Abstract
Background
Listeriosis is a global health threat to both animals and humans, especially in developing countries. This study was designed to isolate Listeria monocytogenes from faeces; environmental samples; and cow, sheep and goat milk, as well as human stool, to study its molecular characteristics and antibiotic sensitivity in the New Valley and Beheira Governorates, Egypt. The isolation and identification of L. monocytogenes were carried out using traditional culture and biochemical methods, followed by antibiography, genus confirmation of some isolates and detection and sequencing of InlB genes via PCR.
Results
Out of 2097 examined samples, the prevalence of L. monocytogenes was 13.4% in animals; the prevalence was 9.2%, 2.4%, 25.4%, 4%, 42.4%, and 6.4% in cattle faeces, cattle milk, sheep faeces, sheep milk, goat faeces, and goat milk, respectively. However, the prevalence of L. monocytogenes was 8.3% in human samples. Both animal and human isolates showed 100% resistance to trimethoprim-sulfamethoxazole, and the isolates showed the highest sensitivity to flumequine (100%), amikacin (99.2%), gentamicin (97.6%), and levofloxacin (94.6%). Multidrug resistance (MDR) was detected in 86.9% of the tested isolates. The 16 S rRNA and inlB genes were detected in 100% of the randomly selected L. monocytogenes isolates. Phylogenetic analysis of three isolates based on the inlB gene showed 100% identity between faecal, milk and human stool isolates.
Conclusions
Faeces and milk are major sources of listeriosis, and the high degree of genetic similarity between animal and human isolates suggests the possibility of zoonotic circulation. The high prevalence of MDR L. monocytogenes in both animal and human samples could negatively impact the success of prevention and treatments for animal and human diseases, thereby imposing serious risks to public health.
Similar content being viewed by others
Background
Listeriosis is a significant food-borne zoonosis with severe clinical consequences. L monocytogenes, which belongs to the genus Listeria, is a rod-shaped, gram-positive bacterium that is motile at 10 °C to 25 °C, non-spore-forming and facultatively anaerobic; it is extensively distributed in many natural environments, such as the water, debris, and soil. This disease can affect a wide range of domestic animals, birds, wild animals, and fish in sporadic cases or outbreaks. Domestic animals are infected mainly by the ingestion of contaminated water and feed. Moreover, animals can be infected by inhalation or venereal transmission, and infected animals shed these bacteria in faeces, milk, urine, uterine discharge, or nasal discharge [1,2,3].
Listeriosis in farm animals is a major problem that increases the risk of transmission to humans. The major sources of human listeriosis are animal products, which are contaminated with animal faeces; L. monocytogenes can be transmitted by direct or indirect contact with infected animals [4, 5]. L. monocytogenes can be shed in the faeces of diseased, recovered, or asymptomatic animals, causing contamination of the environment, thereby increasing intraherd transmission, accidental spread to other herds, and increasing the risk of human infection [6, 7].
In humans, Listeriosis is an international public health concern because it has the greatest fatality rate among food-borne pathogens, with fatality rates of up to 20–30% [8]. The most important sources of human listeriosis are the consumption of contaminated raw or undercooked food such as milk, soft cheese, unpasteurized dairy products, raw vegetables, meat products, and poultry [9]. Moreover, listeriosis causes watery diarrhoea, fever, abdominal pain, and vomiting in noninvasive patients; usually persists for approximately one to three days; and is self-limiting [10]. However, listeriosis causes severe invasive disease and serious clinical signs in children, immunocompromised patients and elderly individuals, including meningitis, endocardia, septicemia, conjunctivitis, and flu-like symptoms [11, 12].
Polymerase chain reaction (PCR) is an effective procedure for the bacteriological examination of Listeria species and for evaluating their pathogenicity. The 16 S rRNA gene is used for the molecular identification and differentiation of L. monocytogenes. The pathogenicity and severity of L. monocytogenes depend mainly on the presence or absence of virulence genes. The internalin B (inlB) gene is a common genetic marker for identifying the pathogenicity of L. monocytogenes, as it plays the most important role in the adhesion, invasion and production of a biofilm that allows for bacterial penetration of host cells [13, 14].
