Abstract
Background
The adverse prognostic impact of diabetes on hypertrophic cardiomyopathy (HCM) is poorly understood. We sought to explore the underlying mechanisms in terms of structural and functional remodelling in HCM patients with coexisting diabetes (HCM-DM).
Methods
A total of 45 HCM-DM patients were retrospectively included. Isolated HCM controls (HCM patients without diabetes) were matched to HCM-DM patients in terms of maximal wall thickness, age, and gender distribution. Left ventricular (LV) and atrial (LA) performance were evaluated using cardiac magnetic resonance feature tracking strain analyses. The associations between diabetes and LV/LA impairment were investigated by univariable and multivariable linear regression.
Results
Compared with the isolated HCM controls, the HCM-DM patients had smaller end-diastolic volume and stroke volume, lower ejection fraction, larger mass/volume ratio and impaired strains in all three directions (all P < 0.05). In terms of the LA parameters, HCM-DM patients presented impaired LA reservoir and conduit strain/strain rate (all P < 0.05). Among all HCM patients, comorbidity with diabetes was independently associated with a low LV ejection fraction (β = − 6.05, P < 0.001) and impaired global longitudinal strain (β = 1.40, P = 0.007). Moreover, compared with the isolated HCM controls, HCM-DM patients presented with more myocardial fibrosis according to late gadolinium enhancement, which was an independent predictor of impaired LV global radial strain (β = − 45.81, P = 0.008), LV global circumferential strain (β = 18.25, P = 0.003), LA reservoir strain (β = − 59.20, P < 0.001) and strain rate (β = − 2.90, P = 0.002).
Conclusions
Diabetes has adverse effects on LV and LA function in HCM patients, which may be important contributors to severe manifestations and outcomes in those patients. The present study strengthened the evidence of the prevention and management of diabetes in HCM patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Hypertrophic cardiomyopathy (HCM) was once considered a malignant disease with limited effective treatment options but is now considered a relatively common contemporary and highly treatable disease with a normal life expectancy benefiting from substantially evolved understanding and management of the disease, particularly in the last two decades [1, 2]. As the clinical course of HCM extended, ageing-related cardiovascular risks are increasing. Comorbidities such as type 2 diabetes mellitus (T2DM) can worsen the clinical features of HCM. Previous studies have shown that HCM patients with T2DM (HCM-DM) had a higher New York Heart Association class, a higher prevalence of comorbidities, and a higher risk of adverse events including end-stage renal disease progression, stroke, heart failure and cardiovascular death [3,4,5,6]. T2DM is associated with left ventricular (LV) hypertrophy, diastolic dysfunction as well as left atrial (LA) enlargement, and overt systolic impairment can occur in advanced stages [7,8,9,10,11,12]. However, the mechanism for the adverse prognostic impact of T2DM on HCM remains incompletely understood.
Cardiac magnetic resonance (CMR) is an established, noninvasive and comprehensive imaging strategy used for the evaluation and follow-up of patients with HCM [13]. CMR feature tracking, an emerging method for strain analysis, has been widely applied to evaluate myocardial deformation in all cardiac chambers including the relatively thin-walled atrium [14]. Compared with conventional functional analysis, strain analysis can provide incremental information for clinical management by detecting early dysfunction and subtle changes in disease progression. We supposed that diabetes-related adverse cardiac alterations may worsen the clinical manifestations of HCM, but few studies have focused on the effects of T2DM on cardiac remodelling in patients with HCM thus far. Therefore, the present study aimed to determine the impact of T2DM on LV and LA function in patients with HCM using CMR feature tracking.
