Abstract
Asthma exacerbations in children are associated with respiratory viral infection and atopy, resulting in systemic immune activation and infiltration of immune cells into the airways. The gene networks driving the immune activation and subsequent migration of immune cells into the airways remains incompletely understood. Cellular and molecular profiling of PBMC was employed on paired samples obtained from atopic asthmatic children (n = 19) during acute virus-associated exacerbations and later during convalescence. Systems level analyses were employed to identify coexpression networks and infer the drivers of these networks, and validation was subsequently obtained via independent samples from asthmatic children. During exacerbations, PBMC exhibited significant changes in immune cell abundance and upregulation of complex interlinked networks of coexpressed genes. These were associated with priming of innate immunity, inflammatory and remodelling functions. We identified activation signatures downstream of bacterial LPS, glucocorticoids and TGFB1. We also confirmed that LPS binding protein was upregulated at the protein-level in plasma. Multiple gene networks known to be involved positively or negatively in asthma pathogenesis, are upregulated in circulating PBMC during acute exacerbations, supporting the hypothesis that systemic pre-programming of potentially pathogenic as well as protective functions of circulating immune cells preceeds migration into the airways. Enhanced sensitivity to LPS is likely to modulate the severity of acute asthma exacerbations through exposure to environmental LPS.
Similar content being viewed by others
Introduction
Exacerbations of asthma in children are most commonly caused by acute respiratory viral infections. The immune mediated response to infection is broadly understood to involve recruitment to the airways of high numbers of innate and adaptive immune cells from a variety of subpopulations. Pro-inflammatory products released from these cells following their activation are held to be responsible for the acute damage to local mucosal tissues that triggers the symptoms characteristic of this disease [1]. Key aspects of the underlying activation process, and the full range of its cellular targets, are incompletely understood. An important question that remains to be resolved concerns what signals are released from the airways and how these impact the circulating immune cells prior to their migration into the airways. Such signals may be suitable targets for therapeutic development efforts.
There is compelling evidence from experimental murine asthma [2], and human atopic asthma [3] in which “exacerbations” are elicited by bronchial allergen challenge, that activation occurs in immune cells prior to their migration towards the airways. One such example is lung homing eosinophils that are activated prior to their release from bone marrow [3]. The extent to which similar processes occur in other cell populations involved in asthma pathogenesis, and the applicability of this general paradigm to “natural” (including viral triggered) asthma exacerbations, remains to be established.
In the present study, we have investigated systemic processes activated during an asthma exacerbation employing a genomics-based systems level approach involving comparative cellular and transcriptomic profiling of peripheral blood mononuclear cells (PBMC) collected from asthmatic children presenting to hospital emergency during an acute exacerbation, versus a second “resting” sample collected subsequently from each participant, at a time remote from the exacerbation. Flow cytometry and bulk RNA-Sequencing (RNA-Seq) combined with network analysis was employed to elucidate the signals impacting the circulating immune cells during acute asthma, by comparing PBMC response patterns between acute and convalescent visits. Our findings suggest that circulating inflammatory cells, preceding their homing to the airways, demonstrate a multitude of pre-programmed asthma-associated gene networks involving myeloid cell activation and differentiation in combination with leukocyte migration signatures. The strongest upstream drivers of these signatures were bacterial lipopolysaccharide (LPS), glucocorticoids and the master regulator of immune responses transforming growth factor beta 1 (TGFB1).
Materials and methods
Sample collection
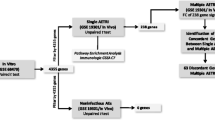
The study population comprised school-age children (n = 19) presenting with an exacerbation of asthma to the Emergency Department at Princess Margaret Hospital for Children, Perth Australia [4]. Inclusion criteria were a gestational age of ≥ 36 weeks and with no known chronic underlying disorder, apart from asthma. Peripheral blood was collected from each participant during their acute exacerbation (acute visit = AV), on average 8.4 h after their most recent administration of systemic glucocorticoid treatment (standard dose of 1 mg/kg of patient’s weight), and at follow-up ≥ 3.9 weeks later (convalescent visit = CV), when the children were clinically stable and had no symptoms. Peripheral blood mononuclear cells (PBMC) were cryobanked as previously described [5]. Following thawing, we recovered > 90% of viable PBMC. We have previously shown that relevant gene expression is not affected by cryopreservation when assessed by real-time quantitative reverse transcription [6].
Atopic status determination
Atopic status was defined as house dust mite (HDM) specific IgE ≥0.35kU/L or total IgE ≥300kU/L and/or skin prick positive HDM and/or cat at the acute visit. Serum total IgE, inhalant allergen (house dust mite) specific IgE, and cat specific IgE were quantified as previously described at the acute visit [5]. Skin prick tests were completed using house dust mite and cat allergen (wheal sizes ≥3 mm = positive) [5]. Asthma was diagnosed by the treating physician at presentation to the Emergency Department.
Viral detection
Nasal specimens collected during the acute exacerbation were analysed for respiratory viruses as previously described [4, 7].
Flow cytometry
Immunostaining of viable PBMC was conducted employing a panel of 12 monoclonal antibodies and appropriate isotype control as previously described [8, 9]. Sample compensation, quality control and manual gating was carried out with FlowJo software (version 10.0.8r1) as detailed in the Additional Material and Additional Fig. 1/Additional Table 1. Flow cytometry standard (fcs) samples were compensated and down-sampled fcs files (50,000 events per sample) were exported from FlowJo and imported into R 4.1.1 using FlowCore [10]. Flow-SOM clustering, dimensionality reduction, cluster visualisation (UMAP) and annotation were carried out using CATALYST [11]. Comparison of differential abundance between clusters was assessed using diffcyt [12].