Antibiotics are very important for the treatment of listeriosis, but unfortunately, the effectiveness of antibiotics decreases as the antimicrobial resistance of Listeria increases. The random use and misuse of antibiotics in communities and animal farms results in increased L. monocytogenes resistance to antibiotics and the development of a critical level of MDR. This resistance to antibiotics poses a real threat to human and animal health, as the number of deaths related to this problem is estimated to be more than 700 thousand annually [15, 16].
The aim of the present study was to identify and confirm L. monocytogenes strains recovered from the milk and faeces of cows, sheep and goats, as well as human stool. Moreover, the prevalence and antibiotic resistance and genetic similarity of the examined isolates were determined.
Results
A total of 262 L. monocytogenes isolates were recovered from 2097 examined samples from different sources, including fresh milk, animal faeces, and human stool. The results in Table (1) show that the overall prevalence of L. monocytogenes was 13.4% and 8.3% in animals and humans, respectively. The prevalence of L. monocytogenes was 31%, 2.4%, 47.1%, 4.04%, 55.8%, and 6.4% in cattle faeces, cattle milk, sheep faeces, sheep milk, goat faeces, and goat milk, respectively, as shown in Table (2).
Seven randomly selected isolates were subjected to PCR to evaluate the presence of the 16 S rRNA and inlB virulence genes. The prevalence of the 16 S rRNA and inlB genes was 100%, as shown in Table (3) and Figs. 1 and 2.
Overall, 130 L. monocytogenes isolates were tested for antibiotic susceptibility to 12 of the most commonly used antibiotics in the study area. Both animal and human isolates showed 100% resistance to trimethoprim-sulfamethoxazole. In contrast, the isolates showed the highest sensitivity to the following antibiotics: 100% sensitivity to flumequine, 99.2% sensitivity to amikacin, 97.6% sensitivity to gentamicin, and 94.6% sensitivity to levofloxacin, as shown in Table 4. The data in Table (5) show that the mean MAR indices of L. monocytogenes isolated from animal and human samples were 0.435 and 0.375, respectively.
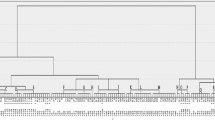
As shown in Figs. 3 and 4, the DNA sequences of the inlB genes, which represent faecal, milk and stool isolates, exhibited 100% identity with many other isolates in GenBank. The accession numbers of the analysed isolates have been deposited in GenBank as follows: faeces samples (accession number OQ190470), milk samples (accession number OQ190469) and human stool samples (accession number OQ190471). The phylogenetic tree revealed two main clusters based on inlB gene sequencing. The first cluster, which includes the isolates of this study, has high genetic similarity between the isolates. The second cluster contains many subclusters.
Discussion
Listeriosis is one of the most severe food-borne diseases and poses a great risk to public health because it is the most fatal food-borne disease worldwide [17].
Our results showed that the overall prevalence of L. monocytogenes was 13.4% in animals, and this result was confirmed by another Egyptian study [18]. In contrast, other studies reported different prevalences (35.5% and 10.4%) of L. monocytogenes in animals [19, 20]. However, the prevalence of L. monocytogenes in human samples was 8.3%, which is lower than that reported in some previous studies (12.5% and 10%) [21, 22]. Other authors were unable to isolate L. monocytogenes from human samples [23, 24]. Our results revealed that the occurrence of L. monocytogenes was greater in animals than in humans, but there was no significant difference between the two groups (P value = 0.275).