Methods
Study population
A total of 45 consecutive HCM-DM patients who underwent CMR evaluation between January 2012 and December 2022 were retrospectively included. Isolated HCM controls (HCM patients without diabetes) were matched to HCM-DM patients according to maximal wall thickness, age, and gender distribution. The diagnosis of HCM was based on the presence of unexplained LV hypertrophy on CMR (a maximal end-diastolic wall thickness ≥ 15 mm, or ≥ 13 mm with a family history of HCM or with a positive genetic test) [15]. The diagnosis of T2DM was based on the clinical chart at the time of CMR examination in accordance with the 2019 European Society of Cardiology guidelines [16]. The exclusion criteria were as follows: (1) a history of myocardial infarction on medical documentation, or significant coronary arterial stenosis (≥ 50%) on invasive coronary angiography or coronary computed tomography angiography; (2) history of septal reduction therapy; (3) concomitant uncontrolled hypertension; (4) congenital heart diseases; (5) infiltrative cardiomyopathies; (6) severe valvular heart diseases; (7) persistent atrial fibrillation; and (8) uninterpretable CMR images. The study was approved by the Biomedical Research Ethics Committee of the local hospital, and the requirement for informed consent was waived because of the retrospective design.
CMR examination and analysis
CMR examinations were performed with 3.0 T scanners (Magnetom Skyra or Tim Trio; Siemens, Erlangen, Germany) with 32-channel phased array coils, using electrocardiographic and respiratory gating. A standardized protocol was used as previous described [17], mainly including balanced steady-state free precession sequences for cine images (temporal resolution = 37–42 ms, echo time = 1.2 ms) and segmented phase-sensitive inversion recovery sequences for late gadolinium enhancement (LGE) images acquired in short-axis views and three LV long-axis views 10–15 min after gadolinium-based contrast agent injection (inversion time = 330–380 ms).
The structural and functional parameters of the LA and LV were assessed by using the commercial software CVI42 (Circle Cardiovascular Imaging, Calgary, Canada). The LV volumes and ejection fraction (LVEF) were quantified on short-axis cine stacks by manually outlining the endocardial and epicardial contours at end diastole and end systole. Papillary muscles and trabeculae were assigned to the blood pool. LGE was measured by manually drawing the endocardial and epicardial contours on the short-axis view and selecting a region of interest in the normal (dark) zone to define the reference signal intensity. The myocardium with a signal intensity ≥ 6 standard deviations above the mean of the reference region was identified as enhanced myocardium. The extent of LGE was recorded as a percentage of the total LV mass.
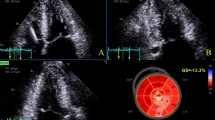
Feature tracking strain analysis was conducted on cine images for LA and LV (Fig. 1). After manually delineating the contours at end diastole, the automatically tracked borders of the myocardium in each phase were carefully checked and adjusted if necessary. The LV peak strains in three directions were derived from three long-axis cines and short-axis cines, including the global radial strain (GRS), global circumferential strain (GCS), and global longitudinal strain (GLS). The LA parameters were analysed on LV 2-chamber and 4-chamber views with pulmonary veins and atrial appendage excluded, including phasic volume, longitudinal strain and strain rate measurements: maximal volume (Vmax), pre-atrial contractile volume (Vpac), minimal volume (Vmin), reservoir strain (εs), conduit strain (εe), booster‒pump strain (εa), peak positive strain rate (SRs), peak early negative strain rate (SRe), and peak late negative strain rate (SRa). The phasic LA EF parameters were computed as follows: LA total EF = (LA Vmax − LA Vmin)/LA Vmax; LA passive EF = (LA Vmax − LA Vpac)/LA Vmax; LA booster EF = (LA Vpac − LA Vmin)/LA Vpac [18, 19].
Measurements of left ventricular and atrial strain. LV left ventricular, GRS global radial strain, GCS global circumferential strain, GLS global longitudinal strain, LA left atrial, εs reservoir strain, εe conduit strain, εa booster-pump strain, SRs peak positive strain rate, SRe peak early negative strain rate, SRa peak late negative strain rate
Inter- and intraobserver variability in strain parameters were independently assessed in 12 randomly selected patients by 2 radiologists with 6 and 10 years of CMR experience (S.Y and K.S), respectively. One of the radiologists repeated the analysis 2 months later to determine the intraobserver variability.
