Abstract
Ischemia with non-obstructive coronary artery (INOCA) is a common cause of hospital admissions, leading to negative outcomes and reduced quality of life. Central to its pathophysiology is endothelial dysfunction, which contributes to myocardial ischemia despite the absence of significant coronary artery blockage. Addressing endothelial dysfunction is essential in managing INOCA to alleviate symptoms and prevent cardiovascular events. Recent studies have identified diabetes mellitus (DM) as a significant factor exacerbating INOCA complications by promoting endothelial impairment and coronary microvascular dysfunction. MicroRNAs (miRNAs) have emerged as potential biomarkers and therapeutic targets in various biological processes, including endothelial dysfunction and cardiovascular diseases. However, research on miRNA biomarkers in INOCA patients is sparse. In this study, we examined a panel of circulating miRNAs involved in the regulation of endothelial function in INOCA patients with and without DM. We analyzed miRNA expression using RT-qPCR in a cohort of consecutive INOCA patients undergoing percutaneous coronary intervention. We detected a significant dysregulation of miR-363-5p and miR-92a-3p in INOCA patients with DM compared to those without DM, indicating their role as biomarkers for predicting and monitoring endothelial dysfunction in INOCA patients with DM.
Similar content being viewed by others
Background
Ischemia with no-obstructive coronary artery (INOCA) is a leading cause of hospitalizations, driving adverse outcomes and reducing quality of life [1,2,3,4,5,6]. Endothelial dysfunction plays a crucial role in INOCA; indeed, despite the absence of significant coronary artery obstruction, in this pathologic condition endothelial cells fail to function optimally [7,8,9,10]. Such dysfunction contributes to the mismatch between myocardial oxygen supply and demand, leading to ischemia and symptoms like chest pain or discomfort. In particular, coronary microvascular dysfunction might be playing a crucial role in the onset of adverse events in INOCA subjects [11,12,13,14]. Understanding and addressing endothelial dysfunction is essential in managing patients with INOCA to improve symptoms and prevent adverse cardiovascular events [3, 4]. To counteract INOCA complications, our group has recently demonstrated that hyperglycemia is one of the mechanisms involved in its pathophysiology [15]. Diabetes mellitus (DM) and hyperglycemia are known to drive endothelial impairment and coronary microvascular dysfunction [16,17,18].
MicroRNAs (miRNAs, miRs) are short non-coding RNAs that post-transcriptionally regulate gene expression by binding to the 3′ untranslated region of target messenger RNAs (mRNAs), leading to its degradation or translational repression [19, 20]. It is currently accepted that miRs exert their activity in many biological processes and, as such, have been proposed as biomarkers and potential targets of novel therapeutic strategies [21, 22]; Moreover, miRs have been linked to endothelial dysfunction and cardiovascular diseases [23,24,25,26,27] and may be also useful to monitor the evolution of cardiovascular diseases and atherosclerosis [28,29,30].
However, currently there are no established biomarkers of endothelial dysfunction in INOCA patients. Hence, in our study, we monitored the expression of a panel of circulating miRs involved in the regulation of endothelial function in a population of individuals with a confirmed diagnosis of INOCA comparing patients with or without DM.
Methods
We evaluated consecutive INOCA patients referred to the Casa di Cura “Montevergine”, Mercogliano (Avellino) and ASL Naples, both in Italy, for percutaneous coronary intervention (PCI). We defined DM according to the American Diabetes Association (ADA) guidelines [31]. Consistent with previous investigations [3, 4, 32], we defined INOCA as:
-
Symptoms of myocardial ischemia;
-
Non-obstructive coronary artery stenosis defined as: <50% diameter reduction and/or fractional flow reserve > 0.80;
-
Objective evidence of myocardial ischemia;
-
Impaired coronary microvascular function defined as: impaired coronary flow reserve (≤ 2.0), abnormal coronary microvascular resistance indices, coronary microvascular spasm, endothelial dysfunction with ≥ 20% luminal constriction during acetylcholine infusion, and/or coronary slow flow phenomenon.