The prevalence of L. monocytogenes was 9.2%, 2.4%, 25.4%, 4.04%, 42.4%, and 6.4% in cattle faeces, cattle milk, sheep faeces, sheep milk, goat faeces, and goat milk, respectively. A nearly similar prevalence in cattle faeces, cattle milk, sheep milk, and goat milk was reported in previous studies [25,26,27]. However, lower prevalence rates in goat faeces (23.3%) and sheep faeces (8%) were reported in other studies [28, 29]. These results indicated that L. monocytogenes was more abundant in faeces than in milk samples. The greater prevalence of L. monocytogenes in faeces than in milk can be attributed to a combination of seasonal effects, farm management practices, animal health and environmental conditions that favour the survival and proliferation of this pathogen in faecal material more than in the environment where milk is produced [20, 30, 31]. Therefore, animal faeces are considered the most potent source of transmission of L. monocytogenes to humans, other animals and milk [32]; thus, faeces from infected animals serve as the main source of L. monocytogenes environmental contamination [33].
This variation in the prevalence rates of L. monocytogenes in other studies might be due to variations in season, geographical location, animal farming practice, sample type, and isolation technique [34].
PCR is an effective method for the molecular confirmation of isolated L. monocytogenes via detection of the 16 S rRNA gene and for studying its pathogenicity via detection of the inlB gene [15]. Our results confirmed that the 16 S rRNA and inlB genes were detected in 100% of the seven randomly selected L. monocytogenes isolates, and these results were previously reported in other studies [35, 36]; however, in another study, inlB genes were detected in only 40% of the examined samples [25]. The differences in the distribution of these genes may be due to the different sample sources, L. monocytogenes serotypes, or mutations in these genes [2, 3].
Listeriosis is one of the most common causes of acute gastroenteritis in humans, and humans are usually treated with antibiotics to overcome infection [10]. Unfortunately, the overuse of antibiotics in animal and human medicine has resulted in the development of antimicrobial-resistant bacteria, which have become a serious problem worldwide.
Antimicrobial resistance refers to the ability of a microorganism to survive and reproduce in the presence of previously effective antibiotic doses [35]. The isolated L. monocytogenes strains showed the highest resistance to trimethoprim-sulfamethoxazole (100%), followed by lincomycin (79.2%) and rifampicin (70%). These results are similar to those reported in other studies [18, 37, 38]. Our results disagree with the results of other studies that reported that trimethoprim-sulfamethoxazole, rifampicin, and lincomycin were effective against L. monocytogenes [36, 39, 40].
The highest sensitivity levels in this study were reported for flumequine (100%), followed by amikacin (99.2%), gentamicin (97.6%), levofloxacin (94.6%), norfloxacin (73.1%), ampicillin (71.5%), and amoxicillin (60%). These results are consistent with those of other authors who reported high sensitivity rates to levofloxacin, ampicillin, and norfloxacin [35, 38, 41]. In contrast, our results disagree with the results of other authors who found that L. monocytogenes isolates were resistant to gentamicin, amikacin, norfloxacin, amoxicillin, ampicillin, and levofloxacin [15, 18, 42, 43].
Our results showed that 86.9% of the L. monocytogenes isolates obtained from animals and humans were MDR, as bacterial isolates that exhibit resistance against three or more different antibiotic classes are considered MDR [44]; nearly the same percentages were reported in one other study [43], and a higher percentage (100%) was reported in another previous study [15]. This high prevalence of MDR in L. monocytogenes is alarming both in the human health and veterinary fields since it increases the difficulty of treating listeriosis [45].
Our data revealed that the mean MAR indices of L. monocytogenes isolated from animal and human samples were 0.435 and 0.375, respectively. These results are in agreement with those of a previous study in which the mean MAR index of L. monocytogenes isolated from animals was 0.47 [46] and another study in which the mean MAR index of L. monocytogenes isolated from humans was ˃ 0.2 [39]. Our results revealed that the mean MAR index of the animal isolates was greater than that of the human isolates, which confirmed the high resistance of L. monocytogenes of animal origin. All L. monocytogenes isolates in our study had an MAR of more than 0.2, which indicates that all the isolates originated from high-risk sources of contamination where antibiotics are often used [40].
