Abstract
Background
Throughout a three-year study period, 1,577 bovine clinical mastitis samples and 302 bulk tank samples were analyzed from ten Brazilian dairy herds. Enterococcus spp. was isolated and identified in 93 (5.9%) clinical mastitis samples. In addition, 258 Enterococcus spp. were isolated from the bulk tank samples of the same herds. The identification of Enterococcus spp. isolated from bulk tanks and milk samples of clinical mastitis were accomplished by phenotypic characteristics and confirmed by MALDI-TOF Mass Spectrometry (MS). Fisher test was performed to verify the difference between bulk tanks and mastitis samples.
Results
The following species were identified from clinical mastitis: E. saccharolyticus (62.4%), E. faecalis (19.4%), E. faecium (15.1%), E. hirae (1.1%), E. mundtii (1.1%), E. durans (1.1%). Furthermore, from 258 bulk tank milk samples, eight enterococci species were isolated: E. faecalis (67.8%), E. hirae (15.1%), E. faecium (4.6%), E. saccharolyticus (4.6%), E. mundtii (3.1%), E. caseliflavus ( 2.7%), E. durans (1.2%), E. galinarum (0.8%).
Conclusions
The difference in species predominance in bulk tank samples (67.8% of E. faecalis) and clinical mastitis (62.4% of E. saccharolyticus) was unexpected and caught our attention. Although Enterococcus spp. are traditionally classified as an environmental mastitis agent, in the present study, E. saccharolyticus behaved as a contagious agent of mastitis, which consequently changed the control patterns to be implemented.
Similar content being viewed by others
Background
Enterococci are commensal bacteria found in the intestines of animals and humans, some strains have become important nosocomial pathogens, frequently isolated from hospital environments. They can cause severe human infections and may present intrinsic resistance or low susceptibility to antimicrobials such as aminoglycosides, cephalosporin and some beta-lactams [1,2,3].
Enterococci are also economically important as etiological agents of animal disease, especially for bovine mastitis, alongside with Staphylococcus and Streptococcus [4]. However, mastitis cases associated with enterococci are still undervalued due to the limitations of phenotypic diagnostic methods [5, 6]. Fortin et al. [7] and Werner et al. [8] reported that between 6.2% and 20% of Enterococcus spp. were misdiagnosed as Streptococcus spp. (such as Strep. uberis, Strep. dysgalactiae, and Strep. bovis) based on phenotypic methods, and Scillieri et al. [6] corroborated the observation.
Several methods have been used to identify these pathogens, such as biochemicals tests, molecular techniques and sequencing essays [9,10,11]. Among techniques, the Matrix-Assisted Laser Desorption/Ionization time-of-flight mass spectrometry (MALDI-ToF), a fast and accurate technology that identifies bacteria by mass spectrometry, is highly recommended to improve microorganism identification. The same technology has been used successfully in research with a similar purpose in other countries [5, 6, 9].
Enterococcus was reported worldwide as a cause of clinical and subclinical mastitis. Researchers reported that enterococci were isolated from 1.2 to 7.6% of all clinical mastitis cases [13,14,15]. Kuyucuoğlu [16] registered a 10.97% prevalence of Enterococcus spp. in 392 milk samples from 274 cows with subclinical mastitis in Turkey. Song et al. [17] reported a 1.73% prevalence of Enterococcus spp. in milk samples from 1153 cows (15 herds) with signs of clinical mastitis or positive for the Lanzhou Mastitis test in China. In a recent study, Pascu et al. [18] isolated Enterococcus spp. from 1.72% to 6.9% of milk samples from 127 cows (4 herds) with clinical and subclinical mastitis in Romania, respectively. Bi et al. [19] reported that 35.7% of 894 bulk tank milk samples collected in China were positive for Enterococcus spp.
This study aimed to detect the presence of enterococci in Brazilian dairy farms throughout the use of MALDI-ToF MS and to assess their potential impact on animal health and milk quality. The findings of the study show the main enterococci species with pathogenic potential for causing mastitis that are circulating in dairy products in São Paulo and Minas Gerais States, in Brazil.