Statistical analysis
Statistical analyses were performed using MedCalc 16.8.4 (Ostend, Belgium). The normality of continuous variables was tested using the Shapiro-Wilk test, histograms, and Q–Q plots. Continuous data are expressed as means ± standard deviations or medians with interquartile ranges (IQRs) appropriately, and differences between HCM patients and HCM-DM patients were compared using the t-test or Mann–Whitney U test. Categorical variables are presented as numbers with percentages, and datasets were compared using the chi-square test or Fisher’s exact test. Univariable and multivariable linear regression analyses (stepwise methods) were performed to determine the associations of variables with LA/LV dysfunction. Only variables with two-sided P values < 0.05 according to the univariate analysis were included in the multivariate analysis. The intraclass correlation coefficient was used to evaluate the inter- and intraobserver variability. A two-tailed P value < 0.05 was considered statistically significant.
Results
Study population
The baseline demographic and clinical characteristics are summarized for each group and compared in Table 1. HCM patients with T2DM presented with higher body mass index, cardiac troponin T (cTnT) levels and blood glucose levels than those without T2DM did (P = 0.02, P = 0.01 and P < 0.001, respectively). Most HCM-DM patients (89%, 40 of 45) were treated with non-insulin medications, mainly metformin (56%, 25 of 45), and only 11% (5 of 45) of the patients were treated with insulin.
LV and LA CMR parameters
In terms of LV parameters, the patients with T2DM had smaller end-diastolic volume (132.2 ± 26.9 ml vs. 142.5 ± 21.4 ml) and stroke volume (83.1 ± 17.7 ml vs. 98.4 ± 15.0 ml), lower ejection fraction (63.2% ± 7.2 vs. 69.2% ± 4.9), and larger mass/volume ratio (MVR; 0.9 ± 0.3 vs. 0.8 ± 0.2) than those without T2DM (all P < 0.05; Table 2). Compared with patients without T2DM, those with T2DM had worse LV strain parameters in all three directions (GRS: 26.7% ± 9.0 vs. 33.3% ± 8.9; GCS: − 16.0% ± 3.8 vs. − 18.5% ± 3.1; GLS: − 10.6% ± 3.0 vs. − 12.8% ± 2.8; all P < 0.001). In addition, the extent of LGE was greater in HCM-DM patients than in isolated HCM patients (2.5% [IQR, 1.0%~6.2%] vs. 1.2% [IQR, 0.3%~3.4%]; P = 0.047).
Although the average LA volumes were not significantly different between HCM patients with and without T2DM (all P > 0.05), impaired LA reservoir and conduit function were detected by strain analyses in patients with T2DM compared with those without T2DM(Table 2; εs: 23.2% ± 11.0 vs. 28.2% ± 7.9; εe: 9.0% [IQR, 4.6%~13.5%] vs. 12.3% [IQR, 10.1%~14.7%]; SRs: 1.2 ± 0.5 s−1 vs. 1.5 ± 0.8 s−1; SRe: − 0.7 s−1 [IQR, − 1.2 ~ − 0.5 s−1] vs. − 0.9 s−1 [IQR, − 1.2 ~ − 0.7 s−1]; all P < 0.05). The LA booster-pump function was similar between the two groups (LA booster EF: 32.3% ± 14.6 vs. 34.9% ± 9.9; εa: 13.1% ± 6.8 vs. 15.4% ± 6.1; SRa: − 1.5 ± 0.8 s−1 vs. − 1.7 ± 0.7 s−1; all P > 0.05). Representative cases from the two groups are shown in Fig. 2.
Association between T2DM and LV/LA dysfunction
The factors associated with LV/LA dysfunction are displayed in Tables 3 and 4.. Among all HCM patients, comorbidity with T2DM was significantly associated with LVEF (r = − 6.05, P < 0.001), GRS(r = − 6.66, P < 0.001), GCS (r = 2.51, P < 0.001), GLS (r = 2.28, P < 0.001), εs (r = − 4.97, P = 0.02), SRs (r = − 0.31, P = 0.02) and εe (r = − 2.66, P = 0.03) in the univariate analysis. In the multivariate analysis, the coexistence of T2DM remained an independent predictor of a low LVEF (β = − 6.05, P < 0.001) and impaired GLS (β = 1.40, P = 0.007). In addition, the extent of LGE was independently associated with impaired GRS (β = − 45.81, P = 0.008), GCS (β = 18.25, P = 0.003), εs (β = − 59.20, P < 0.001), εe (β = − 21.29, P = 0.02) and SRs (β = − 2.90, P = 0.002) when adjusted for confounding factors.