Plasma proteins
Plasma was separated from blood and stored at -80 °C until further processing. Endotoxin was quantified with the Limulus amebocyte lysate (LAL) assay (Hycult). ELISAs for soluble CD14 (sCD14; R&D Systems), Neopterin (IBL International), fibronectin 1 (FN1; R&D Systems) and S100A8/S19 (R&D Systems) were carried out following the manufacturer’s instructions.
Statistical analysis
Population data that were non-normally distributed were presented as medians and interquartile ranges. Differences in sample ranks of cellular frequencies were assessed with a Wilcoxon test for paired comparisons and a P-value ≤ 0.05 was considered statistically significant. Absolute cell numbers (per ml of blood) are presented as ratios (acute: convalescent; mean ± Standard Error of the Mean (SEM)), and relative proportions (frequencies) are presented as median ± Standard Deviation (SD).
RNA isolation and bulk RNA-Sequencing (RNA-Seq)
Total RNA extraction, integrity assessment, and subsequent sequencing employed standard methodology as detailed in the Additional Material.
Differential expression analysis
Raw sequencing reads were aligned to the reference genome (hg19) and summarised as gene-level counts (see Additional Material). Raw read counts were filtered by retaining counts with at least one read per gene. Differentially expressed genes were identified employing the DESeq2 package [13] with False Discovery Rate (FDR) correction for multiple comparisons [14]. DESeq2 is based on a negative binomial distribution and an additive linear model is fit to the data. Samples are contrasted in a paired design comparing acute to convalescence for each participant. DESeq2 differential expression analysis was performed in conjunction with Remove Unwanted Variation (RUV) [15], to adjust the analysis for latent variation (see Additional Material).
Upstream regulator analysis
Upstream regulator analysis (www.ingenuity.com) was employed to identify putative molecular drivers of the observed differentially expressed genes, as detailed previously [16, 17].
Pathways analysis
Comprehensive gene set enrichment/pathways analysis was carried out separately for up and down regulated genes employing InnateDB [18].
Network analysis
To obtain a holistic understanding of the gene expression program we employed weighted gene co-expression network analysis (WGCNA) as detailed in the Additional Material. Exacerbation-associated coexpression modules were identified employing module eigengenes, summarising the overall expression of each module based on the first principal component. Gene network modules were reconstructed employing Network Analyst [18]. For this, the network was constructed using integrated (union) protein-protein (IMEx Interactome DB, zero order network) and transcription factor-gene interactions (JASPAR database), to form a single reconstructed wiring diagram. Edges were filtered by degree to include < 1000 edges and visualised in Cytoscape [19] (version 3.6.1).
Microarray data analysis
We downloaded a microarray dataset (accession no. GSE16032) [6], from the Gene Expression Omnibus, consisting of n = 3 pooled PBMC obtained from n = 25 atopic asthmatic children (age: 7.3 ± 0.7 years, mean ± SEM; 95% atopic; 85% virally infected; 95% of virally infected were rhinovirus positive) sampled at acute exacerbation in hospital emergency and at follow-up when well. Raw data quality control, filtering and annotation of probe sets is described in detail in the Additional Material. Differentially expressed genes were identified with LIMMA [20] with FDR adjusted P-values.
Results
Asthma exacerbation is associated with depletion of circulating immune cells
To assess the immunological response to an acute exacerbation of asthma, we assessed the abundance of circulating immune cells in the PBMC population from 19 children with MD-diagnosed atopic asthma as they presented to the Emergency Department at Princess Margaret Hospital for Children in Perth, Western Australia. The characteristics of the study population are presented in Table 1.
All study participants were inhalant allergy positive (see atopy definition in Methods) and 78.6% tested positive for rhinovirus. One participant tested positive for influenza virus and one for parainfluenza virus (both also testing positive for rhinovirus). There was no correlation between time since systemic glucocorticoid treatment and either IgE levels or SPT outcomes (Additional Fig. 2). A marked reduction of total PBMC counts was observed during the acute exacerbation compared to convalescence (Fig. 1A). For analysis of changes in specific immune cell subset abundance, FlowSOM clustering was used to identify cells with a similar marker expression profile. These were annotated and visualised using UMAP (Fig. 1B). By clustering immune cell abundance, the samples split across the two timepoints (Fig. 1C). Indeed, when comparing abundance of the annotated clusters, significant changes in abundance was observed for all subsets with an increase in proportion during the acute phase observed for B cells and monocytes, whereas a decrease was observed for basophils, T cells and dendritic cell subsets (Fig. 1D), suggesting that these latter subsets may migrate into the airways during an exacerbation. The most dramatic depletion was observed for plasmacytoid dendritic cells, which are potent producers of type I interferons responses during rhinovirus infections [21] (Fig. 1D). Comparable results were also observed using manually gated data (Additional Fig. 3A-B). Time since administration of systemic glucocorticoids was positively correlated with abundance of cDC and monocytes but not other immune cell populations during the acute event (Additional Fig. 4).