Results
Throughout a two-year study period, a total of 4275 bovine clinical mastitis samples as well 302 bulk tank samples were analyzed from ten Brazilian dairy herds, 1577 mastitis-causing pathogens were isolated and identified, showing a predominance of 1244 environmental (78.9%) toward 333 (21.1%) contagious pathogens from the clinical mastitis cases. Enterocccus spp were identified in 394 samples, among other pathogens as Actinomyces spp, Pasteurella, Escherichia coli, Staphylococcus spp and Streptococcus spp.
Of the 108 isolates from clinical mastitis samples and 286 isolates from bulk tanks that were phenotypically diagnosed as Enterococcus spp., were subjected to MALDI-TOF MS.
Of 394 isolates subjected to MALDI-TOF MS, 89.1% (N = 351) were confirmed as different species of Enterococcus, and 10.9% (N = 43) were diagnosed as other bacterial species. The species most misdiagnosed by phenotypic methods were Lactococcus garvieae and Lactococcus lactis.
Through MALDI-ToF Mass Spectrometry, Enterococcus spp. was isolated and identified in 93 (5.9%) clinical mastitis samples. Six different species of enterococci were identified in clinical mastitis samples, respectively: E. saccharolyticus (62.4%), E. faecalis (19.4%), E. faecium (15.1%), E. hirae (1.1%), E. mundtii (1.1%), E. durans (1.1%) (Table 1). In addition, 258 Enterococcus spp. were isolated from the bulk tank samples of the same herds. Eight different species were identified, as shown in Table 2: E. faecalis (67.8%), E. hirae (15.1%), E. faecium (4.6%), E. saccharolyticus (4.6%), E. mundtii (3.1%), E. caseliflavus (2.7%), E. durans (1.2%), E. gallinarum (0.8%). Among the isolated species, Enterococcus faecalis (67.8%) showed the highest frequency, followed by Enterococcus hirae (15.1%). E. saccharolyticus was the enterococci species that showed the highest occurrence (p < 0.0001) among clinical mastitis samples (Table 3).
Out of all 58 clinical mastitis cases identified as E. saccharolyticus using MALDI-TOF MS, 50 presented high-confidence identifications (scores of 2 to 3), while 8 showed low-confidence identifications (scores of 1.70 to 1.99). Regarding consistency, only two samples demonstrated a B-level, while the others exhibited an A-level consistency. In the 12 bulk tank samples where E. saccharolyticus was detected, 10 exhibited high-confidence identifications, and 2 had low-confidence. Only one of these samples showed B-level consistency. These results are detailed in Supplementary Material 1.
Discussion
The occurrence of enterococci in bovine mastitis milk samples varies greatly, with some authors reporting infrequent findings. Petersson-Wolfe et al. [20] referred to 0.3–1.3% prevalence in milk samples from cows with subclinical mastitis in Sweden. Botrel et al. [21] isolated these bacteria in 2.4% of milk samples from cows with clinical mastitis and 3.1% in subclinical cases in France. Hande et al. [22] described Enterococcus spp. in 3.3% of milk samples from subclinical mastitic cows in Turkey. Although with higher isolation frequency (5.9%), our data corroborates with these authors. In contrast, others exhibited higher enterococci levels from mastitis cases in milk samples, such as Kuyucouglu [16], Cameron et al. (2016) [5], Kateete et al. [23], and Różańska et al. [24], who have isolated, respectively, from 10.9%, 15.3%,19.5% and 21.3% of Enterococcus spp in Turkey, Canada, Uganda and Poland.
As previously noted, the accuracy of identifying Enterococcus species through phenotypic diagnostic methods is limited, as acknowledged by various authors [5, 6]. To overcome this challenge, experts recommend leveraging more precise techniques, such as MALDI-ToF, to assess the diversity of species in mastitis and bulk tank samples [6, 25]. A study conducted by Cameron et al. [5] utilized MALDI-ToF and successfully identified 296 environmental streptococci in bovine milk samples from Canada and correctly classified eight enterococci as well (e.g., E. faecalis (6.8%), E. saccharolyticus(2.7%), E. faecium (1%), E. pseudoavium (1%), E. avium (0.3%), E. hirae (0.3%), E.caseiflavus (0.3%), E. vilorum (03%).