Phylogenetic and sequence analyses based on the inlB gene revealed 100% identity between faecal, milk and stool L. monocytogenes isolates. Additionally, the strains shared 100% identity with many isolates from different sources, such as sheep placenta (accession no. URN79349), sheep brain (accession no. OM854788) and human origin (accession no. DQ302514). The high percentage of genetic similarity between isolates might imply that the isolates have not diverged much, as they shared a recent ancestor strain. This hypothesis would explain why the strains retain such similar inlB functionality. This result indicates that although these isolates are from different hosts, they have the ability to facilitate the invasion of host cells, which is key in the virulence of L. monocytogenes. These results suggests zoonotic transmission of L. monocytogenes, which is in agreement with the results reported by other authors and confirmed the importance of zoonotic L. monocytogenes transmission between animals and humans [40].
Conclusion
This study revealed an alarming high prevalence of MDR L. monocytogenes in animal and human samples. Phylogenetic analysis of animal and human samples revealed 100% identity between human and animal isolates, which represent a great danger to public health due to the increasing possibility of zoonotic transmission of L. monocytogenes and treatment failure; therefore, restricting the use of antibiotics as prophylaxes and growth promoters on animal farms is recommended. Additionally, this disease can be prevented by improving farm hygiene and biosafety measures.
Methods
Ethical declaration
The collection of samples and study design were performed in accordance with the “Institutional Review Board” of the Faculty of Medicine at Assiut University. The Institutional Approval Number was 04-2023-200283. All farm owners included in this study were informed of all the study procedures and aims, and permission to collect animal samples was obtained from them verbally.
Study area and design
Samples were collected from September 2022 to November 2023 in New Valley and Beheira Governorate, Egypt. The New Valley is the largest semiarid Governorate in Egypt, consisting of roughly half of Egypt’s area. This area is in the southwestern part of the country, between the Nile, southeastern Libya, and northern Sudan. This study included the main centre of the New Valley Governorate, El-Kharga. Beheira is a large coastal governorate that is located west of the Nile delta and is bounded by the Mediterranean Sea to the north and the Giza governorate to the south, while it is aligned with the Rosetta Nile branch in the east and the Alexandria and El-Alamein Governorates in the west, as shown in Fig. (5).
Sampling
A total of 2097 samples were collected from different farm animals (cows, sheep, and goats) and humans. The samples included cow faeces (n = 541), sheep faeces (n = 319), goat faeces (n = 179), cow’s milk (n = 414), sheep milk (n = 173), and goat milk (n = 77), in addition to human stool samples (n = 394 from contacts and noncontact persons). These samples were collected according to methods described by Abdeen et al. (2021) and Wu et al. (2021) [33, 36].
Bacteriological examination of L. monocytogenes
L. monocytogenes was isolated according to the instructions of the International Organization of Standards (ISO 11290-1) [47]. One gram of each sample was selectively enriched in nine ml of Listeria enrichment broth and incubated at 30 °C for 48 h. From each tube of Listeria enrichment broth culture, a loopful of sample was streaked onto Oxford agar plates and incubated at 37 °C for 48 h. The suspected colonies on Oxford agar were surrounded by a black halo. After purification of the suspected colonies, they were transferred onto tryptic soya agar with 6% yeast extract (TYASE), incubated at 37 °C for 24 h, and then maintained at 4 °C for biochemical identification.
Identification of L. monocytogenes
Biochemical identification of L. monocytogenes
Biochemical identification was achieved using haemolysis tests, motility tests, catalase tests, and sugar fermentation tests according to methods described by Aygun and Pehlivanlar (2006) [48].
