Abstract
Background
Extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) are important zoonotic pathogens that can cause serious clinical infections, also in horses. Preventing the spread of ESBL-E, especially in the equine hospital environment, is key to reducing the number of difficult-to-treat infections. Estimating the local prevalence of ESBL-E in horses is crucial to establish targeted infection control programs at equine hospitals. We conducted a prevalence and risk factor study in equine patients on admission to an equine teaching hospital in Finland through a rectal ESBL-E screening specimen of the horse and a questionnaire.
Results
The prevalence of ESBL-E in admitted horses was 3% (5/161, 95% CI 1–7%); none of the tested factors remained statistically significant in multivariate analysis, although antimicrobial treatment within three months was borderline significant (p = 0.052). Extended-spectrum β-lactamase -producing Klebsiella pneumoniae ST6179:CTX-M-15 was detected in three horses using whole-genome sequencing, which in combination with patient records suggested nosocomial transmission. Escherichia coli isolates were ST1250:CTX-M-1 (n = 1), ST1079:CTX-M-1 (n = 1), and ST1245:CTX-M-14 (n = 1). Multiple virulence genes were detected in the ESBL-E isolates. In the ESBL-E positive horses enrolled in a one-year follow-up study, ESBL-E were unlikely to be isolated in rectal screening specimens after the initial positive specimen.
Conclusions
The prevalence of ESBL-E in horses visiting a veterinary teaching hospital in Finland is low, indicating an overall low prevalence estimate in the country’s equine population. No statistically significant risk factors were identified, likely due to the low number of cases. The duration of ESBL-E carriage is likely to be very short in horses.
Similar content being viewed by others
Background
Extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) are opportunistic gram-negative pathogens. These bacteria are primarily part of the intestinal microbiota in animals and humans and exhibit resistance to multiple antimicrobial substances. The most common extended-spectrum β-lactamase (ESBL)-producing species in horses are Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae [1, 2]. They can cause several types of clinical infections, including neonatal septicaemia, thrombophlebitis, and incisional infections in horses [3,4,5]. The prevention of the development and spread of antimicrobial resistance and multi-drug resistant bacteria among horses is exceptionally important, as there are few suitable antimicrobial agents available for use in equine medicine due to the sensitive digestive system of the horse. To ensure effective treatment of serious bacterial infections, maintaining the effect of these antimicrobial agents is crucial, both in horses and in other animals and humans.
Horses have multiple roles in the modern world; they are used in numerous equine sports (involving even global travel) and in riding hobbies for people of all ages. Horses are also classified as working animals and livestock in some countries [6]. Frequent movement and dense animal populations can promote effective spread of pathogens and infectious diseases [7], including antimicrobial-resistant bacteria. Previous French and Canadian studies revealed medical treatment within three months, number of staff at farm, and recent participation at an equestrian event as risk factors for colonisation with multidrug-resistant or ESBL-producing E. coli in healthy horses [8, 9]. Riding schools are at a greater risk of housing ESBL- or AmpC cephalosporinase-positive horses than breeding facilities in France [9]. People working in the equine sector and hobbyists are in close contact with horses and often exposed to horse faeces, which can pose a public health concern as ESBL-E are transmitted via the faeco-oral route and can spread between humans and animals [10]. Similar ESBL-E isolates were found in a horse, a staff member, and surfaces at an equine clinic in the Czech Republic [11]. Owning a horse is a risk factor for ESBL-E colonisation in humans [12].
The present study was initiated as part of the development of the infection control program at the Equine Veterinary Teaching Hospital (EVTH) at the University of Helsinki, Finland. For the program to be effective in a specific veterinary setting, it should reflect the animal patient population and pathogen introduction risks of that setting [13]. As the EVTH receives equine patients nationwide, estimating the national prevalence of ESBL-E carriage was required, as this has not been previously studied in Finland. Such information is crucial for evaluating the risk that horses pose to both human and animal health. In France, 29% of premises housed healthy horses shedding E. coli non-susceptible to the third-generation cephalosporine ceftriaxone [9]; in the Netherlands the proportion of ESBL/AmpC positive horses was 11% [14]. We hypothesized the number to be lower in Finland than in these countries, since in previous literature the prevalence of ESBL E. coli in healthy dogs and humans in Finland was 5% [15] and 6.3% [16], respectively; we deduced that this could reflect the prevalence also in horses. The occurrence of ESBL-E in horses has not previously been studied extensively in Finland.
Little is known about the dynamics of ESBL-E shedding in horses, as the majority of the data were collected in cross-sectional studies. One three-week study on the effect of antimicrobial treatment regimens in the ESBL-E excretion in hospitalised horses was performed and a two-month investigation on the shedding of E. coli in hospitalised and non-hospitalised horses treated with antimicrobials [17, 18]. We designed a study covering a longer period to evaluate if the likelihood of shedding ESBL-E diminished over time. The excretion of ESBL-E has been studied in dogs and cats and the duration of carriage appears to be limited, lasting from weeks to some months [19, 20]. These studies were conducted in the Netherlands, Portugal, and the United Kingdom.
This study aimed (1) to estimate the prevalence of ESBL-E in horses entering a Finnish equine hospital, (2) to determine the species, phenotypical antimicrobial resistance, resistance and virulence genes and sequence types of the isolated ESBL-E, and (3) to describe the longitudinal shedding of ESBL-E in rectal specimens of horses in Finland.
Results
Prevalence and risk factor study
Description of the study population
Altogether 161 horses were enrolled in the study during October 2020 to April 2021. Among the sampled horses, 53% (n = 85) were geldings, 40% (n = 64) were mares, and 7% (n = 12) were stallions. Mean age of the horses was 10.3 years (range 0 to 30 years), and median age was 10 years. Based on the postal code of the home stable, most of the horses were from the Uusimaa region (68%), which also hosts the EVTH in the metropolitan area (Fig. 1). Most were outpatients (95%), as only eight horses (5%) were admitted to daytime emergency service. The most common presenting complaints were lameness (n = 22), eye examination (n = 20), shoeing (n = 15), suspected or confirmed sand ingestion (n = 14) and planned surgery (n = 12). Close to half of the study population were Warmbloods (44%, n = 71), 16% (n = 25) were Finnhorses, 14% (n = 23) ponies, 10% (n = 16) Standardbreds, and 16% (n = 26) other breeds.
Estimated prevalence of ESBL-E
Out of the 161 horses admitted to the EVTH, five (3%) harboured ESBL-E (95% CI 1–7%). Four of the horses harboured one ESBL-E isolate and one horse (horse no. 2) two ESBL-E isolates (Table 1).