The study was designed and conducted according to the principles outlined in the Declaration of Helsinki.
Circulating miRs were isolated from plasma samples, obtained using EDTA-containing tubes. We extracted miRs using the miRNeasy Serum/Plasma kit (Qiagen, Hilden, Germany) according to the protocol provided by the manufacturer. RNA purity and concentration were evaluated by spectrophotometry using a NanoDrop ND-2000 (ThermoFisher, Waltham, MA); reverse transcription was performed using the miRCURY LNA Universal RT miR PCR kit (Qiagen); miRs expression was analyzed by RT-qPCR, as we described [27]. The quality of miRs was determined using the Agilent Small RNA Kit [33]. We analyzed a panel of miRs that we have previously demonstrated to be involved in the regulation of endothelial dysfunction [26]; miR expression was analyzed by quantitative real-time polymerase chain reaction (RT-qPCR), as described [26]. The RNA Spike-in kit (Qiagen) was used as an exogenous control of RNA extraction following the manufacturer’s instructions. For quality control, we used three synthetic RNA spike-ins (UniSp2, UniSp4, and UniSp5) at different concentrations; as a control for cDNA synthesis, we used UniSp6 spike-in and cel-miR-39-3p.
Relative gene expression was determined using the 2-ΔΔCT method [26]. To normalize, we first selected the miRs that displayed the least variability in their cycle threshold (Ct) values in all samples using the geNorm and NormFinder bioinformatic algorithms [34, 35], which revealed that the most stable (less variable when comparing the two groups) miRs were miR-125a-5p and miR-19a-5p. Then, we used the BestKeeper method [36] to calculate the geometric mean of the pair-wise Ct values (Ct values of miR-125a-5p and miR-19-5p); the relative expression data were calculated with the Ct of each target miR with reference to the BestKeeper value. To confirm the absence of hemolysis in our samples, we assessed the presence of cell free hemoglobin at the spectrophotometer measuring absorbance at 414 nm (A414). Since dyslipidemia could interfere with this kind of evaluation, we also measured the delta quantification cycle (ΔCq) of known blood cell-associated miRs (miR-16-5p and miR-451a) and a control miR (miR-23a-3p) that is known to be invariant in plasma affected by hemolysis.
Statistical analysis
All data were analyzed using the GraphPad Prism software v. 9.0 (Dotmatics, Boston, MA, USA). Data are expressed as means ± SD or as numbers and percentages. The differences in miR levels were analyzed using two-tailed t-tests as appropriate after having verified the normal distribution of values via Kolmogorov–Smirnov test.
Results
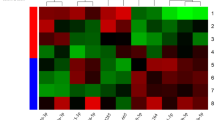
42 patients agreed to enter our study (28 without DM, 14 with DM). Clinical characteristics are shown in Table 1. We measured the expression levels of the panel of miRs that we had previously validated [26] and in INOCA patients with DM (compared to patients without DM) we detected an increased expression of several miRs that have been previously associated with endothelial dysfunction, and a reduced expression of miRs that have been shown to be protective in terms of endothelial function (see heat map in Fig. 1).
Then, we analyzed which of these miRs was significantly upregulated or downregulated when comparing INOCA patients with DM to INOCA patients without DM. We found that miR-363-5p was significantly downregulated (P < 0.001), whereas miR-92 was significantly upregulated (P < 0.001), in DM subjects, as shown in the volcano plot depicted in Fig. 2.
Discussion
In the present study, we have identified, for the first time to our knowledge, circulating miRs involved in endothelial dysfunction that could be useful for monitoring INOCA patients with DM.