Molecular identification of L. monocytogenes
PCR was performed to confirm L. monocytogenes via the detection of 16 S rRNA and to assess its pathogenicity via detection of the inlB virulence gene using the specific primers shown in Table (6) [49, 50]. Genomic DNA was extracted using a QIAamp DNA Mini Kit (catalogue no. 51,304). The Emerald Amp GT PCR master mix (Takara, Code No. RR310A kit) was used. The cycling protocol for the L. monocytogenes 16 S rRNA gene was as follows: 35 cycles of 94 °C for five min during primary denaturation, 94 °C for 30 s during secondary denaturation, 60 °C for 40 s during annealing and 72 °C for 1.2 min during extension, followed by a final extension at 72 °C for 12 min. Moreover, the PCR cycling protocol for the inlB gene was applied at 94 °C for five min during primary denaturation, 94 °C for 30 s during secondary denaturation, 55 °C for 40 s during annealing, and 72 °C for 40 s for 35 cycles during extension, followed by a final extension at 72 °C for ten min. Five microlitres of each amplicon was electrophoresed in a 1% agarose gel, stained with ethidium bromide and visualized and captured on a UV transilluminator. The marker for the PCR products was a 100 bp DNA ladder.
Phylogenetic analysis
A comparative sequencing analysis of one animal and one human L. monocytogenes isolate was performed using the inlB gene. The inlB gene sequences were analysed with the CLUSTRAL W multiple sequence alignment program, version 12.1 of the MegAlign module of Lasergene DNA Star Software Pairwise (Madison, Wisconsin, USA) [51]; phylogenetic analysis was performed using maximum parsimony in MEGA6 [52].
Antibiotic resistance profile of isolated L. monocytogenes
The antibiotic sensitivity test was performed by the disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) instructions [53]. The interpretation of inhibition zone diameters was carried out according to the clinical breakpoint value for Staphylococcus species, as no resistance standards exist in CLSI procedures for L. monocytogenes [54]. The efficacies of the antimicrobial discs used are shown in Table (7). The MAR index was calculated after dividing the number of antimicrobial agents to which the isolate was resistant by the total number of antimicrobial agents used [55, 56].
Statistical analysis
The data were statistically analysed using the chi-square test in SPSS ver. 27 (IBM Corp. Released 2013) to predict associations between variables. The data were treated as a complete randomization design according to methods described by Steel et al. (1997) [57]. P values < 0.05 were considered significant. The significance level was set at < 0.05.
Data availability
The datasets used and/or analyzed in the current study were not publicly published to preserve the privacy of the participants but are available upon reasonable request from the corresponding author.
References
Ravindhiran R, Sivarajan K, Sekar JN, Murugesan R, Dhandapani K. Listeria monocytogenes an Emerging Pathogen: a comprehensive overview on listeriosis, virulence determinants, detection, and anti-listerial interventions. Microb Ecol 2023:1–21.
Dahshan H, Merwad AMA, Mohamed TS. Listeria species in broiler poultry farms: potential public health hazards. J Microbiol Biotechnol. 2016;26(9):1551–6.
Coroneo V, Carraro V, Aissani N, Sanna A, Ruggeri A, Succa S, Meloni B, Pinna A, Sanna C. Detection of virulence genes and growth potential in Listeria monocytogenes strains isolated from ricotta salata cheese. J Food Sci. 2016;81(1):M114–20.
Lourenco A, Linke K, Wagner M, Stessl B. The saprophytic lifestyle of Listeria monocytogenes and entry into the Food-Processing Environment. Front Microbiol 2022, 13.
Boqvist S, Söderqvist K, Vågsholm I. Food safety challenges and one health within Europe. Acta Vet Scand. 2018;60(1):1.
Orsi RH, den Bakker HC, Wiedmann M. Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol. 2011;301(2):79–96.
Lekkas P. The Microbial Ecology of Listeria monocytogenes as impacted by three environments: a cheese microbial community; a farm environment; and a soil microbial community. University of Vermont; 2016.
Barton Behravesh C, Jones TF, Vugia DJ, Long C, Marcus R, Smith K, Thomas S, Zansky S, Fullerton KE, Henao OL. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. J Infect Dis. 2011;204(2):263–7.
Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana K, Prasad SP, Vijila HM. Listeria-review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40(1):4.
McNeill C, Sisson W, Jarrett A. Listeriosis: a resurfacing menace. J Nurse Practitioners. 2017;13(10):647–54.