Characterisation of the ESBL-E isolates and epidemiological investigation
Out of the six phenotypically ESBL-producing isolates, three were E. coli and three K. pneumoniae; all were multi-drug resistant (resistant to at least one antimicrobial in three or more classes) (Table 1, Additional file 1) [21]. All isolates harboured several resistance genes, including multiple towards beta-lactams (CTX-M, TEM, SHV, and OXA). The virulence genes of the isolates are shown in Additional file 2.
The E. coli isolates were of serotypes Ounknown:H26 (HE-3), O6:H49 (HE-4) and O166:H14 (HE-5) and of the following different sequence types: ST1250 (HE-3), ST1079 (HE-4), and ST1245 (HE-5). The isolates represented two phylogroups: B1 (HE-3, HE-4) and E (HE-5). The plasmid replicons IncFIA(HI1) (HE-3), IncFIB(K) (HE-3), IncHI1A (HE-3), IncH1B(R27) (HE-3), IncQ1 (HE-3, HE-5), and IncI1I(Alpha) (HE-4) were identified. No shiga toxin genes were found (Additional file 2).
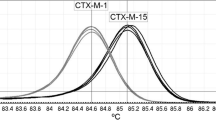
The K. pneumoniae isolates (n = 3) shared nearly identical phenotypic and genotypic antimicrobial resistance patterns (Table 1, Additional file 1) and were of a novel sequence type (ST6179). Despite harbouring aac(6’)-lb-cr aminoglycoside and catB3 amphenicol resistance genes, the K. pneumoniae isolates did not express amikacin or chloramphenicol resistance phenotypically. The isolates HE-8 and HE-15 were indistinguishable by cgMLST, and HE-6 had three allelic differences (out of 2358 alleles) from the other isolates. Plasmid replicons IncFIB and IncFII were identified in each K. pneumoniae isolate.
Since the K. pneumoniae isolates were clonally related, patient data of the EVTH were examined to find a possible epidemiological link between the horses. The data revealed that the horses harbouring the ST6179 K. pneumoniae were hospitalised simultaneously at the EVTH about a month before the sampling date. Horse no. 2 (isolate HE-6) and horse no. 4 (isolate HE-8) had spent three days hospitalised at the same time, and horse no. 5 (isolate HE-15) had been hospitalised for one day at the same time with horses no. 2 and 4.
Risk factors for colonisation with ESBL-E
Descriptive statistics of the variables are presented in Tables 2 and 3.
Based on univariate analysis, antimicrobial treatment within three months (not known vs. no OR 48.61, 95% CI 2.84–833.76, p = 0.007; yes vs. no OR 8.46, 95% CI 1.19–60.36, p = 0.03), surgical procedure within three months (OR 10.48, 95% CI 1.89–58.27, p = 0.007), and imported or visited abroad within three months (OR 11.30, 95% CI 1.21–105.26, p = 0.03) were selected for multivariate analysis (selected variables in Table 4). None remained statistically significant (Table 4).
Additional categorical variables in the risk factor analyses are presented in Table 5.
Longitudinal excretion of ESBL-E
Description of the study population
Thirteen ESBL-E positive horses were enrolled in the longitudinal follow-up study, including the five from the prevalence study and eight horses from the discharge screening of ESBL-E that fulfilled the inclusion criteria.
Five horses completed the entire year of samplings, while eight horses were lost to follow-up (Additional file 4). Further specimens were not received from 2/13 horses (15%) after the initial ESBL-E positive specimen. None of the participating horses developed a clinical infection caused by ESBL-E during the sampling period.
Description of ESBL-E excretion
Most of the participating horses (8/13, 62%) were ESBL-E negative in all specimens obtained after the initial ESBL-E positive specimen (Additional file 4). One horse was persistent ESBL-E positive in two consecutive samplings (a period of four weeks). Two horses appeared ESBL-E positive again after having been ESBL-E negative in one or multiple specimens (minimum and maximum time interval testing ESBL-E negative between ESBL-E positive specimens 11 weeks and 21 weeks, respectively).
The ESBL-E isolates of the horses recruited in the study and the isolates that were obtained after the initial specimen are presented in Table 1. Horse no. 2 shed phenotypically identical K. pneumoniae isolate on week 4 as initially (Table 1, Additional file 4). Horse no. 7 was ESBL-E negative on week 9, but again ESBL-E positive on week 11 (after hospital visit and antibiotic treatment) shedding a phenotypically different E. cloacae isolate than on week 0. Horse no. 10 appeared ESBL-E negative in four consecutive specimens after week 0, but on week 21 shed E. coli (initially E. cloacae). The E. coli isolate in the subsequent specimen on week 26 was phenotypically identical.
Discussion
This is the first study conducted on ESBL-E prevalence in horses in Finland. The low prevalence of ESBL-E (3%; 95% CI 1–7%) in horses admitted to an equine hospital is consistent with our hypothesis, as we expected the result to reflect the prevalence in other species in Finland. None of the tested variables were statistically significant for association with ESBL-E colonisation in horses on admission to an equine hospital. The ESBL-E species observed in our study reflected previous research findings on horses. There was good concordance between antimicrobial resistance phenotype and genotype. We present here a novel sequence type of K. pneumoniae (ST6179), which was likely causative of clonal spread at an equine teaching hospital based on epidemiological and molecular data. In horses enrolled in the one-year follow-up study, ESBL-E were unlikely to be isolated after the initial ESBL-E positive specimen.
As the low prevalence was expected, we decided to use pre-culture enrichment to increase the sensitivity of detecting ESBL-E in the specimens and we also wanted to investigate any ESBL-E isolates appearing in horses in Finland regardless of the magnitude of shedding. In other countries, the prevalence has indeed been higher. In Germany, the colonisation rate on admission to an equine hospital was 11% (no enrichment used); the rate was 20% in Israel (enrichment used) [1, 2]. To date, prevalence data from the Nordic countries are lacking. We suspect that the low prevalence in our study might be partly due to the prudent use of antimicrobials in animals in Finland [22] and partly because Finland is in Northern Europe, with lower intercountry mobility.
The sample population in our prevalence and risk factor study consisted mainly of outpatients living in the greater metropolitan area of Helsinki. Thus, the prevalence cannot be readily generalised to the equine population of Finland, especially since not all the enrolled horses were healthy. However, the study population is representative of the patient material of the EVTH. Since most of the equine population is concentrated in Southern Finland and there is likely more frequent movement between premises, the prevalence of ESBL-E might be even lower in horses from other parts of Finland.
