanced NSCLC [23, 27, 29]. The team from Japan validated the C-PLAN index as a useful biomarker for first-line combined immunotherapy in NSCLC, which also included CRP and ALB [32]. Therefore, cancer treatment needs to consider host systemic inflammation and nutritional status at the same time [31]. In addition to ALB, BMI partly reflects the metabolic nutritional status of the patient. In preclinical studies, obesity may lead to PD-1-mediated T-cell dysfunction and upregulation of PD-1 and PD-Ll expression [33]. High BMI is independently associated with improved survival in multiple clinical cohorts receiving immunotherapy for NSCLC [34,35,36]. Our findings advance the understanding of obesity-induced immune dysfunction in SCLC, highlighting low BMI as a clinical factor to consider before immunotherapy.
Meta-analysis of NSCLC treated with ICIs demonstrated a significant reduction in PFS and OS in patients with high LDH or LIPI scores, and the association of elevated baseline LDH levels with survival remains significant in SCLC [36,37,38,39,40]. Elevated LDH has been observed to be associated with poorer survival in both extensive stage, limited stage, and recurrent SCLC immunotherapy cohorts, which may be associated with higher tumor burden or a greater likelihood of advanced disease in patients with high LDH [41,42,43,44]. Value note that our model incorporates a poorly understood factor RDW-CV. RDW is associated with erythropoiesis and metabolism, and inflammation and poor nutritional status may contribute to its increase and are also associated with poor prognosis in lung cancer [45, 46]. Similarly, this phenomenon has been observed in patients treated with immunotherapy for large B-cell lymphoma and NSCLC [47, 48], but the mechanisms need to be further explored.
The SCLC immunosuppressive phenotype may explain the prognostic differences between immunochemotherapy strategies in SCLC versus NSCLC, with SCLC having a greater tumor diversity, a greater number of immune cells, and more aberrant function [49, 50]. Current research on markers has mostly focused on NSCLC, and biomarkers predictive of clinical benefit in SCLC are limited. In previous studies, some blood indices, such as NLR, SII, and LIPI, are markers of poor patient prognosis [19, 20]. It is important to note that our model had better discriminatory power than the derived indices mentioned above in this cohort, which may be associated with factors such as the model merging more variables, volatility of peripheral blood cell counts, and the combination of clinical measures. Although the mechanism by which peripheral blood parameters predict the efficacy is not clear, the combination of multiple indices helps to overcome the problem of patient heterogeneity, thereby selecting appropriate patients to improve treatment compliance. Moreover, we observed significant differences in survival and immunotherapy response between different risk groups and patients in both ES-SCLC cohorts, and further studies are needed to investigate markers of SCLC immunotherapy response.
Although there is a difference in the baseline between the validation set and the training set, this is because external validation is performed on unknown data to assess the generalization ability of the model. The above six indicators were balanced between the two groups, and the model showed good discrimination ability in the independent dataset, indicating that its performance was good. In addition, developing predictive models requires understanding prognosis and predictors, and to distinguish between the two, we selected ES-SCLC patients receiving chemotherapy alone for comparison. Compared to chemotherapy alone, the model did not predict the benefit of immunochemotherapy in patients, the survival distributions of the two regimens were similar within the high-risk and low-risk groups. And the treatment-by-group interaction test confirmed the prognostic value of this model, rather than predictive value, so this model can predict the risk of patient survival and select appropriate patients for treatment.
In this study, the model combined blood parameters and clinical data to stratify patients receiving immunochemotherapy for ES-SCLC, which performed well in the training set, validation set, and chemotherapy alone set. At the same time, there are some limitations, first of all, as a retrospective study, selection deviation cannot be completely avoided, for example, the time of patient progression is related to the follow-up period, the recording of smoking history relies on electronic medical records, and the existence of survival censored data. Second, the study lacked information on PD-L1 expression, TMB, and microsatellite instability assays, although these markers were poorly predictive of SCLC immunotherapy [10, 18]. In addition, immunologic agents with different targets of PD-1 and PD-L1 inhibitors are heterogeneous [51, 52], although we as well as other studies did not find prognostic differences between the two. Finally, the size of the study was limited, particularly because the validation cohort included only 43 patients, and more centers were required to participate or conduct prospective studies to validate our results.