Moti TB, Bulto AO. Review on Public Importance and Diagnostic Method of Listeria Monocytogenes, Ethiopia. Biomedical Sci. 2022;8(3):73–85.
Li C, Zeng H, Ding X, Chen Y, Liu X, Zhou L, Wang X, Cheng Y, Hu S, Cao Z, Liu R, Yin C. Perinatal listeriosis patients treated at a maternity hospital in Beijing, China, from 2013–2018. BMC Infect Dis. 2020;20(1):601.
Popowska M, Krawczyk-Balska A, Ostrowski R, Desvaux M. InlL from Listeria monocytogenes is involved in biofilm formation and adhesion to mucin. Front Microbiol. 2017;8:660.
Mpundu P, Muma JB, Mukumbuta N, Mukubesa AN, Muleya W, Kapila P, Hang’ombe BM, Munyeme M. Isolation, discrimination, and molecular detection of Listeria species from slaughtered cattle in Namwala District, Zambia. BMC Microbiol. 2022;22(1):160.
Elsayed ME, Abd El-Hamid MI, El-Gedawy A, Bendary MM, ELTarabili RM, Alhomrani M, Alamri AS, Alghamdi SA, Arnout M, Binjawhar DN. New insights into Listeria monocytogenes Antimicrobial Resistance, Virulence attributes and their prospective correlation. Antibiotics. 2022;11(10):1447.
Kayode AJ, Okoh AI. Antimicrobial-resistant Listeria monocytogenes in ready-to-eat foods: implications for food safety and risk assessment. Foods. 2023;12(6):1346.
Jordan K, McAuliffe O. Listeria monocytogenes in foods. Adv Food Nutr Res. 2018;86:181–213.
Badawy B, Gwida M, Sadat A, El-Toukhy M, Sayed-Ahmed M, Alam N, Ahmad S, Ali MS, Elafify M. Prevalence and Antimicrobial Resistance of virulent Listeria monocytogenes and Cronobacter sakazakii in dairy cattle, the Environment, and dried milk with the in vitro application of Natural Alternative Control. Antibiotics. 2022;11(8):1087.
El-Gohary AH, Mohamed AA-E, Ramadan HH, Abuhatab EA. Zoonotic and molecular aspects of listeria species isolated from some farm animals at Dakahlia province in Egypt. Alexandria J Veterinary Sci. 2018;58(1):208.
Palacios-Gorba C, Moura A, Gomis J, Leclercq A, Gómez‐Martín Á, Bracq‐Dieye H, Mocé ML, Tessaud‐Rita N, Jiménez‐Trigos E, Vales G. Ruminant‐associated Listeria monocytogenes isolates belong preferentially to dairy‐associated hypervirulent clones: a longitudinal study in 19 farms. Environ Microbiol. 2021;23(12):7617–31.
Aziz SAAA, Mohamed MBED. Prevalence, virulence genes, and antimicrobial resistance profile of Listeria monocytogenes isolated from retail poultry shops in Beni-Suef city, Egypt. J Adv Veterinary Anim Res. 2020;7(4):710.
Hafner L, Pichon M, Burucoa C, Nusser SH, Moura A, Garcia-Garcera M, Lecuit M. Listeria monocytogenes faecal carriage is common and depends on the gut microbiota. Nat Commun. 2021;12(1):6826.
El-Malek AMA, Ali SFH, Hassanein R, Mohamed MA, Elsayh KI. Occurrence of Listeria species in meat, chicken products and human stools in Assiut city, Egypt with PCR use for rapid identification of Listeria monocytogenes. Veterinary World 2010, 3(8).
Reda WW, Abdel-Moein K, Hegazi A, Mohamed Y, Abdel-Razik K. Listeria monocytogenes: an emerging food-borne pathogen and its public health implications. J Infect Developing Ctries. 2016;10(02):149–54.
EL-Naenaeey E-S, Abdelwahab A, Merwad A, Abdou H. Prevalence of Listeria species in dairy cows and pregnant women with reference to Virulotyping of Listeria monocytogenes in Egypt. Zagazig Veterinary J. 2019;47(3):248–58.