Other studies also described frequencies of these pathogens: E. faecalis 53.4% and E. faecium 18.6% (43 isolates) by Kuyucuoğlu [16]; E. faecalis 44.76%, E. faecium 37.14% and E. hirae 4.76% (105 isolates) by Nam et al. [26]; E. faecalis 29.62%, E. casseliflavus 7.41%, and E. hirae 3.70% (27 isolates) by Ahmed et al. [27].
The data shown in Table 2 indicates that E. faecalis was the enterococci species prevalently isolated from milk samples (19.4%) and bulk tank samples (67.8%), corroborating with the abovementioned authors. However, E. saccharolyticus was the most prevalent species (62.4%) isolated from clinical mastitis samples. Therefore, the source of milk samples should be considered and indicated, i.e., were the milk samples collected from bulk tanks, clinical or subclinical mastitis cases, or milking machines milk samples.
E. faecalis is commonly identified as the primary enterococci found in mastitic milk. This microbe is typically present in organic bedding and has an opportunistic nature, allowing it to invade mammary glands and cause persistent infections. Elhadidy and Zahran [28] demonstrated a correlation between biofilm formation and adherence followed by invasion to mammary gland epithelium, and that ability contributes to recurrent infections and pathogenesis of E. faecalis mastitis.
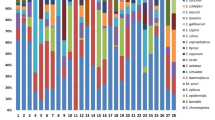
The scrutiny of Table 3 data shows that the highest occurrence of enterococci clinical mastitis cases among the ten studied dairy herds were detected in farms A (24.7%) and E (20.4%). However, considering enterococci isolation from bulk tank samples, the highest enterococci isolation occurred in the C (20.9%) and D (20.9%) dairy herds. Based on the presented data, the abundance of enterococci in the dairy herd environment may not be the sole cause of clinical mastitis cases in cows. There are other factors to consider, such as the presence and variety of virulence factors among different enterococci species and strains exhibiting increased pathogenicity. Additionally, the frequency of isolation data for E. saccharolyticus and E. faecalis, as presented in Table 3, supports these observations.
Although E. faecalis was the dominant species isolated (67.8%) in bulk tank samples, it was found to be present in only 19.4% of the clinical mastitis samples studied. On the other hand, E. saccharolyticus (62.4%) was the predominant (p < 0.0001) enterococci species isolated from clinical mastitis cases, whereas merely 4.7% of these enterococci species were isolated from bulk tank samples.
Upon reviewing the data in Table 1, it becomes evident that the prevalence of enterococci species isolated from clinical mastitis cases in ten dairy herds is noteworthy. Notably, E. saccharolyticus was identified in clinical mastitis samples from nearly all dairy herds (7/10) and emerged as the primary isolated species in six of these herds. Additionally, it is worth mentioning that E. saccharolyticus was present in 83% of clinical cases in dairy herd A, where the highest incidence of enterococci clinical mastitis was observed. Furthermore, analyzing the data of herd A, five animals had E. saccharolyticus prolonged clinical mastitis, characterized by subsequent isolation of this microorganism within intervals ranging from one to five months (five months persistence of clinical mastitis was observed in three of these five animals). It is worth noting that E. saccharolyticus was isolated from bulk tank samples twice in dairy herd A, once during the first year of the research period and again throughout the second year. As a result, the prevalence of E. saccharolyticus in clinical cases and its prolonged presence in the mammary gland leading to clinical mastitis indicate that this species of enterococci is a significant mastitis pathogen and requires further attention.
The fecal origin of enterococci is a known fact. However, in dairy farms, Enterococcus species may occupy specific ecological niches. In the study of Juliano et al. [25]. , E. saccharolyticus was the most prevalent in milk samples, and the odds ratio of isolation observed by the authors suggests that E. saccharolyticus can disseminate in the herd in a contagious fashion, with teat cups acting as fomites in the transmission process [25]. Corroborating with the observation of E. saccharolyticus as a contagious pathogen and with a specific niche in dairy farms, DIAZ-CAO et al. [13], in a survey comprising 163,741 samples from dairy cattle quarters, observed that E. saccharolyticus was consistently isolated in mastitis samples with somatic cell count > 100.000, with percentage of isolation in clinical samples higher than E. faecium. Jackson et al. [29] assessed the prevalence of enterococci species isolated from fecal samples of 122 dairy herds in the US, 88.7% of the samples were positive, and 10 species were observed. The most prevalent species was E. hirae, followed by E. faecalis and E. faecium. E. saccharolyticus was not isolated, supporting the hypothesis that enterococci species can have specific niches and unique epidemiological features.