Conclusions
Based on promising results of immunochemotherapy strategies in ES-SCLC, our developed a nomogram to stratify patients and thus prompt patient prognosis. These clinical and blood markers are easy to measure, but need to be validated in more cohorts.
Data availability
No datasets were generated or analysed during the current study.
Availability of data and materials
The corresponding author can provide the datasets used and/or analyzed during the current study upon reasonable request.
No datasets were generated or analysed during the current study.
Abbreviations
- ALB:
-
Albumin
- ALI:
-
Advanced lung inflammatory index
- ALT:
-
Alanine aminotransferase
- BMI:
-
Body mass index
- CR:
-
Complete response
- CRP:
-
C-reactive protein
- DCA:
-
Decision curve analysis
- DCR:
-
Disease control rate
- ECOG:
-
Eastern Cooperative Oncology Group
- EOS:
-
Eosinophils
- ICI:
-
Immune checkpoint inhibitor
- Lasso:
-
Least absolute shrinkage and selection algorithm
- LDH:
-
Lactate dehydrogenase
- LIPI:
-
Lung immune prognostic index
- LMR:
-
Lymphocyte-monocyte ratio
- mGPS:
-
Modified Glasgow prognostic score
- NLR:
-
Neutrophil-lymphocyte ratio
- NSE:
-
Neuron-specific enolase
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PCT:
-
Platelet volume
- PD-L1:
-
Programmed death ligand 1
- PFS:
-
Progression-free survival
- PLR:
-
Platelet-lymphocyte ratio
- PNI:
-
Prognostic nutritional index
- PR:
-
Partial response
- RDW-CV:
-
Coefficient variation of red blood cell distribution width
- RECIST:
-
Response Evaluation Criteria in Solid Tumor
- ROC:
-
Receiver operating curve
- SCLC:
-
Small cell lung cancer
- SD:
-
Stable disease
- SII:
-
Systemic immune-inflammatory index
- TMB:
-
Tumor mutation burden
- VIF:
-
Variance inflation factor
References
Zou K, Sun P, Huang H, Zhuo H, Qie R, Xie Y, et al. Etiology of lung cancer: Evidence from epidemiologic studies. Journal of the National Cancer Center. 2022;2(4):216–25. https://doi.org/10.1016/j.jncc.2022.09.004.
Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer–what limits limited disease? Lung Cancer. 2002;37(3):271–6. https://doi.org/10.1016/s0169-5002(02)00072-7.
Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664–72. https://doi.org/10.1002/cncr.29098.
Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. https://doi.org/10.1038/s41572-020-00235-0.
Wang Q, Gümüş ZH, Colarossi C, Memeo L, Wang X, Kong CY, et al. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J Thorac Oncol. 2023;18(1):31–46. https://doi.org/10.1016/j.jtho.2022.10.002.
Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346-60.e7. https://doi.org/10.1016/j.ccell.2020.12.014.
Amarasena IU, Chatterjee S, Walters JA, Wood-Baker R, Fong KM. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev. 2015;2015(8):Cd006849. https://doi.org/10.1002/14651858.CD006849.pub3.
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA. 2022;328(12):1223–32. https://doi.org/10.1001/jama.2022.16464.
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol. 2020;15(4):618–27. https://doi.org/10.1016/j.jtho.2019.12.109.
Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(☆). Ann Oncol. 2021;32(5):631–41. https://doi.org/10.1016/j.annonc.2021.01.071.
Mathieu L, Shah S, Pai-Scherf L, Larkins E, Vallejo J, Li X, et al. FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer. Oncologist. 2021;26(5):433–8. https://doi.org/10.1002/onco.13752.
Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. https://doi.org/10.1016/s1470-2045(20)30539-8.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9. https://doi.org/10.1056/NEJMoa1809064.
Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47. https://doi.org/10.1016/s1470-2045(22)00224-8.
Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–44. https://doi.org/10.1016/j.jtho.2018.10.003.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. https://doi.org/10.1016/s1470-2045(20)30445-9.
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol. 2017;35(34):3823–9. https://doi.org/10.1200/jco.2017.72.5069.
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol. 2020;38(21):2369–79. https://doi.org/10.1200/jco.20.00793.