The K. pneumoniae isolates harboured resistance genes for multiple classes of antimicrobials and were co-resistant to fluoroquinolones, sulfamethoxazole-trimethoprim, tetracyclines, and gentamicin. The most common ESBL gene families in horses are CTX-M and SHV, and particularly the genes blaCTX−M−1, blaCTX−M−15, and blaSHV−12 [2, 4, 9, 17, 23,24,25,26]. This is consistent with the findings of our study, as the K. pneumoniae isolates harboured blaCTX−M−15 and two of the E. coli isolates had the blaCTX−M−1 gene. The broad-spectrum β-lactamase encoding gene blaTEM−206 has been reported among K. pneumoniae isolates in urban riverine environment in India [27] and in a neonatal intensive care unit in Italy [28]. Our study is the first to report this gene in K. pneumoniae in animals. The gene has been detected in E. coli in livestock, specifically in pig farms, cattle, and chickens [29,30,31]. It appears that the gene can adapt to genomes of different bacterial species in various hosts and spread effectively in the animal community and the environment.
Two of the K. pneumoniae isolates were indistinguishable by core-genome multi-locus sequence typing (cgMLST), and the third isolate was also clonally related as it differed only by three alleles (out of 2358). These findings and the recent concurrent hospitalisation indicate clonal spread of ESBL-E and nosocomial transmission at the EVTH. One case report on suspected nosocomial infections caused by ESBL-E in a German equine hospital has been published [32]. In Israel, it was observed that ESBL-producing Salmonella enterica spread clonally between seven horses at a veterinary equine teaching hospital [33]. These discoveries underline the importance of the equine hospital setting as a potential risk environment for spread of ESBL-E. Horse faeces likely contaminate the environment, and selection pressure is often increased due to antimicrobial treatments. Furthermore, hospitalised horses are usually more susceptible to infections, and multiple persons are treating patient horses simultaneously. It is alarming that ESBL-E strains with extensive drug resistance characteristics are circulating in the equine population, as few antimicrobial substances are suitable and available for use in horses.
We also detected the presence of type 3 fimbrial gene cluster (mrk) in the K. pneumoniae isolates, and these fimbriae may promote colonisation and attachment to host cells in a horse and biofilm formation [34, 35]. For example, this gene cluster has been detected in a clinical wound infection isolate of K. pneumoniae ST1228 from a horse in Austria [4], which suggests that the isolates in our study may cause infections in horses even though they were discovered from rectal screening specimens. This finding is worrisome, especially considering that nosocomial spread of these multi-resistant bacteria was suspected.
The discovered ST1679 clone belongs to the same clonal group CG307 as a commonly recognised high-risk clone K. pneumoniae ST307 [4, 36, 37]. A CTX-M-15-associated strain of ST307 caused an outbreak involving clinical manifestations at the EVTH in 2014 [3]. As ST1679 only differs from ST307 by one allele in MLST, they are genetically very similar, which leads us to the question if there has been an ongoing evolution of ESBL-producing K. pneumoniae at the EVTH throughout the years. The outbreak clone ST307 expressed an identical phenotypical resistance pattern as the ST1679 clone in the present study. However, detailed molecular comparison is lacking as the ST307 clone at the EVTH has not been subjected to whole-genome sequencing. To gain knowledge on plasmid evolution and its possible connection to equine patients, further genomic investigations in the subject, specifically plasmid analyses, are warranted.
In the risk factor study, none of the tested variables were statistically associated with ESBL-E colonisation on admission, which is likely be at least partly due to the low prevalence compared to the sample size. Furthermore, the study was conducted during the COVID-19 pandemic and during winter. In Finland there are fewer equine events organised during the winter due to lack of indoor facilities. Had the study been conducted in summer, an increase in the number of equine contacts could potentially have affected the results.
However, antimicrobial treatment, surgical operation, or visiting abroad (or import) within three months were statistically significant in univariate analysis. This provides some indication to their relevance as factors to consider when establishing a risk class for a patient admitted to an equine hospital. Antimicrobial treatment is associated with multidrug-resistant E. coli or ESBL-E colonisation in previous studies [1, 38]. Horses undergoing surgical treatment commonly also receive antimicrobials, which leads to an association between these factors and thus not ending up as independent risk factors in our study. Nevertheless, we suggest that the aforementioned factors should be considered when estimating the risk of ESBL-E colonisation of an admitted equine patient. It is likely that some of the variables could have become statistically significant with increased power. It should be noted that the CIs of the ORs were broad due to the rarity of ESBL-E positive horses.
Even though the longitudinal study of individual horses colonised with ESBL-E was descriptive in nature, the results of our study indicate that it is unlikely that ESBL-E isolates persist in horses for a long time, and the exposure risk in the equine community is estimated to be minimal. Most of the horses remained ESBL-E negative after the initial specimen. However, it is noteworthy that the excretion of ESBL-E in dogs is highly dynamic, which is a complicating finding from epidemiological and infection control perspectives, as it makes an animal’s ESBL-E status less predictable [19]. A study conducted in the UK showed that the odds of resistance in E. coli diminish approximately two weeks after antimicrobial treatment in non-hospitalised horses [18]. Another UK study indicated that being stabled in the same yard as a horse that has recently been hospitalised increased the risk of being colonised with ESBL E. coli [38]. It may be worthwhile to consider if ESBL-E positive horses should be managed separately for some weeks at home stables. However, more investigations on the dynamics of ESBL-E over time in healthy horses are needed, as healthy horses at home stable are not subject to the same infection pressure as patients in equine hospitals.
Many of the horses in the longitudinal study visited an equine clinic or hospital several times during the sampling period. Horse number 10 was initially colonised with ESBL E. cloacae (P-2771) and tested ESBL-E negative for several months but tested ESBL-E positive again in week 21. However, the bacterial species was different, this time being ESBL E. coli (HE-14). The horse visited an equine hospital between samplings, which raises the possibility that the horse was recolonised. This is the most likely explanation, as the strain did not appear in the previous enriched specimens and the horse did not receive antimicrobials before the new ESBL-E positive specimen, which could have created selection pressure. However, the recolonisation could mask the shedding of the initial ESBL-E strain (P-2771) and thus complicate the interpretation of the study.
There were some limitations in the execution of the longitudinal study. The sample population was particularly small due to limited resources and many horses were lost to follow-up. Thus, no statistical analyses were performed. Recall bias should also be considered, as we interviewed the owners on the antimicrobial treatments and hospital visits only after the study was finished. To achieve accurate records, we could have conducted a phone interview after each sampling or created a form and ask the owner to send to the laboratory with the specimen.