Endothelial dysfunction is a hallmark of diabetic vascular complications. In DM, dysregulated miR expression contributes to endothelial dysfunction through various mechanisms, including the modulation of pathways involved in endothelial fitness, inflammation, oxidative stress, and angiogenesis. For example, miR-126, miR-155, and miR-21 have been implicated in regulating endothelial cell function and angiogenesis by targeting genes involved in endothelial nitric oxide synthase (eNOS) signaling, inflammatory pathways, and vascular endothelial growth factor (VEGF) signaling [37].
Furthermore, miRs can influence endothelial integrity by targeting genes involved in oxidative stress and inflammation [38, 39], both of which are key drivers of endothelial dysfunction in DM. Emerging evidence suggests that circulating miRs may serve as biomarkers for endothelial dysfunction and diabetic vascular complications; in fact, several studies have identified dysregulated miR expression profiles in the circulation of diabetic patients with endothelial dysfunction [40,41,42], thereby providing insights into the pathogenesis and progression of diabetic vascular complications.
The identification of two miRs, namely miR-92 and miR-363-5p, that are differently expressed in INOCA patients with and without DM, is fully consistent with previous reports. Endothelial-derived extracellular miR-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells and reduces oxidative stress [43, 44]. On the other hand, miR-363-5p reduction results in a significant decrease in endothelial cell tube formation [45]. Henceforth, the aforementioned miRs may be useful markers in the management of INOCA patients, with or without diabetes. Emphasizing the novelty of our work, we did not find any other study investigating miRs in INOCA patients.
The relationship between DM, endothelial dysfunction, and the pathogenesis of INOCA is complex and multifaceted. Indeed, endothelial dysfunction, characterized by impaired endothelium-dependent vasodilation and pro-inflammatory and pro-thrombotic states, is a common feature of both DM and INOCA [46, 47]. In DM, chronic hyperglycemia, insulin resistance, and dyslipidemia further contribute to endothelial dysfunction through various mechanisms, including increased oxidative stress, inflammation, and activation of the renin-angiotensin-aldosterone system [48, 49]. Of note, coronary slow flow (CSF), assessed by invasive coronary angiography has been frequently seen as a potential indicator of coronary microvascular dysfunction in INOCA patients [50,51,52]; however, a recent prospective study has demonstrated that CSF is not a reliable angiographic surrogate of abnormal coronary flow reserve (CFR) or index of microcirculatory resistance (IMR) [53].
Endothelial dysfunction and DM play central roles in the pathophysiology of INOCA by promoting coronary microvascular dysfunction, characterized by impaired vasodilation, increased vasoconstriction, and altered microvascular structure [46, 54, 55]. Dysfunction of the coronary microvasculature results in inadequate myocardial perfusion despite the absence of significant obstructive coronary artery disease, leading to angina-like symptoms and ischemia [56, 57]. Additionally, impaired endothelial function in DM is associated with enhanced endothelial permeability and increased vascular inflammation, which may contribute to myocardial injury and fibrosis, further compromising myocardial perfusion and function in INOCA [58, 59]. DM-related endothelial dysfunction may also exacerbate, and interact with, other risk factors for INOCA, such as hypertension, obesity, and dyslipidemia [60], further aggravating coronary microvascular dysfunction and ischemia. Again, we are the first group to highlight the importance of miR profiles in INOCA.
Nevertheless, our work is not exempt from limitations, including the small size of our population and having limited the panel of miRs to the ones that were implied in endothelial dysfunction. Equally important, further research is needed to elucidate the specific mechanisms underlying the exact role of the miRs that we have demonstrated to be differently expressed in DM and non-DM INOCA patients.
Conclusions
Understanding the role of miRs in endothelial dysfunction may provide novel insights into the pathogenesis of diabetic vascular complications and offer potential therapeutic targets for intervention in INOCA and other diabetic vascular complications.
Data availability
The data that support the findings of this study are available from the last author upon reasonable request.
References
Al-Mallah MH, Nayfeh M, Alrifai M. The role of cardiac PET in diagnosis and prognosis of patients with ischemia with no obstructive coronary arteries (INOCA). Am Heart J Plus. 2024;43:100399.