Xiong Q, Huang Z, Xin L, Qin B, Zhao X, Zhang J, et al. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother. 2021;70(3):713–20. https://doi.org/10.1007/s00262-020-02706-5.
Qi WX, Xiang Y, Zhao S, Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol Immunother. 2021;70(11):3199–206. https://doi.org/10.1007/s00262-021-02926-3.
Botticelli A, Salati M, Di Pietro FR, Strigari L, Cerbelli B, Zizzari IG, et al. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J Transl Med. 2019;17(1):99. https://doi.org/10.1186/s12967-019-1847-x.
Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–24. https://doi.org/10.1158/2326-6066.Cir-16-0325.
Olgun P, Diker O. Sixth-Week Immune-Nutritional-Inflammatory Biomarkers: Can They Predict Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors? Curr Oncol. 2023;30(12):10539–49. https://doi.org/10.3390/curroncol30120769.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–64. https://doi.org/10.1038/s41591-020-1131-x.
Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4(4):617–20. https://doi.org/10.3892/ol.2012.792.
Zhao J, He Y, Yang X, Tian P, Zeng L, Huang K et al. Assessing treatment outcomes of chemoimmunotherapy in extensive-stage small cell lung cancer: an integrated clinical and radiomics approach. J Immunother Cancer. 2023;11(9). https://doi.org/10.1136/jitc-2023-007492.
Tanimura K, Takeda T, Yoshimura A, Honda R, Goda S, Shiotsu S et al. Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study. Cancers (Basel). 2023;15(17). https://doi.org/10.3390/cancers15174358.
Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42(6):760–7. https://doi.org/10.1016/j.ejca.2006.01.002.
Madeddu C, Busquets S, Donisi C, Lai E, Pretta A, López-Soriano FJ et al. Effect of Cancer-Related Cachexia and Associated Changes in Nutritional Status, Inflammatory Status, and Muscle Mass on Immunotherapy Efficacy and Survival in Patients with Advanced Non-Small Cell Lung Cancer. Cancers (Basel). 2023;15(4). https://doi.org/10.3390/cancers15041076.
Lim JU, Kang HS, Shin AY, Yeo CD, Kim SK, Kim JW, et al. Investigation of poor predictive factors in extensive stage small cell lung cancer under etoposide-platinum-atezolizumab treatment. Thorac Cancer. 2022;13(23):3384–92. https://doi.org/10.1111/1759-7714.14697.
McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–40. https://doi.org/10.1016/j.ctrv.2012.08.003.
Sonehara K, Ozawa R, Hama M, Nozawa S, Agatsuma T, Nishie K, et al. C-PLAN index as a prognostic factor for patients with previously untreated advanced non-small cell lung cancer who received combination immunotherapy: A multicenter retrospective study. Thorac Cancer. 2023;14(6):636–42. https://doi.org/10.1111/1759-7714.14798.
Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–51. https://doi.org/10.1038/s41591-018-0221-5.
Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83(6):1345–50. https://doi.org/10.1093/ajcn/83.6.1345.
Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020;6(4):512–8. https://doi.org/10.1001/jamaoncol.2019.5241.
Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. https://doi.org/10.1186/s40425-019-0527-y.
Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. 2019;8(4):1467–73. https://doi.org/10.1002/cam4.2024.
Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5(10):1481–5. https://doi.org/10.1001/jamaoncol.2019.1747.
Zhang X, Guo M, Fan J, Lv Z, Huang Q, Han J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark. 2016;16(3):415–23. https://doi.org/10.3233/cbm-160580.
Li LL, Yu CF, Xie HT, Chen Z, Jia BH, Xie FY, et al. Biomarkers and factors in small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. 2023;12(10):11211–33. https://doi.org/10.1002/cam4.5800.
Lee S, Shim HS, Ahn BC, Lim SM, Kim HR, Cho BC, et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: a single-center experience. Cancer Immunol Immunother. 2022;71(5):1093–101. https://doi.org/10.1007/s00262-021-03052-w.
Sun B, Hou Q, Liang Y, Xue S, Yao N, Wei L, et al. Prognostic ability of lung immune prognostic index in limited-stage small cell lung cancer. BMC Cancer. 2022;22(1):1233. https://doi.org/10.1186/s12885-022-10351-7.