Knowledge on the local prevalence and risk factors for ESBL-E carriage in horses is crucial to establish targeted infection control programs in equine settings. As ESBL-E can spread effectively in the equine community, we encourage equine veterinarians and scientists to collect more epidemiological and molecular data and to conduct further investigations on the dynamics of ESBL-E in equine hospitals and clinics utilising whole-genome sequencing. Rapid sequencing could aid in identifying the source of nosocomial spread or the magnitude of the spread of certain strains in outbreaks, as well as in monitoring persistence of (resistant) pathogens. A deeper understanding on the spread of ESBL genes and bacteria will allow for sufficient infection control measures and improved short- and long-term surveillance to reduce the risk of nosocomial transmission. Routine sequencing of pathogens is still not standard practice in most equine practices but could be implemented in active and passive surveillance strategies along with phenotypic susceptibility analysis. Sharing the best practices of designing and implementing infection control programs at equine clinics or hospitals could benefit the broader equine medicine community and finally the equine patients at clinics and hospitals. Information from the last three months on antimicrobial treatments, surgical treatments, and international travel are suggested to be recorded for patients entering equine hospitals to evaluate the need for preventative actions.
Conclusion
The prevalence of ESBL-E in horses visiting a veterinary teaching hospital in Finland was low, which suggests a low national prevalence among horses in Finland. While no statistically significant risk factors were identified, previous antimicrobial treatment was implicated as a possible risk factor. The situation is thus overall favourable, but equine practitioners should adhere to antimicrobial stewardship guidelines to maintain or improve the situation. As nosocomial transmission of ESBL-E was suspected in three horses, the importance of infection prevention and control at equine hospitals and clinics should be emphasized. Horses seem to carry ESBL-producing bacteria only for a short time, but hospital visits and antimicrobial treatment may prolong carriage or expose the horse to other ESBL-producing bacteria.
Methods
Study design, study population and sample size
Prevalence and risk factor study
This was a cross-sectional prevalence study on equine patients entering the EVTH between October 2020 and April 2021 that were sampled for asymptomatic ESBL-E carriage. The EVTH is the only equine teaching hospital in Finland and receives slightly under 3000 patients per year. The hospital treats both primary (from the metropolitan area) and referral equine patients (nationwide).
Additionally, after obtaining research consent, the person accompanying the horse was presented with a horse-focused questionnaire based on previous literature on possible risk factors for ESBL-E colonisation (Additional file 3); putative risk factors for the analysis included the signalment of the horse, factors concerning the hospital visit, characteristics of the home stable and the horse’s close contacts, visits outside the home stable, as well as recent procedures and medications. The questionnaire was tested prior to the start of the study by horse-owners employed by the EVTH to ensure that the questions and concepts were understood as meant and that all necessary response alternatives were covered. As the test was successful, the original questionnaire was used without modifications. The person accompanying the equine patient was interviewed by KE, EAK, or a trained licentiate thesis student. If there was no person present with the patient, the owner was contacted by phone to inquire for research consent and to present the questionnaire. Study participation was voluntary.
The study population consisted of a convenience sample of equine patients entering the EVTH. Both outpatients and emergency service patients of all ages, breeds, and sexes were included. Horses entering the hospital during off-hours (outside Monday to Friday 8–16, or on national holidays), those that were not given research consent, or both, were excluded from the study.
The sample size was estimated with EpiTools (https://epitools.ausvet.com.au/oneproportion). In 2018, there were approximately 74 400 horses in Finland [39]. With a prevalence estimate of 5% and desired precision of 3%, a sample size of 200 horses was calculated.
Longitudinal study
A prospective observational study with a study period of one year was designed based on literature on ESBL-E shedding in animals and available personnel resources [17,18,19,20]. The ESBL-E positive horses of the prevalence study were enrolled in the longitudinal study. As an epidemiological investigation was initiated at the EVTH due to phenotypically similar findings in the prevalence study, patient screening at discharge was launched to reveal possible ESBL-E positive hospitalised horses. To increase the number of participating horses in the longitudinal study, a separate convenience sample of horses that were ESBL-E positive at the discharge screening were included, in addition to the horses from the prevalence study. The inclusion criteria were (1) rectal colonisation of the horse with ESBL-E, (2) consent for participating in the study from the owner, and (3) absence of active clinical infection caused by any ESBL-E at the time of enrolment.
The sampling scheme started from the sampling date of the initial ESBL-E positive specimen (i.e., the starting date was unique to all participating horses). For the first six months, specimens were asked to be obtained every month, resulting in six specimens. After the first six months, two additional specimens were to be obtained three months apart (i.e., nine months and twelve months after the initial sampling date).
A phone interview with the owner was conducted once the study period was finished to record possible antimicrobial treatments and hospital visits of the horse during the study period.
Ethical review
The study was approved by the Viikki Campus Research Ethics Committee of the University of Helsinki (statement no. 10/2020). The guidelines of the Finnish Advisory Board on Research Integrity for good scientific practice were followed in the study design [40].
Collection of ESBL-E specimens
Bacteriological specimens were obtained from the rectum of the horse using a sterile cotton swab (M40 Transystem™ with Amies gel, Copan Italia S.p.a., Italy). Persons obtaining the specimens were trained by KE or EAK and given written instructions.
For the prevalence study, the sampling was performed on admission without delay and before any procedures.
For the longitudinal study, the owners of the ESBL-E positive horses in the one-year follow-up study were trained for rectal swab sampling and additionally received written instructions. A sampling kit of cotton swabs and material for shipping the specimens to the Clinical Microbiology Laboratory (CML) of the Veterinary Faculty of the University of Helsinki was either handed over or sent to the owner. The shipping was instructed to be performed without delay for the specimen to arrive at the laboratory within 48 h from the sampling. Although the owners were reminded of obtaining the follow-up specimens, the acquisition of them was dependent on the owner’s willingness to continue in the study.
Microbiological methods
Bacterial culturing
Bacterial specimens were cultured at the CML. The specimen was first placed in 3 ml of buffered peptone water (Oxoid Ltd., UK), enriched at 36 ± 2 °C overnight, and then plated onto ESBL selective growth medium (Brilliance™ ESBL Agar, Oxoid Deutschland GmbH, Germany). The agar plates were incubated at 36 ± 2 °C and interpreted at 24 and 48 h.
Species identification and susceptibility testing
Suspected ESBL-E colonies were identified using matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry (Bruker MALDI Biotyper Microflex LT, Bruker Daltonik GmbH, Germany). A score value ≥ 2.0 was considered as highly confident identification. Confirmed ESBL-E species were subcultured onto tryptone soya agar with sheep blood (Oxoid Deutschland GmbH, Germany), grown at 36 ± 2 °C for 24 h.