Zhang W, Liu L, Yin G, Mohammed AQ, Xiang L, Lv X, Shi T, Galip J, Wang C, Mohammed AA, et al. Triglyceride-glucose index is associated with myocardial ischemia and poor prognosis in patients with ischemia and no obstructive coronary artery disease. Cardiovasc Diabetol. 2024;23(1):187.
Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ. 2021;375:e060602.
Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El-Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA study. Circulation. 2021;144(13):1008–23.
AlShaikh S, Rohm CL, Sutton NR, Burgess SN, Alasnag M. INOCA: Ischemia in non-obstructive coronary arteries. Am Heart J Plus. 2024;42:100391.
McChord J, Ong P. Advancing precision medicine in INOCA research: the INOCA-IT registry. Int J Cardiol. 2024;404:131975.
Shimokawa H. Roles of endothelial and smooth muscle cell dysfunction and vasa vasorum in vasomotor disorders in ischemia with no obstructive coronary artery disease. Vascul Pharmacol. 2023;153:107234.
Almeida AG. MINOCA and INOCA: Role in Heart failure. Curr Heart Fail Rep. 2023;20(3):139–50.
Rehan R, Yong A, Ng M, Weaver J, Puranik R. Coronary microvascular dysfunction: a review of recent progress and clinical implications. Front Cardiovasc Med. 2023;10:1111721.
Avtaar Singh SS, Nappi F. Pathophysiology and outcomes of endothelium function in Coronary Microvascular diseases: a systematic review of Randomized controlled trials and Multicenter Study. Biomedicines 2022, 10(12).
Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary microvascular dysfunction across the Spectrum of Cardiovascular diseases: JACC State-of-the-art review. J Am Coll Cardiol. 2021;78(13):1352–71.
Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–70.
Kong H, Cao J, Tian J, Yong J, An J, Song X, He Y. Relationship between coronary microvascular dysfunction (CMD) and left ventricular diastolic function in patients with symptoms of myocardial ischemia with non-obstructive coronary artery disease (INOCA) by cardiovascular magnetic resonance feature-tracking. Clin Radiol. 2024;79(7):536–43.
Mehta PK, Huang J, Levit RD, Malas W, Waheed N, Bairey Merz CN. Ischemia and no obstructive coronary arteries (INOCA): a narrative review. Atherosclerosis. 2022;363:8–21.
Mone P, Lombardi A, Salemme L, Cioppa A, Popusoi G, Varzideh F, Pansini A, Jankauskas SS, Forzano I, Avvisato R, et al. Stress hyperglycemia drives the risk of hospitalization for chest Pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diabetes Care. 2023;46(2):450–4.
Mone P, De Gennaro S, Frullone S, Marro A, Santulli G. Hyperglycemia drives the transition from pre-frailty to frailty: the monteforte study. Eur J Intern Med. 2023;111:135–7.
Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41(8):1387–93.
Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29(9):2064–71.
Santulli G. microRNAs distinctively regulate vascular smooth muscle and endothelial cells: functional implications in Angiogenesis, atherosclerosis, and In-Stent restenosis. Adv Exp Med Biol. 2015;887:53–77.
Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G. Functional role of microRNAs in regulating Cardiomyocyte Death. Cells 2022;11(6):983.
Varzideh F, Kansakar U, Donkor K, Wilson S, Jankauskas SS, Mone P, Wang X, Lombardi A, Santulli G. Cardiac remodeling after myocardial infarction: functional contribution of microRNAs to inflammation and fibrosis. Front Cardiovasc Med. 2022;9:863238.
Avvisato R, Mone P, Jankauskas SS, Varzideh F, Kansakar U, Gambardella J, De Luca A, Matarese A, Santulli G. miR-4432 targets FGFBP1 in human endothelial cells. Biology (Basel) 2023;12(3):459
Santulli G, Wronska A, Uryu K, Diacovo TG, Gao M, Marx SO, Kitajewski J, Chilton JM, Akat KM, Tuschl T, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest. 2014;124(9):4102–14.
Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117(10):870–83.
Wang C, Liu C, Shi J, Li H, Jiang S, Zhao P, Zhang M, Du G, Fu S, Li S, et al. Nicotine exacerbates endothelial dysfunction and drives atherosclerosis via extracellular vesicle-miRNA. Cardiovasc Res. 2023;119(3):729–42.
Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, Marzocco S, De Gennaro S, Famiglietti M, Macina G, et al. Empagliflozin improves the MicroRNA signature of endothelial dysfunction in patients with heart failure with preserved ejection fraction and diabetes. J Pharmacol Exp Ther. 2023;384(1):116–22.
Jankauskas SS, Mone P, Avvisato R, Varzideh F, De Gennaro S, Salemme L, Macina G, Kansakar U, Cioppa A, Frullone S, et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech Ageing Dev. 2023;212:111818.
Napoli C, Schiano C, Soricelli A. Increasing evidence of pathogenic role of the Mediator (MED) complex in the development of cardiovascular diseases. Biochimie. 2019;165:1–8.
Mansueto G, Benincasa G, Della Mura N, Nicoletti GF, Napoli C. Epigenetic-sensitive liquid biomarkers and personalised therapy in advanced heart failure: a focus on cell-free DNA and microRNAs. J Clin Pathol. 2020;73(9):535–43.
Napoli C, Benincasa G, Schiano C, Salvatore M. Differential epigenetic factors in the prediction of cardiovascular risk in diabetic patients. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):239–47.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. 6. Glycemic targets: standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97–110.
Bonanni A, d’Aiello A, Pedicino D, Di Sario M, Vinci R, Ponzo M, Ciampi P, Lo Curto D, Conte C, Cribari F et al. Molecular Hallmarks of Ischemia with non-obstructive coronary arteries: the INOCA versus obstructive CCS challenge. J Clin Med 2022;11(6):1711
Kansakar U, Gambardella J, Varzideh F, Avvisato R, Jankauskas SS, Mone P, Matarese A, Santulli G. miR-142 targets TIM-1 in human endothelial cells: potential implications for stroke, COVID-19, Zika, Ebola, Dengue, and other viral infections. Int J Mol Sci 2022;23(18):10242
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034.
Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–50.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–15.
Santulli G. MicroRNAs and endothelial (dys) function. J Cell Physiol. 2016;231(8):1638–44.
Minjares M, Wu W, Wang JM. Oxidative stress and MicroRNAs in endothelial cells under metabolic disorders. Cells 2023;12(9):1341
Medina-Leyte DJ, Zepeda-Garcia O, Dominguez-Perez M, Gonzalez-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci 2021, 22(8).
Nemecz M, Stefan DS, Comarita IK, Constantin A, Tanko G, Guja C, Georgescu A. Microvesicle-associated and circulating microRNAs in diabetic dyslipidemia: miR-218, miR-132, miR-143, and miR-21, miR-122, miR-155 have biomarker potential. Cardiovasc Diabetol. 2023;22(1):260.
Silambarasan M, Tan JR, Karolina DS, Armugam A, Kaur C, Jeyaseelan K. MicroRNAs in Hyperglycemia Induced endothelial cell dysfunction. Int J Mol Sci. 2016;17(4):518.
Satake E, Pezzolesi MG, Md Dom ZI, Smiles AM, Niewczas MA, Krolewski AS. Circulating miRNA profiles Associated with hyperglycemia in patients with type 1 diabetes. Diabetes. 2018;67(5):1013–23.
Kriegel AJ, Baker MA, Liu Y, Liu P, Cowley AW Jr., Liang M. Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension. 2015;66(4):793–9.
Wang C, Wu H, Xing Y, Ye Y, He F, Yin Q, Li Y, Shang F, Shyy JY, Yuan ZY. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci Rep. 2022;12(1):344.