Haggag YN, Nossair MA, Shehab SA. Is raw milk still vehicle for transmitting Listeria species to pregnant women? Alexandria J Veterinary Sci 2019, 61(1).
Elbar S, Elkenany R, Elhadidy M, Younis G. Prevalence, virulence and antibiotic susceptibility of Listeria monocytogenes isolated from sheep. Mansoura Veterinary Med J. 2020;21(2):48–52.
El Sawaak AA, El Desoky I, Abd Elgwaad AM, Ahmed HA, Shalaby MI. Detection of Virulence Associated genes in Listeria Monocytogenes isolated from diseased farm animals. Kafrelsheikh Veterinary Med J. 2016;14(1):297–314.
Farag H, Abdallah M, Nossair M. Prevalence of listeriosis in some farm animals. Damanhour J Veterinary Sci. 2021;6(1):17–20.
Castro H, Jaakkonen A, Hakkinen M, Korkeala H, Lindström M. Occurrence, persistence, and contamination routes of Listeria monocytogenes genotypes on three Finnish dairy cattle farms: a longitudinal study. Appl Environ Microbiol. 2018;84(4):e02000–02017.
Obaidat MM, Stringer AP. Prevalence, molecular characterization, and antimicrobial resistance profiles of Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157: H7 on dairy cattle farms in Jordan. J Dairy Sci. 2019;102(10):8710–20.
Schoder D, Guldimann C, Märtlbauer E. Asymptomatic carriage of Listeria monocytogenes by animals and humans and its impact on the Food Chain. Foods. 2022;11(21):3472.
Bandelj P, Jamnikar-Ciglenecki U, Ocepek M, Blagus R, Vengust M. Risk factors associated with fecal shedding of L isteria monocytogenes by dairy cows and calves. J Vet Intern Med. 2018;32(5):1773–9.
Diriba K, Awulachew E, Diribsa K. The prevalence of Listeria species in different food items of animal and plant origin in Ethiopia: a systematic review and meta-analysis. Eur J Med Res. 2021;26(1):1–9.
Abdeen EE, Mousa WS, Harb OH, Fath-Elbab GA, Nooruzzaman M, Gaber A, Alsanie WF, Abdeen A. Prevalence, antibiogram and genetic characterization of Listeria monocytogenes from food products in Egypt. Foods. 2021;10(6):1381.
Owusu-Kwarteng J, Wuni A, Akabanda F, Jespersen L. Prevalence and characteristics of Listeria monocytogenes isolates in raw milk, heated milk and nunu, a spontaneously fermented milk beverage, in Ghana. Beverages. 2018;4(2):40.
Wu L, Bao H, Yang Z, He T, Tian Y, Zhou Y, Pang M, Wang R, Zhang H. Antimicrobial susceptibility, multilocus sequence typing, and virulence of listeria isolated from a slaughterhouse in Jiangsu, China. BMC Microbiol. 2021;21(1):1–13.
Shourav AH, Hasan M, Ahmed S. Antibiotic susceptibility pattern of Listeria spp. isolated from cattle farm environment in Bangladesh. J Agric Food Res. 2020;2:100082.
Abd Al-Mayahi FS, Jaber SM. Multiple drug resistance of Listeria monocytogenes isolated from aborted women by using serological and molecular techniques in Diwaniyah city/Iraq. Iran J Microbiol. 2020;12(4):305.
Nain Z, Islam MA, Karim MM. Antibiotic resistance profiling and molecular phylogeny of biofilm forming bacteria from clinical and non-clinical environment in southern part of Bangladesh. Int J Enteric Pathogens. 2019;7(2):37–43.
Manyi-Loh CE, Okoh AI, Lues R. Occurrence and Multidrug Resistance in strains of Listeria monocytogenes recovered from the anaerobic Co-digestion Sludge contained in a single Stage Steel Biodigester: implications for Antimicrobial Stewardship. Microorganisms. 2023;11(3):725.