Environmental streptococci, comprising Streptococcus uberis, S. dysgalactiae, and Enterococcus spp., are frequent subclinical and clinical mastitis agents in dairy cows [6, 8, 30, 31]. These bacteria are abundant in the dairy herd environment and are conventionally classified as environmental pathogens. However, either S. uberis or S. dysgalactiae can be sporadically agents of contagious mastitis transmitted cow-to-cow during milking [32, 33]. Based on our observations of E. saccharolyticus isolated from clinical cases, specifically in dairy herds A and F, it behaved more like a contagious pathogen than an environmental one. As such, additional research is needed to fully understand the relevant epidemiological aspects, which are especially important for implementing effective control measures.
Identifying the enterococci species causing mastitis is necessary for a proper pattern of control measures to be successfully employed. Therefore, using modern and accurate technologies such as MALDI-TOF, in parallel with the classical phenotypic identification methods, may enhance the identification of enterococci at the species level.
Comparing research results can be complex, as many factors must be considered, including differences in location and time, the sanitary conditions of the herds being studied, the sampling methodology used, and the identification techniques employed. Despite these challenges, it is clear that enterococci species play a significant role in causing mastitis, and their potential impact on public health cannot be ignored. These bacteria have been known to cause severe infections in humans, such as bacteremia, endocarditis, and urinary tract infections, and they may also possess intrinsic resistance or low susceptibility to antimicrobial treatments [1,2,3, 5] .
Conclusions
The Matrix-assisted desorption ionization-time of flight technic was able to identify the main species of enterococci present in mastitis in the studied herds. The predominance of E. saccharolyticus as an agent of clinical mastitis, as well as their long persistence in affected animals suggests a particular relevance of these enterococci species as a mastitis pathogen, reinforcing previous observation that E. saccharolyticus has specific ecological niches and acts as a contagious microbe. Additionally, the high occurrence of E. faecalis in bulk tank samples represents a potential public health risk due to their high antimicrobial resistance and highlights the need for better hygiene measures in these equipment to maintain milk quality.
Methods
This study was developed following the Ethics Committee on Animal Use (CEUA) guidelines and approved by the School of Veterinary Medicine and Animal Science (FMVZ), São Paulo State University (UNESP), Botucatu, São Paulo State, Brazil; protocol number 0136/2017.
Inclusion criteria
A convenience sample of ten dairy farms located in Sao Paulo and Minas Gerais states was used for the study. The eligibility criteria for the farms were breed (Holstein), herd size (> 200 lactating cows that produced > 20 kg of milk/d, bulk tank somatic cell count (SCC) < 400,000 cells/mL), and use of milking equipment. For all farms, cows were milked thrice daily and housed in sand-bedded freestalls.
Sampling strategy
Milk samples were collected from all dairy cows that experienced clinical mastitis from September 2017 to September 2019. These samples were aseptically collected and frozen by farm personnel. After udder hygiene procedures (examination of the first milk streams, pre-dipping, and drying of the teats), the teat end was disinfected with cotton pads soaked with 70% alcohol. Subsequently, the first streams of milk were discarded, and 15 mL of milk were collected in a sterile plastic vial.
Additionally, bulk tank samples were collected monthly during the study period.