Intra- and interobserver variability
The intra- and interobserver reproducibility of the LV and LA strain parameters are presented in Table 5. The intraclass correlation coefficient ranged from 0.827 to 0.981 for intraobserver agreement and from 0.818 to 0.970 for interobserver agreement, which indicated good reproducibility.
Discussion
The current study investigated the effects of T2DM on LV and LA remodelling in HCM patients using CMR, featuring the largest sample size described to date. Several important observations are evident: (1) the LVEF and LV strains in all three directions were impaired in HCM patients with T2DM compared with those without T2DM; (2) CMR strain analysis revealed an early reduction in LA reservoir and conduit function in the HCM-DM group; (3) T2DM was independently associated with LVEF and GLS; and (4) HCM-DM patients presented with greater myocardial scar indicated by LGE, which was an independent predictor of GRS, GCS, εs and SRs, respectively.
The presence of diabetes was associated with adverse LV remodelling in HCM patients. Our results showed lower LV volumes and larger mass/volume ratio in HCM-DM patients than HCM-alone patients. This finding was consistent with a Mendelian randomization study which demonstrated an association between insulin resistance and adverse changes in LV structure [20]. Similar to our results, a prospective study revealed a greater reduction in LV contractile function and a greater degree of fibrosis burden in 20 HCM-DM patients [21]. In addition, our data showed a higher myocardial injury degree in HCM-DM patients indicated by a higher level of cTnT, which was independently associated with GRS and GCS, respectively. Diabetes-related microvascular dysfunction may be a possible explanation. This was theoretically supported by Jex et al., who described more impaired stress myocardial perfusion in HCM-DM patients than in patients with isolated HCM [21]. Further study was expected to investigate the impact of diabetes-related microvascular dysfunction on LV contractile abnormalities in HCM patients. In addition, our study showed a greater degree of LV fibrosis in the HCM-DM patients than in the isolated HCM controls, which could be the result of diabetes-related microvascular dysfunction.
Recently, literatures have reported a higher prevalence of atrial fibrillation in HCM patients with diabetes comorbidity than in those without [3, 4, 22]. The current study provided insights into an early condition for the pathophysiological alteration. We observed impaired LA reservoir and conduit function in HCM-DM patients, whereas the LA size was similar between patients with and without coexisting diabetes. LA reservoir and conduit functions contribute to LV filling and are useful indicators of LV diastolic function. The increased LV diastolic stiffness, reflected by impaired LA reservoir and conduit function, can lead to symptoms of heart failure and provide an elucidation of the higher New York Heart Association class in HCM-DM patients. This was also consistent with an echocardiographic study that revealed the prognostic value of LA reservoir strain for predicting incident heart failure events in HCM patients [23]. In addition, a greater degree of LV fibrosis burden in HCM-DM patients was independently associated with LA reservoir function in the present study, which could provide a mechanism for the intimate relationship between LV myocardial fibrosis and future atrial fibrillation in a previous HCM study [24].
Previous findings suggested the presence of myocardial microvascular dysfunction, myocardial hypertrophy and fibrosis, left ventricular diastolic dysfunction primarily and systolic impairment later in diabetes, independent of diabetes-related cardiovascular diseases [25,26,27]. Similarly, our results added that T2DM was an independent determinant of LVEF and GLS in HCM patients, respectively. Diabetes-induced microvascular ischaemia may be the underlying mechanism since subendocardial fibres lying in a longitudinal orientation contribute to the main myocardial contraction and are most vulnerable to pathological changes of ischaemia [11, 28, 29]. We also found that a greater extent of LGE in HCM-DM patients was an independent predictor of GRS, GCS, εs and SRs. The possible mechanisms are as follows: (1) Diabetes can aggravate fibrosis progression in HCM patients since myocardial fibrosis is a well-recognized pathological change in diabetes [25, 26, 30]; (2) LGE on the images of HCM patients typically manifests as a patchy midwall pattern in areas of hypertrophy and the insertion points of the septum [31], which can affect radial and circumferential myocardial motion; and (3) LV fibrosis in HCM-DM patients can further increase myocardial stiffness, which could lead to diastolic dysfunction reflected by impaired LA strain. In summary, the present study demonstrated the association between adverse LV/LA remodelling and T2DM in HCM patients. The results support the active prevention and close surveillance of T2DM throughout life as an integral part of the management of HCM. A prospective study might be warranted to confirm the clinical value of CMR for monitoring the adverse impact of T2DM on HCM patients and guiding early therapy to prevent adverse events.