The isolates were tested for antimicrobial susceptibility on Mueller-Hinton agar (Oxoid Ltd., UK). The standard laboratory disc panel for gram-negative species consisted of ampicillin (10 µg), amoxicillin/clavulanic acid (30 µg), cefpodoxime (10 µg), meropenem (10 µg), enrofloxacin (5 µg), sulfamethoxazole-trimethoprim (25 µg), amikacin (30 µg), gentamicin (10 µg), chloramphenicol (30 µg), tetracycline (20 µg), and doxycycline (30 µg) (Oxoid Ltd., UK). Susceptibility for colistin was investigated using Colistin ETEST® (bioMérieux SA, France). The CLSI disc diffusion guidelines were followed (tetracycline [41], colistin [42], the rest of the panel [43, 44]). In addition, phenotypic identification of ESBL production was performed using double-disc diffusion test (including cefotaxime 30 µg, cefotaxime/clavulanic acid 30/10 µg, ceftazidime 30 µg, and ceftazidime/clavulanic acid 30/10 µg) and MASTDISCS® Combi (Mast Group Ltd., UK). E. coli NCTC 13351 and E. coli ATCC 25922 were used as ESBL-positive and ESBL-negative quality control strains, respectively, and for the quality control of the standard disc diffusion panel. The ESBL-E isolates were frozen in skim milk at −80 °C until further investigation.
DNA extraction and whole-genome sequencing
The DNA of the ESBL-E isolates in the prevalence study was extracted and purified using DNeasy® Blood & Tissue Kit (QIAGEN GmbH, Germany). The pre-treatment was performed according to the manufacturer’s instructions for gram-negative bacteria. The remainder of the preparation protocol was performed according to Purification of Total DNA from Animal Tissues (SpinColumn Protocol) in the user handbook. The extraction was automated using a QIAcube® nucleic acid extraction unit (QIAGEN GmbH, Germany). DNA quantity was measured with an Invitrogen Qubit™ 4 fluorometer (Life Technologies Holdings Pte Ltd., Singapore) and quality with a NanoDrop™ spectrophotometer (NanoDrop Technologies, USA; for HE-6, HE-8, HE-15) and DeNovix DS-11+ (DeNovix Inc., USA; for HE-3, HE-4, HE-5). DNA was eluted in Buffer AE (QIAGEN GmbH, Germany).
Whole-genome sequencing was performed by Novogene (UK) Company Limited. The NEBNext® Ultra™ DNA Library Prep Kit (New England BioLabs, USA) was used for library preparation. Sequencing was performed using Illumina NovaSeq 6000 platform (Illumina, Inc., USA) to generate paired-end 150 bp reads.
The raw reads of E. coli were deposited into the European Nucleotide Archive (ENA). Accession numbers of the isolates are provided in Table 1 (project accession number PRJEB71437).
Bioinformatics
Raw reads were processed in Ridom SeqSphere+ (software version 7.7.5, Ridom GmbH, Germany) with default parameters, including data quality assessment with FastQC (version 0.11.7) [45], trimming of adapters with Trimmomatic (version 0.36) [46], and de-novo assembly with SKESA (version 2.3.0) [47]. Statistics of the assembly phase are presented in Additional file 5.
Bioinformatic analyses were performed on the assembled sequences using web-based tools (Center for Genomic Epidemiology (CGE), DTU, Denmark) and in Ridom SeqSphere + with default settings. Antimicrobial resistance genes were identified with NCBI AMRFinderPlus (version 3.2.3) [48] and in ResFinder (version 4.4.1) [49, 50], plasmid replicons in PlasmidFinder (version 2.1) [51], and virulence genes in VirulenceFinder (for E. coli, version 2.0.3) [52, 53] and with VFDB (for K. pneumoniae, version 2020-Feb-28, http://www.mgc.ac.cn/VFs/). Serotyping of E. coli was run on SeroTypeFinder by CGE (version 2.0.1) [54] and phylogrouping of E. coli was performed on the web-based ClermonTyping tool [55].
Multi-locus sequence typing (MLST) was performed in MLST (version 2.0) by CGE [56]. The sequences of K. pneumoniae were submitted to the Institut Pasteur MLST database (https://bigsdb.pasteur.fr/klebsiella) for assignment of novel sequence types (accession numbers provided in Table 1).
For cgMLST producing a minimum spanning tree in Ridom SeqSphere+, the K. pneumoniae genomes were mapped using the ST307 reference strain NR5632 (GenBank accession no. CP025143) in K. pneumoniae sensu lato cgMLST (version 1.0). The cutoff value for relatedness in K. pneumoniae was set to ≤ 10 alleles (57,58).
Epidemiological data
Epidemiological (patient) data were obtained from the patient information system of the EVTH (Provet Net, Nordhealth Finland Oy, Finland) and combined with laboratory data of the CML within the same information system.
Statistical analyses
The 95% confidence interval (CI) for the prevalence estimate was calculated using an EpiTools calculator (https://epitools.ausvet.com.au/ciproportion) with Wilson score method due to low prevalence estimate.
The questionnaire data were pre-processed in Microsoft Excel by KE and EAK and analysed by 4Pharma Oy (Finland). Statistical analyses were performed using SAS System for Windows (version 9.4, SAS Institute Inc., Cary, NC, USA). A control was defined with the outcome “ESBL-E negative” and a case with “ESBL-E positive” in the screening specimen.
Each risk factor for ESBL-E colonisation was first analysed individually using Firth’s logistic regression. This method was chosen due to extremely rare ESBL-E cases to minimise the analytical bias caused by rare events. Following the univariate analyses, a multivariate model was constructed.
The multivariate model was constructed in a stepwise manner, including the risk factors in the selection process that had p-values (Wald) < 0.05 in univariate modelling. The variable selection was performed using a traditional implementation of stepwise selection. The Significance Level for Entry (SLE) was set to 0.15 and the Significance Level to Stay (SLS) was set to 0.20. If any effect at any step in the model was not significant at the SLE, then the least significant of the effects was removed from the model, and the algorithm proceeded to the next step. After necessary deletions, another effect, whose addition yielded the most significant F value, was added to the model, and the algorithm proceeded to the next step. The stepwise process ended when none of the effects outside the model had an F statistic significant at the SLE and every effect in the model was significant at the SLS. The stepwise selection was performed using the GLMSELECT procedure in SAS.
Multivariate analysis was performed using Firth’s logistic regression. Odds ratios (OR) with 95% CIs were calculated.