Inflammatory cells are trafficking and leaving the peripheral blood. (A) Number of PBMC per ml of blood during the acute (AV) and convalenscent (CV) timepoint. (B, C) Visualisation or FlowSOM clusters using UMAP (B) and clustering of immune cell subset abundance for each individual in (C). (D) Frequency of specific immune cell subset cluster for each individual compared at the two timepoints. Significance of differences are calculated using Wilcoxon test for paired analysis and displayed as: ***, < 0.001; **, < 0.01 and *, < 0.05
Identification of differentially expressed genes and putative molecular drivers. Differentially expressed genes were identified comparing acute exacerbation versus convalescence in (A) with DESeq2 analysis and (B) LIMMA in independent samples (GSE16032). (A/C) Volcano plots showing the –log10(adjusted P-value) plotted against the log2 fold change, in (A) atopic asthmatic children (n = 19), and in (C) independent samples (GSE16032) consisting of atopic asthmatic children (n = 25). Upregulated genes = red. (B/D) Top molecular drivers identified employing the Ingenuity Knowledge Base. Red = activated driver, blue = inhibited driver. Absolute activation Z-scores ≥ 2.0 and P-values ≥ 0.01 were deemed significant. FTP = fluticasone propionate, DEX = dexamethasone. (E) Venn diagram illustrating the overlap of target genes from the drivers LPS, TGFB1 and glucocorticoid. (F) LPS was measured with limulus amebocyte lysate (LAL) assay (Endotoxin Unit = EU) and (G) the LPS binding protein (LPB) was increased in plasma at AV relative to CV. The P-value is derived from a Wilcoxon test for paired analysis, ***,<0.001
Network analysis reveals five exacerbation-associated gene modules. Network analysis (WGCNA) was performed utilising the top ~ 4000 most variable genes, in (A) heatmap of the gene networks with hierarchical clustering gene dendrograms where each column and row represent single genes. Red colour indicates highly co-expressed clusters of genes or gene networks across the diagonal, and lighter colour in white/yellow indicates low co-expression, in (A, B) the data clustered into 11 co-expression modules denoted modules A-K, and the overall expression of the modules was determined using eigengene values contrasting acute exacerbation versus convalescence. Wilcoxon signed ranked test was used to test for differences between the acute and convalescent visits (AV/CV). *,<0.05, **,<0.01. In (C) putative molecular drivers were identified for the significant modules with Ingenuity Pathways Analysis from the Ingenuity Knowledge Base. Red = activated driver, blue inhibited driver. Absolute activation Z-scores ≥ 2.0 and P-values ≥ 0.01 were deemed significant. FTP = fluticasone propionate, DEX = dexamethasone, CpG ODN = CpG oligodeoxynucleotide. (D) Reconstruction of the wiring diagram of the innate immunity module J and visualisation using Cytoscape. Coloured node = present in the original network (zero order network), white node = transcription factors
Acute asthma exacerbations induce transcriptional changes in PBMC
To assess transcriptional changes to the gene expression program during asthma exacerbations, PBMC were profiled by RNA-Seq. Comparison of gene expression profiles in PBMC between acute exacerbation and convalescent visits identified 2,160 differentially expressed genes; 1,388 genes were upregulated and 772 downregulated (false discovery rate (FDR) < 0.05; Fig. 2A, Additional Table 2). Of those, the IL1 receptor IL1R2 (adj.P = 4.11 × 10− 11), IL13RA1 (adj.P = 3.64 × 10− 3) and CCR2 (adj.P = 1.05 × 10− 3) were among the differentially expressed genes where CCR2 is known for its lung homing function (Additional Table 2). Pathways analysis demonstrated that the upregulated genes were enriched for hemostasis (p = 3.64 × 10− 13), antibody-mediated complement activation (p = 3.38 × 10− 7), Fcgamma receptor (FCGR) dependent phagocytosis (p = 8.31 × 10− 7), EGFR1 signalling (p = 2.53 × 10− 5), endogenous TLR signalling (p = 1.40 × 10− 3) and B cell receptor signalling (p = 1.62 × 10− 3). Downregulated genes were enriched for components of the translation machinery (p = 7.12 × 10− 6), IL-12-mediated signalling (p = 2.63 × 10− 4) and T cell receptor signalling (p = 3.81 × 10− 2). To identify putative molecular drivers of the differential gene expression patterns the data were further interrogated using upstream regulator analysis (Fig. 2B). The top-ranking activated drivers were glucocorticoids (dexamethasone (DEX), fluticasone propionate (FTP)), lipopolysaccharide (LPS)/E.coli B4 LPS, colony stimulating factor 2 (CSF2), proliferation associated genes hypoxia-inducible factor 1 alpha (HIF1A), EGFR long non-coding downstream RNA (ELDR), yes-associated protein 1 (YAP1) and transforming growth factor beta 1 (TGFB1), Th2 cytokines interleukin (IL)-4 and IL-5, as well as the pro-inflammatory enzyme transglutaminase 2 (TGM2) and cytokine IL-6. To confirm the findings in an independent cohort, we utlised a microarray dataset from a study we previously published (GSE16032)[6], comparing transcriptomic profiles of acute exacerbation and convalescent PBMC samples from asthmatic children. Reanalysis of these data identified 1,476 genes differentially expressed at exacerbation (Fig. 2C), and upstream regulator analysis revealed a list of molecular drivers (Fig. 2D) that included 5 of the top 20 drivers (DEX, LPS, TGM2, IL6, SLC15A5) in the data set from the present study. Of note, type 1 interferon pathways (IRF7, IFNA) featured in the top drivers in the replication cohort, which had a 95% incidence rate of rhinovirus infection and similar age range (see Methods), whilst in the current study, IFNA did not meet the significance threshold (z-score = 1.84). The regulator analysis from the two independent cohorts hence suggests that the drivers LPS, and glucocorticoids (DEX/FTP) are likely relevant drivers of the transcriptional program observed during asthma exacerbations in atopic children.