Costa A, Afonso J, Osorio C, Gomes AL, Caiado F, Valente J, Aguiar SI, Pinto F, Ramirez M, Dias S. Mir-363-5p regulates endothelial cell properties and their communication with hematopoietic precursor cells. J Hematol Oncol. 2013;6(1):87.
Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, Catalini C, Gjeloshi K, Albanese G, Di Martino A et al. Coronary microvascular dysfunction in diabetes Mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines 2022;10(9):2274
Gunawardena T, Merinopoulos I, Wickramarachchi U, Vassiliou V, Eccleshall S. Endothelial dysfunction and coronary vasoreactivity - A review of the history, physiology, diagnostic techniques, and clinical relevance. Curr Cardiol Rev. 2021;17(1):85–100.
Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008;37(3):685–711, ix-x.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
Shereef AS, Abdelmajeed MG, Alshair MH, El D, Khalil II, Wageeh WA, Shehata S. Coronary slow flow and its correlation with reduced left ventricle global longitudinal strain: a case-control study. Echo Res Pract. 2024;11(1):2.
Chakrala T, Prakash R, Valdes C, Pepine CJ, Keeley EC. Circulating biomarkers in coronary microvascular dysfunction. J Am Heart Assoc. 2023;12(12):e029341.
Ashokprabhu ND, Quesada O, Alvarez YR, Henry TD. INOCA/ANOCA: mechanisms and novel treatments. Am Heart J Plus 2023;30:100302
Harkin KL, Loftspring E, Beaty W, Joa A, Serrano-Gomez C, Farid A, Hausvater A, Reynolds HR, Smilowitz NR. Visual estimates of coronary slow Flow Are Not Associated with Invasive Wire-based diagnoses of coronary microvascular dysfunction. Circ Cardiovasc Interv; 2024;17:e013902
Godo S, Suda A, Takahashi J, Yasuda S, Shimokawa H. Coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol. 2021;41(5):1625–37.
Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, Huang L, Liu Y. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8(1):152.
Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med 2020;9(9):2880
Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, et al. Coronary microvascular dysfunction is Associated with myocardial ischemia and abnormal coronary perfusion during Exercise. Circulation. 2019;140(22):1805–16.
Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216–31.
Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3(6):853–76.
Sarebanhassanabadi M, Mirjalili SR, Marques-Vidal P, Kraemer A, Namayandeh SM. Coronary artery disease incidence, risk factors, awareness, and medication utilization in a 10-year cohort study. BMC Cardiovasc Disord. 2024;24(1):101.
Acknowledgements
This work was funded by the SOLOMAX SociaLNetwOrk of MedicAlEXperiences Prog. N. F/260007/05/X51?CUP: B49J21031700008 and Innovation Agreement D.M. 02.8.2019 Life Sciences. MISE.
Funding
The Santulli’s Laboratory is supported in part by the National Heart, Lung, and Blood Institute (NHLBI: R01-HL146691, T32-HL144456) and National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823). This publication was produced with the co-funding European Union—Next Generation EU, in the context of The National Recovery and Resilience Plan, Investment Partenariato Esteso PE8 “Conseguenze e sfide dell’invecchiamento”, Project Age-It (Ageing Well in an Ageing Society).
Author information
Authors and Affiliations
Contributions
M.F., G.G., F.A.C., A.B., A.F., M.E., R.R., A.R. F.V., U.K., C.D.G., F.M., T.T., E.V., G.I., and P.M data analysis and enrollment; M.F., J.G., M.C., V.V., G.S., P.M wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Ethical Committee Campania Nord approved the protocol.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ferrone, M., Ciccarelli, M., Varzideh, F. et al. Endothelial microRNAs in INOCA patients with diabetes mellitus. Cardiovasc Diabetol 23, 268 (2024). https://doi.org/10.1186/s12933-024-02331-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02331-x