Laboratory methods
According to the National Mastitis Council (1999) [34] guidelines, milk samples were cultured for microbiological diagnosis. Samples were initially plated on blood agar and MacConkey and subjected to biochemical reactions for phenotypic identification of mastitis-causing pathogens. The colonies that were morphologically compatible with enterococci were analyzed using Gram stain microscopy and catalase and hemolysins tests. Afterward, samples were submitted to the following biochemical assays proposed by Facklam and Collins [35] and updated by Teixeira et al. [36]: growth in halophyte broth (6.5% NaCl), esculin hydrolysis, hydrolysis of L-pyrrolidonyl-beta-naphthylamide (PYR), arginine decarboxylase test, pigment production, motility, tetrazolium reduction, mannitol, arabinose, raffinose, and sorbitol fermentation test. Bulk tank samples were plated (0.1 mL) on Enterococcosel agar (Becton, Dickinson and Company, New Jersey, USA) for selective isolation of Enterococcus spp. Four esculin-positive colonies from each plate were selected for identification; one was removed from the center and three from the periphery of the plate, at 120° angles) [34]. Plates were incubated for 24 h at 37 °C, and colonies were selected for further identification as described above.
The isolates that were presumptively diagnosed as Enterococcus spp., and for species differentiation, colonies were analyzed by Matrix-assisted desorption ionization-time of flight (MALDI-TOF). Samples were analyzed using the MALDI-TOF equipment (Bruker Daltonics, Bremen, Germany). The microorganisms were identified by their protein profile (mass spectra) by the Microflex LT MS software, flexControl (version 3.4), which is compared with a database of microbial spectra present in the equipment (MALDI Biotyper Real-Time Classification (RTC) and Offline Classification (OC; version 3.1) [37]. These analyses were performed at the Qualileite FMVZ-USP laboratory.
To perform the analysis, sterile wooden sticks were used to apply the isolated colony to the plates used for agent identification, each plate containing 96 wells (Bruker Daltonics Inc., Billerica, MA). After applying the colony, the plate was left to dry at room temperature. Subsequently, 1.0 µL of 40% formic acid solution was applied into each well for extraction and left to dry. Then, 1.0 µL of matrix solution α-cyano-4-hydroxycinnamic acid diluted in 50% acetonitrile, 47.5% water, and 2.5% trifluoroacetic acid (Sigma-Aldrich Canada Inc., Oakville, ON, Canada) was added onto each well. Once the plate dried, the analysis was performed by mass spectrometry. For interpreting the results, the manufacturer advised the following scores: 2.000 to 3.000 was considered probable species identification; 1.700 to 1.999 indicated genus identification; <1.700 was considered unreliable identification (5).
Statistical analysis and sample size calculation
In order to accurately test the hypothesis of a significant difference in Enterococcus strain distribution between mastitis and environmental samples, the estimated accuracy of pertinent statistics was taken into account when determining the necessary sample size. To achieve an 80% statistical power and an alpha level of 5%, it was determined that each group (mastitis or environmental) would require twenty-three Enterococcus samples. This sample size would allow for testing the hypothesis that there is a 40% difference in the prevalence of the predominant Enterococcus strain between the two sample types. These calculations were performed using the online tool StatsToDo (www.statstodo.com).
A Fisher’s test was conducted to compare the distribution of Enterococcus species strains in mastitis and bulk tank samples. The analysis was conducted using SAS software, version 9.4, developed by the SAS Institute in Carry, NY. A significance level of 0.05 was considered for the analysis.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MALDI-ToF:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
References
Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. AntimicrobialResistant Pathogens Associated with Healthcare-Associated infections: Annual Summary of Data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011.
Arias CA, Murray BE. The rise of the Enterococcus: beyond Vancomycin resistance. Nat Rev Microbiol. 2012;10:266–78.
Prieto AMG, van Schaik W, Rogers MRC, Coque TM, Baquero F, Corander J, et al. Global emergence and dissemination of Enterococci as nosocomial pathogens: attack of the clones? Front Microbiol. 2016;7 MAY:1–15.
Ruegg PL. A 100-Year review: Mastitis detection, management, and prevention. J Dairy Sci. 2017;100(1):10381–97.
Cameron M, Saab M, Heider L, McClure JT, Rodriguez-Lecompte JC, Sanchez J. Antimicrobial Susceptibility Patterns of Environmental Streptococci Recovered from Bovine Milk Samples in the Maritime Provinces of Canada. Front Vet Sci. 2016;3 SEP.
Smith JS, Moroni P, Nydam D. Lactococcus: an emerging mastitis pathogen. Dairy Bus Holstein World. 2016;October:36–8.