To further explore the overlap, downstream targets of these regulators were identified. The data showed that there were 193 (32.5%) common targets between the drivers LPS and glucocorticoids (Fig. 2E), LPS had the larger number of unique downstream targets (n = 206, 34.7%) compared to glucocorticoids (n = 195, 32.8%). As LPS was identified as the major driver of the transcriptomic profile observed during asthma exacerbation, we assessed plasma levels of endotoxin/LPS and LPS associated proteins. Although there was no difference in LPS levels (measured by limulus amebocyte lysate assay, Fig. 2F), LPS binding protein (LBP) was significantly increased during the acute exacerbation compared with convalesence (Fig. 2G). No changes in plasma concentration of sCD14, neopterin, fibronectin 1 (FN1) and S100A8/S100A9 were observed (Additional Fig. 5). Given that LPB markedly enhances the sensitivity of myeloid cells to LPS [22], the data suggests that LPS responses might be exaggerated during acute asthma exacerbations.
In our study the participants were treated with steroids, and corticosteroids are known to have an impact on the gene expression in PBMC, we therefore computed eigengene values (summarising the first principal component) from the LPS and DEX/FTP signatures, and correlated these with the time since steroid treatment. The data showed that the time since steroid administration did not correlate with either the LPS nor the DEX/FTP signatures (Additional Fig. 6).
Network analysis reveals five acute exacerbation-associated gene modules
To obtain a systems level understanding of the exacerbation responses, we constructed a coexpression network using WGCNA employing the most variable genes of the transcriptomic data derived from the PBMC samples across both visits (Fig. 3A). The resulting network contained 4,679 genes, organised into 11 co-expression modules (labelled A-K; Fig. 3A-B). To summarise the overall expression of each gene module, eigengene values were calculated and compared between the two visits. The data showed that five modules, modules C, F, G, J and K were significantly perturbed at the acute visit compared to the convalescent visit (Fig. 3B).
To probe the biological functions associated with the gene network modules, pathways analysis was performed using InnateDB (Additional Table 3). This analysis suggested that the five disease-associated modules were enriched with the following pathways: Module C - the complement pathway; Module F - the cell cycle; Module G - antibody-mediated complement activation and B cell receptor signalling; Module J – innate immunity; and Module K - hemostasis/ extracellular receptor interactions. Of note, there was also an antiviral/type 1 interferon module H, however, this module was not differentially perturbed between visits.
To identify module driver genes we employed upstream regulator analysis (Fig. 3C). The data showed that the top-ranking drivers, identified in the differential expression analysis, namely LPS, TGM2, TGFB1, IL-4/IL-5, and the glucocorticoids, partitioned to some extent into separate modules. Key activated drivers of module C, the complement module, were: glucocorticoids, LPS, CpG oligodeoxynucleotides (CpG ODN) and IL-4. Module F only contained one significant driver: methylprednisolone. Module G, the antibody module, was putatively driven by proliferation/growth factors including ELDR, ERBB2, VEGF and HGF and pro-inflammatory CEBPB. The top drivers of Module J, the innate immunity module: LPS, DEX, inflammatory CEBPA, IL1B, TNF, as well as type 1 interferon (IFNA, MYD88), and finally Module K, the ECM module: the top regulators included the transcription factors GATA binding protein 1 (GATA1) and TGFB1 (Fig. 3C).
The network wiring diagrams of the significant modules (C, F, G, J, K) were reconstructed using Network Analyst, applying a zero order network of literature-curated protein-protein interactions and transcription factors (Fig. 3D, Additional Fig. 7). The innate immunity module J, that is predicted to be regulated by LPS is shown in Fig. 3D. Overall, the data showed common transcription factors that target all the network modules including GATA-binding factor 2 (GATA2), Forkhead Box C1 (FOXC1), Yin Yang 1 (YY1) and Forkhead Box L1 (FOXL1).
Given that PBMC comprise a heterogeneous cell population, and variations in cellular composition may potentially confound the analyses, we repeated our analyses with or without adjustment for cellular proportions of monocytes, T and B cells. The differential expression analysis results were overlaid on a module-by-module basis and the only substantive effect observed was when adjusting for monocyte numbers, and as a result, modules C (adjusted p = 0.07) and K (adjusted p = 0.12) were no longer differentially expressed (Additional Fig. 8), suggesting that expression of these network modules was monocyte-associated.
Discussion
A feature of the acute phase of asthma exacerbations is the rapid migration of immune cell populations into airways tissues, resulting in transient reduction in circulating numbers of PBMC including T-cells and dendritic cell populations with less impact observed on monocyte and B cell populations. Monocytes are recognised to be an important component of the overall cellular infiltrate that accumulates in the airways following exacerbation [23], and the difference observed here relative to other myeloid (and lymphoid) populations may reflect variations in underlying migration kinetics, or in bone marrow output. Hematopoietic stem and progenitor cells in the bone marrow are known to act as sensors for peripheral inflammation/infection and will increase the proliferation of myeloid lineage cells that are released into system circulation to deal with environmental stimuli [24].