Sale MP, Ibrahim A, Adedeji BA, Hamza FA. Prevalence and antibiogram of Listeria monocytogenes isolated from ready-to-eat vegetables and fermented milk in Yola, Nigeria. Microbes and Infectious Diseases; 2023.
Kayode AJ, Okoh AI. Assessment of multidrug-resistant Listeria monocytogenes in milk and milk product and one health perspective. PLoS ONE. 2022;17(7):e0270993.
Jubair N, Rajagopal M, Chinnappan S, Abdullah NB, Fatima A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evidence-Based Complementary and Alternative Medicine 2021, 2021.
Pagliano P, Ascione T, Boccia G, De Caro F, Esposito S. Listeria monocytogenes meningitis in the elderly: epidemiological, clinical and therapeutic findings. Infez Med. 2016;24(2):105–11.
Dunka H, Bello M, Lawan M. Prevalence and antibiogram of Listeria monocytogenes contamination of liver, spleen, ruminal content and effluent in Jos, Nigeria. J Vet Med Anim Sci. 2021;4:1072.
Barre L, Angelidis AS, Boussaid D, Brasseur ED, Manso E, Besse NG. Applicability of the EN ISO 11290-1 standard method for Listeria monocytogenes detection in presence of new Listeria species. Int J Food Microbiol. 2016;238:281–7.
Aygun O, Pehlivanlar S. Listeria spp. in the raw milk and dairy products in Antakya, Turkey. Food Control. 2006;17(8):676–9.
Kumar A, Grover S, Batish VK. Exploring specific primers targeted against different genes for a multiplex PCR for detection of Listeria monocytogenes. 3 Biotech. 2015;5:261–9.
Pangallo D, Kaclikova E, Kuchta T, Drahovska H. Detection of Listeria monocytogenes by polymerase chain reaction oriented to the gene inlB. New Microbiol. 2001;24(4):333–9.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–80.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25 Wayne, PA: Clinical and Laboratory Standards Institute 2016.
Du X-j, Zhang X, Wang X-y, Su Y-l, Li P, Wang S. Isolation and characterization of Listeria monocytogenes in Chinese food obtained from the central area of China. Food Control. 2017;74:9–16.
Singh S, Yadav AS, Singh SM, Bharti P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res Int. 2010;43(8):2027–30.
Cusack T, Ashley E, Ling C, Rattanavong S, Roberts T, Turner P, Wangrangsimakul T, Dance D. Impact of CLSI and EUCAST breakpoint discrepancies on reporting of antimicrobial susceptibility and AMR surveillance. Clin Microbiol Infect. 2019;25(7):910–1.
Steel RG, Torrie JH, Dickey DA. Principles and procedures of statistics: a biometrical approach; 1997.
Acknowledgements
The authors also thank all members of the Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine at New Valley University for their valuable assistance in this study.
Funding
No funding has been provided.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors collaborated in work planning, experimental design, measurement of parameters, and writing of the manuscript. MSD, YFE, AMO, and NKA conceived and designed the experiments. MSD, NKA, YFE measured the parameters. SAS and AMO statistically analyzed the data. SAS, NKA and MSD wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was conducted in accordance with the guidelines of “Institutional Review Board” of the Faculty of Medicine in Assiut University. The Institutional Approval Number of (04-2023-200283). All the farm owners included in this study were informed of all the study procedures and aims, and permission to collect animal samples was obtained from them verbally. All methods were performed in accordance with the ARRIVE guidelines for the reporting of animal experiments (https://arriveguidelines.org). The study was conducted in accordance with the Declaration of Helsinki for medical research involving human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sotohy, S.A., Elnaker, Y.F., Omar, A.M. et al. Prevalence, antibiogram and molecular characterization of Listeria monocytogenes from ruminants and humans in New Valley and Beheira Governorates, Egypt. BMC Vet Res 20, 297 (2024). https://doi.org/10.1186/s12917-024-04138-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04138-0