Fortin M, Messier S, Paré J, Higgins R. Identification of Catalase-Negative, non-beta-hemolytic, gram-positive Cocci isolated from milk samples. J Clin Microbiol. 2003;41:106–9.
Werner B, Moroni P, Gioia G, Lavín-Alconero L, Yousaf A, Charter ME, et al. Short communication: genotypic and phenotypic identification of environmental streptococci and association of Lactococcus lactis ssp. lactis with intramammary infections among different dairy farms. J Dairy Sci. 2014;97:6964–9.
Ke D, Picard FJ, Martineau F, Menard C, Roy PH, Ouellette M, Bergeron MG. Development of a PCR Assay for Rapid Detection of Enterococci. Journal of Clinical Microbiology. 1999; 37: 3497–3503, 1999.
Day A, Sandoe J, Cove JH, Phillips-Jones MK. Evaluation of a biochemical test scheme for identifying clinical isolates of;Enterococcus faecalis and Enterococcus faecium. Lett Appl Microbiol. 2001;33:392–6.
Kim MA, Rosa V, Min KS. Characterization of Enterococcus faecalis in different culture conditions. Sci Rep.2020;10.
El Zowalaty ME, Lamichhane B, Falgenhauer L, Mowlaboccus S, Zishiri Ot, Forsythe S, Hemly Y. Antimicrobial resistance and whole genome sequencing of novel sequence types of Enterococcus faecalis, Enterococcus faecium, and Enterococcus durans isolated from livestock. Sci Rep.2023;13.
Diaz-Cao JM, Barreal ML, Pombo B, Prieto A, Alonso JM, Iglesias A, et al. Evaluation and cluster analysis of inflammatory reactions of dairy cattle mastitis pathogens in milk samples submitted for microbiological examination. Span J Agric Res. 2020;17:e0505.
Bradley AJ, Green MJ. Mastitis control: from science to practice. The Netherlands: Wageningen Academic; 2008.
Truchetti G, Bouchard E, Descôteaux L, Scholl D, Roy J-P. Efficacy of extended intramammary ceftiofur therapy against mild to moderate clinical mastitis in Holstein dairy cows: a randomized clinical trial. Can J Vet Res. 2014;78:31–7.
Kuyucuoğlu Y. Antibiotic resistances of enterococci isolated from bovine subclinical mastitis. Eurasian J Vet Sci. 2011;27:231–4.
Song X, Huang X, Xu H, Zhang C, Chen S, Liu F, et al. The prevalence of pathogens causing bovine mastitis and their associated risk factors in 15 large dairy farms in China: an observational study. Vet Microbiol. 2020;247(June):108757.
Pascu C, Herman V, Iancu I, Costinar L. Etiology of Mastitis and Antimicrobial Resistance in dairy cattle farms in the western part of Romania. Antibiotics. 2022;11:57.
Bi Y, Wang YJ, Qin Y, Guix Vallverdú R, Maldonado García J, Sun W, et al. Prevalence of Bovine Mastitis Pathogens in Bulk Tank Milk in China. PLoS ONE. 2016;11:e0155621.
Petersson-Wolfe CS, Currin J. Environmental Streptococci and Enterococcus spp.: a practical Summary for Controlling Mastitis. Va Coop Ext. 2012;:2.
Botrel M-A, Haenni M, Morignat E, Sulpice P, Madec J-Y, Calavas D. Distribution and Antimicrobial Resistance of Clinical and subclinical mastitis pathogens in dairy cows in Rhône-Alpes, France. Foodborne Pathog Dis. 2010;7:479–87.
Hande G, Arzu F, Nilgün G, Serhat AS, Alper Ç, Ece K, et al. Investigation on the etiology of subclinical mastitis in Jersey and Hybrid Jersey dairy cows. Acta Vet Brno. 2015;65:358–70.
Kateete DP, Kabugo U, Baluku H, Nyakarahuka L, Kyobe S, Okee M, et al. Prevalence and Antimicrobial susceptibility patterns of Bacteria from Milkmen and cows with clinical mastitis in and around Kampala, Uganda. PLoS ONE. 2013;8:e63413.