A key finding in this study was the demonstration of a complex pre-programmed coexpression network operative within PBMC circulating at the time of acute asthma. The genes in this network were organised into a range of immunoinflammatory pathways, in particular those associated with activation of innate immune and inflammatory function(s), cell migration and tissue homing, and antibody-mediated complement activation. Principal to this, was the demonstration that the top drivers from the differential expression analysis (LPS, glucocorticoids) were partitioned into respective modules J, C, G and K, and corresponding to unique biological expression signatures. Given that the children were treated with steroids, which could potentially impact on cellular immune responses and gene expression profiles, we investigated the relationship between time since administration of systemic glucocorticoids and ensuing cellular and molecular responses. We found that the time since administration of steroids was positively correlated with the abundance of cDC and monoctes, but there was no relationship with either the LPS nor DEX/FTP signatures, and therefore the LPS signature could not be explained by the steroid treatment. Analyses adjusting for monocytes identified myeloid cells as the source of modules C and K, and this conclusion is consistent with our earlier studies on PBMC samples from exacerbating children, employing cell sorting in conjunction with RT-qPCR profiling [6].
LPS-driven activation signatures are prominent at the acute exacerbation timepoint, notably LPS appears as a significant driver associated positively with downstream genes in myeloid-associated module C (Fig. 3). Interestingly, no change in circulating LPS levels was observed, thus this does not support the ‘leaky gut’ theory of dysfunctional gut mucosal barrier during respiratory infections leading to circulating LPS. In contrast, we observed an increase in LPS binding protein, which markedly enhances the sensitivity of myeloid cells to LPS [22], detected at the acute timepoint relative to convalescense. Enhanced sensitivity to LPS is likely to modulate the severity of acute asthma exacerbations through exposure to environmental LPS [25] and/or the presence of pathogenic gram negative bacteria in the airway microbiome [26].
This LPS signature is consistent with contributions from secondary bacterial infections as the exacerbation response progresses, as inferred from earlier studies [26,27,28,29], and/or a marker for endogenous TLR signalling [25]. Moreover, McCauley and colleugaues recently demonstrated interactions between viruses and bacteria in children with high-risk for exacerbations, showing that bacterial family members Moraxella and Haemophilus are enriched in nasal specimen of children that test positive for a respiratory virus and progress to experience exacerbations [30]. Furthermore, our group has recently shown that in vitro LPS stimulation of PBMC from children at high-risk of developing asthma are associated with signalling plasticity and immature type 1 interferon signalling gene networks [31], which can be modulated by in vivo treatment of a bacterial lysate mixture that in turn reduces the risk of lower respiratory tract infections [32].
In relation to cell trafficking-associated signatures, we additionally surveyed exacerbation-associated gene expression profiles for the activation status of specific chemokine receptor genes, and it was noteworthy that the receptor gene exhibiting a high level of expression was CCR2, which in other studies has been strongly associated with lung homing [6, 33]. These findings are generally consistent with a model in which signals generated as part of the exacerbation process are sensed by myeloid precursors in bone marrow, thus altering the programming of immature emigrants prior to their release into the blood and subsequent migration to the exacerbation site [34].
The precedent for the operation of such a systemic mechanism during asthma exacerbations are studies in mouse and humans relating to pre-activation of eosinophil precursors in bone marrow following bronchial challenge of sensitised atopics with aeroallergen. In that experimental system, IL-5-secreting aeroallergen-specific Th2 cells are initially activated in the airways and then migrate to bone marrow where they mediate local IL-5-driven pre-activation of eosinophils, which then traffic back to the lung to mediate the eosinophil-driven asthma late phase response [35].
Upregulated cell cycling module F (Fig. 3B) is consistent with the enhanced release into the circulation of immature PBMC subpopulations triggered by this viral-initiated exacerbation event. In this regard, it is notable that at this stage of the exacerbation cycle antiviral signatures are present within the overall coexpression network (within module H in Fig. 3B), but they were not consistently differentially expressed across all participants. The attenuation of the antiviral signatures in a large subset of the participants at this sampling time may reflect the preferential depletion of circulating pDC which are the primary source of type 1 interferons, possibly reflecting the rapid trafficking of these protective cells, which constitute the line-of-defence against viruses, to the infection site [36]. Alternatively, antiviral responses may be deficient in a subset of the children as suggested from previous studies [37,38,39], or the observed heterogeneity may also be due to the time of collection relative to onset of infections, since anti-viral interferon responses peak within 48 h [38].
The most significantly upregulated network modules C and G are regulated by glucocorticoids and growth factors, respectively. The presence of mainly immunoglobulin-related gene signatures in the module G may reflect the transient boosting of IgE production which is a notable component of the exacerbation response [6, 40]. The strong upregulation of glucocorticoid-associated module C is likely a direct result of the high dose steroid treatment (standard dose of 1 mg/kg of child’s weight), given to all participants upon hospital presentation with acute asthma (on average 7.7 h prior to blood collection), potentially with additional effects from the endogenous hormone. Glucocorticoids are known to inhibit pro-inflammatory pathways and adaptive immunity, whilst preserving innate mechanisms in airway epithelial cells [41]. It is noteworthy that this module contained the upregulated C1Q complex (Additional Table 3), which is the first component of the classical complement cascade and the prototypical innate anti-microbial defence mechanism, its functions including promotion of opsonisation/phagocytosis, lysis of bacteria/infected cells, augmentation of antibody responses [42], but also inhibition of Th2-associated airways eosinophilia [43] and IFN mediated signalling in pDC [44].