Data availability
The raw reads generated during the current study are available in the European Nucleotide Archive (ENA) at EMBL-EBI under study accession number PRJEB71437 (https://www.ebi.ac.uk/ena/browser/view/PRJEB71437) and in the Institut Pasteur MLST database (https://bigsdb.pasteur.fr/klebsiella). The isolate accession numbers are provided in Table 1.
Abbreviations
- cgMLST:
-
Core-genome multi-locus sequence typing
- ESBL:
-
Extended spectrum β-lactamase
- ESBL-E:
-
Extended spectrum β-lactamase -producing Enterobacterales
- MLST:
-
Multi-locus sequence typing
- MRSA:
-
Methicillin resistant Staphylococcus aureus
References
Shnaiderman-Torban A, Navon-Venezia S, Dor Z, Paitan Y, Arielly H, Abu Ahmad W, et al. Extended-spectrum β-lactamase-producing Enterobacteriaceae Shedding in Farm horses Versus hospitalized horses: prevalence and risk factors. Anim (Basel). 2020;10(2):282. https://doi.org/10.3390/ani10020282.
Walther B, Klein KS, Barton AK, Semmler T, Huber C, Wolf SA, et al. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Acinetobacter baumannii among horses entering a veterinary teaching hospital: the contemporary trojan horse. PLoS ONE. 2018;13(1):e0191873. https://doi.org/10.1371/journal.pone.0191873.
Thomson K, Eskola K, Eklund M, Suominen K, Määttä M, Junnila J, et al. Characterisation of and risk factors for extended-spectrum β-lactamase producing Enterobacterales (ESBL-E) in an equine hospital with a special reference to an outbreak caused by Klebsiella pneumoniae ST307:CTX-M-1. Acta Vet Scand. 2022;64(1):4. https://doi.org/10.1186/s13028-022-00621-6.
Loncaric I, Cabal Rosel A, Szostak MP, Licka T, Allerberger F, Ruppitsch W, et al. Broad-spectrum cephalosporin-resistant Klebsiella spp. Isolated from diseased horses in Austria. Anim (Basel). 2020;10(2):332. https://doi.org/10.3390/ani10020332.
DebRoy C, Roberts E, Jayarao BM, Brooks JW. Bronchopneumonia Associated with Extraintestinal Pathogenic Escherichia Coli in a horse. J Vet Diagn Invest. 2008;20(5):661–4. https://doi.org/10.1177/104063870802000524.
Timoney PJ. Infectious diseases and International Movement of horses. Equine Infect Dis. 2014;544–551e1. https://doi.org/10.1016/B978-1-4557-0891-8.00063-4.
Dominguez M, Münstermann S, de Guindos I, Timoney P. Equine disease events resulting from international horse movements: systematic review and lessons learned. Equine Vet J. 2016;48(5):641–53. https://doi.org/10.1111/evj.12523.
de Lagarde M, Fairbrother JM, Arsenault J, Prevalence. Risk factors, and characterization of Multidrug resistant and ESBL/AmpC producing Escherichia coli in healthy horses in Quebec, Canada, in 2015–2016. Animals (Basel) (2020) 10(3):523. https://doi.org/10.3390/ani10030523
de Lagarde M, Larrieu C, Praud K, Schouler C, Doublet B, Sallé G, et al. Prevalence, risk factors, and characterization of multidrug resistant and extended spectrum β-lactamase/AmpC β‐lactamase producing Escherichia coli in healthy horses in France in 2015. J Vet Intern Med. 2019;33(2):902–11. https://doi.org/10.1111/jvim.15415.
Grönthal T, Österblad M, Eklund M, Jalava J, Nykäsenoja S, Pekkanen K, et al. Sharing more than friendship – transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Eurosurveillance. 2018;23(27):1700497. https://doi.org/10.2807/1560-7917.ES.2018.23.27.1700497.
Dolejska M, Duskova E, Rybarikova J, Janoszowska D, Roubalova E, Dibdakova K, et al. Plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. J Antimicrob Chemother. 2011;66(4):757–64. https://doi.org/10.1093/jac/dkq500.
Huijbers PMC, de Kraker M, Graat EAM, van Hoek AHAM, van Santen MG, de Jong MCM, et al. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in humans living in municipalities with high and low broiler density. Clin Microbiol Infect. 2013;19(6):E256–9. https://doi.org/10.1111/1469-0691.12150.
Stull JW, Weese JS. Hospital-Associated infections in Small Animal Practice. Vet Clin North Am Small Anim. 2015;45(2):217–33. https://doi.org/10.1016/j.cvsm.2014.11.009.
Hordijk J, Farmakioti E, Smit LAM, Duim B, Graveland H, Theelen MJP, et al. Fecal carriage of extended-Spectrum-β-Lactamase/AmpC-Producing Escherichia coli in horses. Appl Environ Microbiol. 2020;86(8):e02590–19. https://doi.org/10.1128/AEM.02590-19.
Jalava J, Miettinen S, Pelkonen S, Rantala M. Prevalence of third-generation cephalosporin resistant Escherichia coli and their resistance mechanisms in dogs in Finland, P1107 abstract. 22nd ECCMID Conference; 2012 Mar 31 – Apr 3; London, UK.
Gröndahl-Yli-Hannuksela K, Lönnqvist E, Marttila H, Rintala E, Rantakokko-Jalava K, Vuopio J. Performance of the check-direct ESBL screen for BD MAXTM for detection of asymptomatic faecal carriage of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae. J Glob Antimicrob Resist. 2020;408–13. https://doi.org/10.1016/j.jgar.2020.04.015.
Damborg P, Marskar P, Baptiste KE, Guardabassi L. Faecal shedding of CTX-M-producing Escherichia coli in horses receiving broad-spectrum antimicrobial prophylaxis after hospital admission. Vet Microbiol. 2012;154(3):298–304. https://doi.org/10.1016/j.vetmic.2011.07.005.
Johns I, Verheyen K, Good L, Rycroft A. Antimicrobial resistance in faecal Escherichia coli isolates from horses treated with antimicrobials: a longitudinal study in hospitalised and non-hospitalised horses. Vet Microbiol. 2012;159(3):381–9. https://doi.org/10.1016/j.vetmic.2012.04.010.
Baede VO, Wagenaar JA, Broens EM, Duim B, Dohmen W, Nijsse R, et al. Longitudinal study of extended-Spectrum-β-Lactamase- and AmpC-Producing Enterobacteriaceae in Household Dogs. Antimicrob Agents Chemother. 2015;59(6):3117–24. https://doi.org/10.1128/AAC.04576-14.