Stratmann JA, Timalsina R, Atmaca A, Rosery V, Frost N, Alt J, et al. Clinical predictors of survival in patients with relapsed/refractory small-cell lung cancer treated with checkpoint inhibitors: a German multicentric real-world analysis. Ther Adv Med Oncol. 2022;14:17588359221097192. https://doi.org/10.1177/17588359221097191.
Hurkmans DP, Sassen SDT, de Joode K, Putter L, Basak EA, Wijkhuijs AJM et al. Prospective real-world study on the pharmacokinetics of pembrolizumab in patients with solid tumors. J Immunother Cancer. 2021;9(6). https://doi.org/10.1136/jitc-2021-002344.
Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. https://doi.org/10.3109/10408363.2014.992064.
Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS ONE. 2013;8(11):e80240. https://doi.org/10.1371/journal.pone.0080240.
Beltran BE, Paredes S, Castro D, Cotrina E, Sotomayor EM, Castillo JJ. High Red Cell Distribution Width is an Adverse Predictive and Prognostic Factor in Patients With Diffuse Large B-Cell Lymphoma Treated With Chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2019;19(9):e551–7. https://doi.org/10.1016/j.clml.2019.06.005.
Liu S, Zhao L, Zhou G. Peripheral blood markers predict immunotherapeutic efficacy in patients with advanced non-small cell lung cancer: A multicenter study. Front Genet. 2022;13:1016085. https://doi.org/10.3389/fgene.2022.1016085.
Remon J, Aldea M, Besse B, Planchard D, Reck M, Giaccone G, et al. Small cell lung cancer: a slightly less orphan disease after immunotherapy. Ann Oncol. 2021;32(6):698–709. https://doi.org/10.1016/j.annonc.2021.02.025.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289–97. https://doi.org/10.1038/s41568-019-0133-9.
Qiu G, Wang F, Xie X, Liu T, Zeng C, Chen Z, et al. A retrospective real-world experience of immunotherapy in patients with extensive stage small-cell lung cancer. Cancer Med. 2023;12(14):14881–91. https://doi.org/10.1002/cam4.5843.
Zhou F, Zhao W, Gong X, Ren S, Su C, Jiang T, et al. Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer. J Immunother Cancer. 2020;8(2).https://doi.org/10.1136/jitc-2020-001300
Acknowledgements
We thank all participants for their efforts and contributions to this study, as well as Professor Junqi He and his team at Capital Medical University for their support.
Funding
This work was supported by Beijing Municipal Science and Technology Commission [grant number Z211100002921013], Tongzhou Lianggao Talents Project [grant number YH201920] and Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes [grant number JYY2023-14 and JYY2023-15] to TM Zhang.
Beijing Municipal Science and Technology Commission,Z211100002921013,Tongzhou Lianggao Talents Project,YH201920,Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes,JYY2023-15
Author information
Authors and Affiliations
Contributions
XL: Conceptualization, Data curation, Formal analysis, Writing-original draft; LT: Data curation, Writing-original draft; SW: Data curation, Formal analysis; JY: Data curation; BL2: Software, Supervision; QW: Methodology, Resources; MH: Supervision, Validation; JW: Data curation; JY: Project administration, Writing-review & editing; BL1: Conceptualization, Writing-review and editing; TZ: Conceptualization, Writing-review and editing. All authors read and approved the final manuscript.
XL: Conceptualization, Data curation, Formal analysis, Writing-original draft; LT: Data curation, Writing-original draft; SW: Data curation, Formal analysis; JY: Data curation; BL2: Software, Supervision; QW: Methodology, Resources; MH: Supervision, Validation; JW: Data curation; JY: Project administration, Writing-review & editing; BL1: Conceptualization, Writing-review & editing; TZ: Conceptualization, Writing-review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committees of Beijing Chest Hospital, Capital Medical University and Beijing Friendship Hospital, Capital Medical University before data collection began (YJS-2022–19). Given its retrospective nature, two institutional committees waived informed consent for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Tong, L., Wang, S. et al. Integration of clinical and blood parameters in risk prognostication for patients receiving immunochemotherapy for extensive stage small cell lung cancer: real-world data from two centers. BMC Med 22, 381 (2024). https://doi.org/10.1186/s12916-024-03612-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03612-8