Of interest in this study was the identification, within the co-expression network, of the immunomodulatory cytokine TGFB1 amongst the most dominant drivers of the myeloid-associated module K (Fig. 3C). TGFB1 is a highly pleiotropic cytokine with myriad functions that are likely to be location specific. There is significant literature associating genetic/epigenetic variations in TGFB1 and its downstream signalling pathway (SMAD3) to asthma [45,46,47] and atopy [48] and heightened susceptibility of allergic disease [49]. Our group has recently demonstrated, utilising a dichotomous system of high versus low risk atopy/non-atopic susceptibility to asthma (BN/PVG strain rats), that co-exposure to aeroallergen (ovalbulmin) and rhinovirus infection (rodent equivalent = attenuated mengovirus), resulted in exaggerated transcriptional profiles in susceptible lungs and regulation of pathways by TGFB1/SMAD3 signal was identified in high risk animals [50]. TGFB1 has also been implicated in tissue remodelling (i.e. fibrosis) of the lung parenchyma and airways in asthmatics [51, 52], and various studies have shown elevated expression of TGFB1 in BAL and biopsies [53, 54], and also in serum from asthmatic participants [55]. In the airways, the main source of TGFB1 is thought to be from eosinophils [56], and depletion of eosinophils in asthmatics, employing anti-IL-5, results in decreased TGFB1, suggesting eosinophils are important contributors to airways remodelling [57]. The presence of TGFB1 as a major upstream driver of the exacerbation-associated networks suggests that it has also been expressed and had its effects on myeloid precursor populations in the bone marrow prior to their release into the blood. Several studies have also shown that TGFB1 potentiates replication of rhinovirus by suppressing key antiviral genes, type I and III interferons and inducing cell cycle arrest [58,59,60]. This is of relevance as rhinovirus infection was confirmed in 78.6% of study participants at the acute event. It has been demonstrated that viral infection in vitro impairs the action of glucocorticoids, independent of virus type [61], which emphasises the need for novel treatment options since the standard treatment for asthma exacerbations involves glucocorticoid therapy [62].
Our study has limitations that should be acknowledged. The gene profiling was based on PBMC, a heterogeneous cell population, and hence differential gene expression signatures observed may be influenced by changes in the cellular composition, although these were controlled for statistically. We cannot discern the transitional stage at which we sampled the PMBC; some PBMC could be primed for migration to the tissues, whilst others may be emerging from the bone marrow, and some PBMC may be recirculating from inflamed tissues and yet other cells could simply remain in circulation. Molecular profiling on sorted cell populations or single cell RNA-Seq would overcome this limitation. In addition, tracing studies labelling bone marrow or PBMC could be carried out in further studies. Furthermore, no control samples from healthy children with or without a viral infection were available. Nevertheless, acute samples were paired with convalescent samples providing internal controls for each participant. Lastly, the study participants were recruited in the Emergency Department with severe illness, providing mechanistic insights into real-world immune activation. However, as a consequence, all of our participants were treated with steroids before blood was collected and therefore, the clinical assessments, cellular immune and molecular responses, cellular immune and molecular responses were performed post-steroid treatment and may have been impacted by steroids. To address this, we evaluated the impact of steroid treatment and found no significant relationship between total serum IgE and atopy with the time since steroid administration. With regards to cellular and molecular responses, whilst time since steroid treatment was positively correlated with the abundance of cDC and monocytes, there was no significant relationship with the LPS activation signature.
In summary, this study demonstrates considerable changes in circulating immune cell subset abundance during an acute asthma exacerbation, which is accompanied by differential gene expression and modulation of signalling networks, some of which are driven by LPS/LBP, glucocorticoids and TGFB1. This study advances our understanding of underlying disease mechanisms in asthma and provides a new approach for identification of targets for mechanistic studies and drug repurposing programs. Moreover, we demonstrate that components of the inflammatory cell activation process, associated with asthma exacerbations, are triggered systemically, prior to their recruitment into the lung, which would enable therapeutic targeting of relevant precursor cell populations systemically as opposed to only after their recruitment to the airways, as a valid approach towards improved asthma control. Importantly, the demonstration of increased systemic LPS binding protein during the acute exacerbation phase, may enhance the sensitivity to LPS and is likely to modulate the severity of acute asthma exacerbations in children.
Data Availability
The datasets supporting the conclusions of this article are available in the Gene Expression Omnibus repository [GSE96530, GSE16032; http://www.ncbi.nlm.nih.gov/geo/]. Supplementary methodology is supplied in the Additional Material.
Abbreviations
- AV:
-
Acute visit
- CV:
-
Convalescent visit
- FDR:
-
False discovery rate
- GC:
-
Glucocorticoids
- IgE:
-
Immunoglobulin E
- LPS:
-
Lipopolysaccharide
- PBMC:
-
Peripheral blood mononuclear cells
- RNA-Seq:
-
RNA Sequencing
- TGFB1:
-
Transforming growth factor beta 1
- WGNCA:
-
Weighted gene network co-expression analysis
References
Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annu Rev Pathol. 2015;10:511–45.
Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17(4):404–13.
Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O’Byrne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106(5 Suppl):242–6.
Martin AC, Laing IA, Khoo SK, Zhang G, Rueter K, Teoh L, et al. Acute asthma in children: Relationships among CD14 and CC16 genotypes, plasma levels, and severity. Am J Respir Crit Care Med. 2006;173(6):617–22.
Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365(9454):142–9.