Menezes J, Frosini SM, Belas A, Marques C, da Silva JM, Amaral AJ, et al. Longitudinal study of ESBL/AmpC-producing Enterobacterales strains sharing between cohabiting healthy companion animals and humans in Portugal and in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2023;42(8):1011–24. https://doi.org/10.1007/s10096-023-04629-2.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Finnish Food Authority. (2023). FINRES-Vet 2022, Finnish Veterinary Antimicrobial Resistance Monitoring and Consumption of Antimicrobial Agents. Finnish Food Authority publications 4/2023. https://helda.helsinki.fi/server/api/core/bitstreams/a95d09e1-9253-42e1-ac71-d49ea9303cb4/content [Accessed December 8, 2023].
Boyen F, Smet A, Hermans K, Butaye P, Martens A, Martel A, et al. Methicillin resistant staphylococci and broad-spectrum β-lactamase producing Enterobacteriaceae in horses. Vet Microbiol. 2013;167(1):67–77. https://doi.org/10.1016/j.vetmic.2013.05.001.
Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect. 2012;18(7):646–55. https://doi.org/10.1111/j.1469-0691.2012.03850.x.
Sadikalay S, Reynaud Y, Guyomard-Rabenirina S, Falord M, Ducat C, Fabre L, et al. High genetic diversity of extended-spectrum β-lactamases producing Escherichia coli in feces of horses. Vet Microbiol. 2018;219:117–22. https://doi.org/10.1016/j.vetmic.2018.04.016.
Isgren CM, Edwards T, Pinchbeck GL, Winward E, Adams ER, Norton P, et al. Emergence of carriage of CTX-M-15 in faecal Escherichia coli in horses at an equine hospital in the UK; increasing prevalence over a decade (2008–2017). BMC Vet Res. 2019;15(1):268. https://doi.org/10.1186/s12917-019-2011-9.
Mondal AH, Siddiqui MT, Sultan I, Haq QMR. Prevalence and diversity of blaTEM, blaSHV and blaCTX-M variants among multidrug resistant Klebsiella spp. from an urban riverine environment in India. Int J Environ Health Res. 2019;29(2):117–29. https://doi.org/10.1080/09603123.2018.1515425.
Corbella M, Caltagirone M, Gaiarsa S, Mariani B, Sassera D, Bitar I, et al. Characterization of an outbreak of extended-spectrum β-Lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit in Italy. Microb Drug Resist. 2018;24(8):1128–36. https://doi.org/10.1089/mdr.2017.0270.
Von Salviati C, Friese A, Roschanski N, Laube H, Guerra B, Käsbohrer A, et al. Extended-spectrum beta-lactamases (ESBL)/AmpC beta-lactamases-producing Escherichia coli in German fattening pig farms: a longitudinal study. Berl Munch Tierarztl Wochenschr. 2014;127(9–10):412–9.
Kholik K, Srianto P, Aulanniam A, Abdul Rantam F, Madyawati SP. Characterization and phylogenetics of beta-lactamase Temoneira gene in Escherichia coli of the Bali cattle on Lombok island, Indonesia. Iraqi J Vet Sci. 2023;37(2):487–93. https://doi.org/10.33899/ijvs.2022.135062.2441.
Li L, Wang B, Feng S, Li J, Wu C, Wang Y, et al. Prevalence and characteristics of extended-spectrum β-Lactamase and plasmid-mediated Fluoroquinolone Resistance genes in Escherichia coli isolated from chickens in Anhui Province, China. PLoS ONE. 2014;9(8):e104356. https://doi.org/10.1371/journal.pone.0104356.
Walther B, Lübke-Becker A, Stamm I, Gehlen H, Barton AK, Janssen T, et al. Suspected nosocomial infections with multi-drug resistant E. Coli, including extended-spectrum beta-lactamase (ESBL)-producing strains, in an equine clinic. Berl Munch Tierarztl Wochenschr. 2014;127(11–12):421–7.
Dor Z, Shnaiderman-Torban A, Kondratyeva K, Davidovich-Cohen M, Rokney A, Steinman A, et al. Emergence and spread of different ESBL-Producing Salmonella enterica serovars in hospitalized horses sharing a highly transferable IncM2 CTX-M-3-Encoding plasmid. Front Microbiol. 2020;11:616032. https://doi.org/10.3389/fmicb.2020.616032.
Allen BL, Gerlach GF, Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991;173(2):916–20. https://doi.org/10.1128/jb.173.2.916-920.1991.
Gual-de-Torrella A, Delgado-Valverde M, Pérez-Palacios P, Oteo-Iglesias J, Rojo-Molinero E, Macià MD, et al. Prevalence of the fimbrial operon mrkABCD, mrkA expression, biofilm formation and effect of biocides on biofilm formation in carbapenemase-producing Klebsiella pneumoniae isolates belonging or not belonging to high-risk clones. Int J Antimicrob Agents. 2022;60(4):106663. https://doi.org/10.1016/j.ijantimicag.2022.106663.
Butaye P, Stegger M, Moodley A, Damborg P, Williams A, Halliday-Simmonds I, et al. One health genomic study of human and animal Klebsiella pneumoniae isolated at Diagnostic Laboratories on a small Caribbean Island. Antibiot (Basel). 2021;11(1):42. https://doi.org/10.3390/antibiotics11010042.
Wyres KL, Hawkey J, Hetland MAK, Fostervold A, Wick RR, Judd LM, et al. Emergence and rapid global dissemination of CTX-M-15-associated Klebsiella pneumoniae strain ST307. J Antimicrob Chemother. 2019;74(3):577–81. https://doi.org/10.1093/jac/dky492.
Maddox TW, Pinchbeck GL, Clegg PD, Wedley AL, Dawson S, Williams NJ. Cross-sectional study of antimicrobial-resistant bacteria in horses. Part 2: risk factors for faecal carriage of antimicrobial-resistant Escherichia coli in horses. Equine Vet J. 2012;44(3):297–303. https://doi.org/10.1111/j.2042-3306.2011.00440.x.
Hippolis SH, Luke Hevostalous. ry, Suomen Ratsastajainliitto ry, (2019). Hevostalous lukuina 2018. https://www.hippos.fi/uploads/sites/1/2021/03/29bba42d-hevostalous_lukuina_2018.pdf [Accessed December 19, 2023].
Varantola K, Launis V, Helin M, Spoof SK, Jäppinen S. Responsible conduct of research and procedures for handling allegations of misconduct in Finland. Guidelines of the Finnish Advisory Board on Research Integrity 2012. Publications of the Finnish National Board on Research Integrity TENK; 2013. p. 40.
Clinical and Laboratory Standards Institute (CLSI). (2018). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, CLSI standard VET01. 5th ed. Wayne, PA, USA.