Różańska H, Lewtak-Piłat A, Kubajka M, Weiner M. Occurrence of enterococci in mastitic cow’s milk and their antimicrobial resistance. J Vet Res. 2019;63:93–7.
Juliano LCB, Gouvêa FLR, Latosinski GS, Fabri FHH, Salvador TB, Guimaraes F, et al. Species diversity and antimicrobial resistance patterns of Enterococcus spp. isolated from mastitis cases, milking machine and the environment of dairy cows. Lett Appl Microbiol. 2022;75:924–32.
Nam HM, Lim SK, Moon JS, Kang HM, Kim JM, Jang KC et al. Antimicrobial Resistance of Enterococci isolated from Mastitic Bovine Milk Samples in Korea. Zoonoses Public Health. 2010;57.
Ahmed W, Neubauer H, Tomaso H, El Hofy FI, Monecke S, Abd El-Tawab AA, et al. Characterization of Enterococci- and ESBL-Producing Escherichia coli isolated from milk of Bovides with mastitis in Egypt. Pathogens. 2021;10:97.
Elhadidy M, Elsayyad A. Uncommitted role of enterococcal surface protein, Esp, and origin of isolates on biofilm production by Enterococcus faecalis isolated from bovine mastitis. J Microbiol Immunol Infect. 2013;46:80–4.
Jackson CR, Lombard JE, Dargatz DA, Fedorka-Cray PJ. Prevalence, species distribution and antimicrobial resistance of enterococci isolated from US dairy cattle. Lett Appl Microbiol. 2011;52:41–8.
Reyher KK, Dufour S, Barkema HW, Des Côteaux L, DeVries TJ, Dohoo IR, et al. The National Cohort of dairy Farms—A data collection platform for mastitis research in Canada. J Dairy Sci. 2011;94:1616–26.
Oliveira L, Hulland C, Ruegg PL. Characterization of clinical mastitis occurring in cows on 50 large dairy herds in Wisconsin. J Dairy Sci. 2013;96:7538–49.
Blowey R, Edmondson P. Mastitis control in dairy herds. (2a edição). 2010.
Zadoks RN, Gillespie BE, Barkema HW, Sampimon OC, Oliver SP, Schukken YH. Clinical, epidemiological and molecular characteristics of Streptococcus uberis infections in dairy herds. Epidemiol Infect. 2003;130:335–49.
National MC. Laboratory handbook on bovine mastitis. 3th ed. Walton: Nat Mastitis Council; 1999.
Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–4.
Teixeira LM, Carvalho M da, Facklam GS, Shewmaker RR. PL. Enterococcus. Man of Clin Microbiol. 2015;:403–21.
Tomazi T, Gonçalves JL, Barreiro JR, Braga PAC, Prada e Silva LF, Eberlin MN, Santos MV. Identification of coagulase-negative staphylococci from bovine Intramammary infection by Matrix-assisted laser desorption ionization – time of Flight Mass Spectrometry. J Clin Microbiolgy. 2014;52:1658–63.
Acknowledgements
We thank the farmers and their staff for collaborating with the study activities.
Funding
This study was funded by the São Paulo State Foundation (FAPESP) grant 2015/19688-8, 2017/08823-7.
Author information
Authors and Affiliations
Contributions
F.F.G. designed and performed the experiment, analyzed the data and wrote the manuscript. G.N.M., S.F.J., S.T.G., F.M.D. assisted with sample collection and the experiment. M.S.R.M. and F.M.H.M. analyzed the data, wrote and revised the manuscript. F.S.P. performed the statistics. J.C.F.P., L.S.B., M.G.R., V.L.M.R., R.T.H., D.S.L., H.L., designed the experiment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent
This study was developed following the Ethics Committee on Animal Use (CEUA) guidelines and approved by the School of Veterinary Medicine and Animal Science (FMVZ), São Paulo State University (UNESP), Botucatu, São Paulo State, Brazil; protocol number 0136/2017.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guimarães, F.F., Moraes, G.N., Joaquim, S.F. et al. Identification of Enterococcus spp. by MALDI-TOF mass spectrometry isolated from clinical mastitis and bulk tank milk samples. BMC Vet Res 20, 378 (2024). https://doi.org/10.1186/s12917-024-04217-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04217-2