Subrata LS, Bizzintino J, Mamessier E, Bosco A, McKenna KL, Wikstrom ME, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol. 2009;183(4):2793–800.
Chidlow GR, Harnett GB, Shellam GR, Smith DW. An economical tandem multiplex real-time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses. 2009;1(1):42–56.
Leffler J, Jones AC, Hollams EM, Prastanti F, Le Souef PN, Holt PG, et al. Basophil counts in PBMC populations during childhood acute wheeze/asthma are associated with future exacerbations. J Allergy Clin Immunol. 2018;142(5):1639–41. e5.
Leffler J, Read JF, Jones AC, Mok D, Hollams EM, Laing IA, et al. Progressive increase of FcepsilonRI expression across several PBMC subsets is associated with atopy and atopic asthma within school-aged children. Pediatr Allergy Immunol. 2019;30(6):646–53.
Hahne F, LeMeur N, Brinkman RR, Ellis B, Haaland P, Sarkar D, et al. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinformatics. 2009;10:106.
Chevrier S, Crowell HL, Zanotelli VRT, Engler S, Robinson MD, Bodenmiller B. Compensation of signal spillover in suspension and imaging mass cytometry. Cell Syst. 2018;6(5):612–20. e5.
Weber LM, Nowicka M, Soneson C, Robinson MD. Diffcyt: Differential discovery in high-dimensional cytometry via high-resolution clustering. Commun Biol. 2019;2:183.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8(9):1765–86.
Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32(9):896–902.
Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinf (Oxford England). 2014;30(4):523–30.
Troy NM, Hollams EM, Holt PG, Bosco A. Differential gene network analysis for the identification of asthma-associated therapeutic targets in allergen-specific T-helper memory responses. BMC Med Genomics. 2016;9:9.
Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond-recent updates and continuing curation. Nucleic Acids Res. 2013;41(D1):D1228–D33.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Xi Y, Troy NM, Anderson D, Pena OM, Lynch JP, Phipps S, et al. Critical role of plasmacytoid dendritic cells in regulating gene expression and innate immune responses to human rhinovirus-16. Front Immunol. 2017;8:1351.
Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56.
Eguiluz-Gracia I, Bosco A, Dollner R, Melum GR, Lexberg MH, Jones AC, et al. Rapid recruitment of CD14(+) monocytes in experimentally induced allergic rhinitis in human subjects. J Allergy Clin Immunol. 2016;137(6):1872–81e12.
Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 2019;20(7):802–11.
Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. 2015;125(2):571–82.
Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–15.
Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–95.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5(1):e8578.
Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133(4):1008–13.
McCauley KE, Flynn K, Calatroni A, DiMassa V, LaMere B, Fadrosh DW, et al. Seasonal airway microbiome and transcriptome interactions promote childhood asthma exacerbations. J Allergy Clin Immunol. 2022;150(1):204–13.
Read JF, Serralha M, Mok D, Holt BJ, Cruickshank M, Karpievitch YV, et al. Lipopolysaccharide-induced interferon response networks at birth are predictive of severe viral lower respiratory infections in the first year of life. Front Immunol. 2022;13:876654.
Troy NM, Strickland D, Serralha M, de Jong E, Jones AC, Read J, et al. Protection against severe infant lower respiratory tract infections by immune training: mechanistic studies. J Allergy Clin Immunol. 2022;150(1):93–103.
Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, et al. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181(1):610–20.
Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18(5):726–35.
Gauvreau GM, Denburg JA. Hemopoietic progenitors: the role of eosinophil/basophil progenitors in allergic airway inflammation. Expert Rev Clin Immunol. 2005;1(1):87–101.
Xi Y, Upham JW. Plasmacytoid dendritic cells and asthma: a review of current knowledge. Expert Rev Respir Med. 2020;14(11):1095–106.
Khoo SK, Read J, Franks K, Zhang G, Bizzintino J, Coleman L, et al. Upper Airway Cell Transcriptomics identify a Major New Immunological phenotype with strong clinical Correlates in Young Children with Acute Wheezing. J Immunol. 2019;202(6):1845–58.
Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, et al. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129(6):1506–14. e6.
Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130(2):489–95.
Hales BJ, Martin AC, Pearce LJ, Rueter K, Zhang G, Khoo SK, et al. Anti-bacterial IgE in the antibody responses of house dust mite allergic children convalescent from asthma exacerbation. Clin Exp Allergy. 2009;39(8):1170–8.
Zhang N, Truong-Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol. 2007;179(1):578–89.
Walport MJ, Complement. First of two parts. N Engl J Med. 2001;344(14):1058–66.
Mascarell L, Airouche S, Berjont N, Gary C, Gueguen C, Fourcade G, et al. The regulatory dendritic cell marker C1q is a potent inhibitor of allergic inflammation. Mucosal Immunol. 2017;10(3):695–704.
Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, et al. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60(10):3081–90.
Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21.
DeVries A, Wlasiuk G, Miller SJ, Bosco A, Stern DA, Lohman IC, et al. Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J Allergy Clin Immunol. 2017;140(2):534–42.
Altman MC, Gill MA, Whalen E, Babineau DC, Shao B, Liu AH, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol. 2019;20(5):637–51.
Li H, Romieu I, Wu H, Sienra-Monge JJ, Ramirez-Aguilar M, del Rio-Navarro BE, et al. Genetic polymorphisms in transforming growth factor beta-1 (TGFB1) and childhood asthma and atopy. Hum Genet. 2007;121(5):529–38.
Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5(195):195ra94.
de Jong E, Lauzon-Joset JF, Leffler J, Serralha M, Larcombe AN, Christophersen CT, et al. IRF7-associated immunophenotypes have dichotomous responses to virus/allergen coexposure and OM-85-induced reprogramming. Front Immunol. 2021;12:699633.
Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44(2):127–33.
Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, et al. Integrin alphavbeta5-mediated TGF-beta activation by airway smooth muscle cells in asthma. J Immunol. 2011;187(11):6094–107.
Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):591–9.
Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–86. quiz 87.
Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117(1):111–8.
Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25(9):477–82.
Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–36.
Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–47.
Thomas BJ, Lindsay M, Dagher H, Freezer NJ, Li D, Ghildyal R, et al. Transforming growth factor-beta enhances rhinovirus infection by diminishing early innate responses. Am J Respir Cell Mol Biol. 2009;41(3):339–47.
Bedke N, Sammut D, Green B, Kehagia V, Dennison P, Jenkins G, et al. Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PLoS ONE. 2012;7(9):e44580.
Xia YC, Radwan A, Keenan CR, Langenbach SY, Li M, Radojicic D, et al. Glucocorticoid insensitivity in virally infected airway epithelial cells is dependent on transforming growth factor-beta activity. PLoS Pathog. 2017;13(1):e1006138.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention., 2021. Available from: www.ginasthma.org.
Acknowledgements
Special thanks to Kristina Rueter, Jack Goldblatt and Gary Geelhoed for their role in the recruitment and follow-up of the study participants.
Funding
The study was funded by the National Health and Medical Research Council (NHMRC).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: AB, DHS, PGH; Acquisition of data: ACJ, IAL, JB, SKK; Data analysis and interpretation: ACJ, JL, IAL, SKK, PNL, PDS, PGH, DHS, AB; Drafting the manuscript for important intellectual content: ACJ, JL, IAL, PGH, DHS, AB; all authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study and protocol were approved by the Child and Adolescent Health Service Human Research Ethics Committee (CAHS HECS, Approval no: RGS 2369) at Perth Children’s Hospital with written informed consent from at least one parent or guardian. Parents/guardians gave written informed consent in accordance with the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
AB is a co-inventor on a patent application that is related to this work. AB is a co-founder, equity holder, and director of the startup company Respiradigm Pty Ltd and subsidiary First Breath Health Pty Ltd that is related to this work. AB is the founder of the startup company INSiGENe Pty Ltd that is unrelated to this work. All remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
: Extended Methods
Additional file 2: Fig. 1
. Exemplary gating strategy for all subsets analysed. Example gating strategy from acute peripheral blood mononuclear cells labelled with a panel of antibodies to identify lymphoid and myeloid cells subsets
Additional file 3: Fig. 2
. Correlation with Atopy readouts and systemic glucocorticoid treatment. Total serum IgE and SPT cumulative wheal size did not corelate with time since administration of systemic glucocorticoids
Additional file 4: Fig. 3
. Inflammatory cells are trafficking and leaving the peripheral blood. Peripheral blood mononuclear cells (PBMC) were sampled from atopic asthmatics at presentation to hospital Emergency during an exacerbation, the acute visit (AV), and following recovery at the convalescent visit (CV). Multi-colour flow cytometry was employed to quantify inflammatory cell subsets, in (A) ratio of acute: convalescent (cells per ml blood), data are represented as mean ± SEM, and (B) cellular frequency (percentage) of PBMC. The P-values are derived from a Wilcoxon test for paired analysis. ****,<0.0001, ***,<0.001, **,<0.01, *,<0.05
Additional file 5: Fig. 4
. Correlation with cell subset abundance during the acute event and systemic glucocorticoid treatment. Abundance of immune cell subsets correlated with time since administration of systemic glucocorticoids. Correlation was assessed using Pearson’s parametric correlation on samples for which data on steroid treatment was available, N = 18, **, p < 0.01, *, p < 0.05
Additional file 6: Fig. 5
. Plasma levels measured of soluble CD14 (sCD14), neopterin, fibronectin 1 (FN1) and S100A8/S19
Additional file 7: Fig. 6
. Correlation of LPS and steroid module eigengene signatures from the upstream regulator analysis during the acute event and time since glucocorticoid treatment. Time since steroid administration does not correlate with either the DEX/FT or the LPS signature. Correlation was assessed using Pearson’s parametric correlation, n.s. = not significant
Additional file 8: Fig. 7
. Reconstruction of the wiring diagram of modules C, F, G and K. Coloured node = present in the original network (zero order network), white node = transcription factors
Additional file 9: Fig. 8
. Identification of exacerbation-associated modules with or without adjustment for cellular composition. The data was analysed with DESeq2/RUVSeq and adjusted for cellular composition, in (a) unadjusted networks, (b) adjustment for proportions of monocytes, (c) adjustment for proportions of B cells, and (d) adjustment for proportions of T cells. The dashed horizontal line indicates an adjusted p-value < 0.05. **median adjusted P-value < 0.01, *median adjusted P-value < 0.05, ns = not significant
Additional file 10: Table 1
. Multi-parameter flow cytometry panel. Table 2. Differentially expressed genes comparing acute versus convalescent visits. Table 3. Pathways analysis for modules A-K
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jones, A.C., Leffler, J., Laing, I.A. et al. LPS binding protein and activation signatures are upregulated during asthma exacerbations in children. Respir Res 24, 184 (2023). https://doi.org/10.1186/s12931-023-02478-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-023-02478-3