The European Committee on Antimicrobial Susceptibility Testing (EUCAST). (2021). Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. http://www.eucast.org
Clinical and Laboratory Standards Institute (CLSI). (2020). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, CLSI supplement VET01S. 5th ed. Wayne, PA, USA.
Clinical and Laboratory Standards Institute (CLSI). (2020). Performance Standards for Antimicrobial Susceptibility Testing, CLSI supplement M100. 30th ed. Wayne, PA, USA.
Babraham Bioinformatics. FastQC. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Accessed November 30, 2023].
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. https://doi.org/10.1093/bioinformatics/btu170.
Souvorov A, Agarwala R, Lipman DJ. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018;19(1):153. https://doi.org/10.1186/s13059-018-1540-z.
Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, et al. Validating the AMRFinder Tool and Resistance Gene Database by using Antimicrobial Resistance genotype-phenotype correlations in a Collection of isolates. Antimicrob Agents Chemother. 2019;63(11):e00483–19. https://doi.org/10.1128/AAC.00483-19.
Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72(10):2764–8. https://doi.org/10.1093/jac/dkx217.
Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–500. https://doi.org/10.1093/jac/dkaa345.
Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In Silico Detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–903. https://doi.org/10.1128/AAC.02412-14.
Malberg Tetzschner AM, Johnson JR, Johnston BD, Lund O, Scheutz F. In Silico Genotyping of Escherichia coli isolates for Extraintestinal virulence genes by Use of whole-genome sequencing data. J Clin Microbiol. 2020;58(10):e01269–20. https://doi.org/10.1128/jcm.01269-20.
Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, et al. Real-time whole-genome sequencing for routine typing, Surveillance, and outbreak detection of Verotoxigenic Escherichia coli. J Clin Microbiol. 2020;52(5):1501–10. https://doi.org/10.1128/jcm.03617-13.
Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. Rapid and Easy in Silico Serotyping of Escherichia coli isolates by Use of whole-genome sequencing data. J Clin Microbiol. 2015;53(8):2410–26. https://doi.org/10.1128/JCM.00008-15.
Beghain J, Bridier-Nahmias A, Le Nagard H, Denamur E, Clermont O. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia Genus strain phylotyping. Microb Genom. 2018;4(7):e000192. https://doi.org/10.1099/mgen.0.000192.
Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J Clin Microbiol. 2020;50(4):1355–61. https://doi.org/10.1128/jcm.06094-1157.
Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, et al. Tracking a Hospital Outbreak of Carbapenem-Resistant Klebsiella pneumoniae with Whole-Genome Sequencing. Sci Transl Med. 2012;4(148):148ra116. https://doi.org/10.1126/scitranslmed.3004129.
Zhou H, Liu W, Qin T, Liu C, Ren H. Defining and evaluating a Core Genome Multilocus sequence typing Scheme for whole-genome sequence-based typing of Klebsiella pneumoniae. Front Microbiol. 2017;8:371. https://doi.org/10.3389/fmicb.2017.00371.
Eskola K, Grönthal T, Heikinheimo A, Mykkänen A. Whole genome sequencing in extended-spectrum beta-lactamase (ESBL) producing Enterobacterales outbreak detection in an equine hospital. Poster presented at: 13th International Meeting on Microbial epidemiological markers (IMMEM XIII). September 14–17 2022, Bath, United Kingdom.
Eskola K, Grönthal T, Heikinheimo A, Mykkänen A. Whole genome sequencing in extended-spectrum beta-lactamase (ESBL) producing Enterobacterales outbreak detection in an equine hospital. Poster presented at: Finnish Veterinary Congress 2022. November 2–4 2022, Helsinki, Finland.
Kaira O. Laajakirjoisia beetalaktamaaseja tuottavat enterobakteerit hevosilla Suomessa. University of Helsinki Open Repository: University of Helsinki; 2023. http://hdl.handle.net/10138/357241 [Cited June 13, 2024].
Acknowledgements
We are grateful to veterinary licentiate students Oona Kaira and Siiri Leino and the owners and the grooms of the horses for obtaining screening specimens. We also thank the staff of the CML for culturing and interpreting the specimens and Kirsi Ristkari for performing the DNA extraction. The Language Centre of the University of Helsinki is acknowledged for proofreading the manuscript. We thank the Institut Pasteur teams for the curation and maintenance of BIGSdb-Pasteur databases at https://bigsdb.pasteur.fr/.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).We would like to thank the Finnish Veterinary Foundation for financial support for this study. The funding body did not play a role in the design, analysis, or reporting of the study. Open access was funded by Helsinki University Library.
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final version of the manuscript.KE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review and editing. EAK: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. AH: Resources, Supervision, Validation, Writing – review and editing. AM: Conceptualization, Investigation, Methodology, Supervision, Writing – review and editing. TH: Formal analysis, Software, Writing – review and editing. TG: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Viikki Campus Research Ethics Committee of the University of Helsinki (statement no. 10/2020). The guidelines of the Finnish Advisory Board on Research Integrity for good scientific practice were followed in the study design [40]. Informed owner consent to participate was obtained on the questionnaire form (Additional file 3) for all participating horses.
Prior publication
Parts of the preliminary results of the study (prevalence of ESBL-E on admission, whole-genome sequencing of K. pneumoniae) have been presented at the poster sessions of the 13th International Meeting on Microbial Epidemiological Markers (IMMEM XIII), Bath, United Kingdom [59] and Finnish Veterinary Congress 2022, Helsinki, Finland [60] and published in a non-peer-reviewed licentiate thesis project (in Finnish) related to veterinary studies (prevalence of ESBL-E on admission, questionnaire) [61].
Consent for publication
Not applicable.
Competing interests
Author TH is employed by Oy 4Pharma Ltd. The remaining authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
. Antimicrobial resistance genes of the extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) isolates in the prevalence study
Additional file 2
. Virulence genes of the extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) isolates in the prevalence study
Additional file 3
. Questionnaire (translated from Finnish to English) for determining risk factors for extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) carriage in admitted horses at the Equine Veterinary Teaching Hospital
Additional file 4
. Excretion of extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) in horses recruited in a one-year (52 weeks) follow-up study
Additional file 5
. Statistics of the de-novo assembly of the extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) isolates in the prevalence study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eskola, K., Aimo-Koivisto, E., Heikinheimo, A. et al. Prevalence, risk factors, and characterisation of extended-spectrum β-lactamase -producing Enterobacterales (ESBL-E) in horses entering an equine hospital and description of longitudinal excretion. BMC Vet Res 20, 412 (2024). https://doi.org/10.1186/s12917-024-04260-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04260-z