The limitations of the current study were as follows: (1) quantitative stress perfusion was not available in this retrospective analysis, and the association between microvascular dysfunction and adverse cardiac function should be elucidated in future studies; (2) the present study was limited to conducting subgroup analysis by diabetes features because of incomplete data regarding the duration of T2DM; (3) there is potential bias, as patients were recruited from a single tertiary referral center; and (4) the relatively small population size and low adverse event rate in the HCM patients limited prognostic analysis. Further studies with large sample sizes are supposed to provide a more detailed analysis of the associations between impaired imaging parameters and poor outcomes.
Conclusions
T2DM worsened LV and LA function in HCM patients and was independently associated with impaired LVEF and GLS. Compared with the isolated HCM controls, the HCM-DM patients presented with a greater extent of LGE, which was an independent predictor of impaired GRS, GCS, εs and SRs. These findings may help elucidate the mechanism underlying the adverse prognostic impact of T2DM on HCM and support the prevention and management of T2DM in HCM patients.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HCM:
-
Hypertrophic cardiomyopathy
- T2DM:
-
Type 2 diabetes mellitus
- CMR:
-
Cardiac magnetic resonance
- LV:
-
Left ventricular
- LA:
-
Left atrial
- LGE:
-
Late gadolinium enhancement
- GRS:
-
Global radial strain
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- Vmax:
-
Maximal volume
- Vpac:
-
Pre-atrial contractile volume
- Vmin:
-
Minimal volume
- εs:
-
Reservoir strain
- εe:
-
Conduit strain
- εa:
-
Booster-pump strain
- SRs:
-
Peak positive strain rate
- SRe:
-
Peak early negative strain rate
- SRa:
-
Peak late negative strain rate
- IQR:
-
Interquartile range
- cTnT:
-
Cardiac troponin T
- MVR:
-
Mass/volume ratio
References
Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Dearani JA, Rowin EJ, Maron MS, Sherrid MV. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):390–414.
Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–68.
Wasserstrum Y, Barriales-Villa R, Fernandez-Fernandez X, Adler Y, Lotan D, Peled Y, Klempfner R, Kuperstein R, Shlomo N, Sabbag A, et al. The impact of diabetes mellitus on the clinical phenotype of hypertrophic cardiomyopathy. Eur Heart J. 2019;40(21):1671–7.
Lopes LR, Losi MA, Sheikh N, Laroche C, Charron P, Gimeno J, Kaski JP, Maggioni AP, Tavazzi L, Arbustini E, et al. Association between common cardiovascular risk factors and clinical phenotype in patients with hypertrophic cardiomyopathy from the European Society of Cardiology (ESC) EurObservational Research Programme (EORP) Cardiomyopathy/Myocarditis registry. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):42–53.
Lee HJ, Kim HK, Kim BS, Han KD, Rhee TM, Park JB, Lee H, Lee SP, Kim YJ. Impact of diabetes mellitus on the outcomes of subjects with hypertrophic cardiomyopathy: a nationwide cohort study. Diabetes Res Clin Pract. 2022;186:109838.
Wang S, Cui H, Ji K, Song C, Ren C, Guo H, Zhu C, Wang S, Lai Y. Impact of type 2 diabetes mellitus on mid-term mortality for hypertrophic cardiomyopathy patients who underwent septal myectomy. Cardiovasc Diabetol. 2020;19(1):64.
Karwi QG, Ho KL, Pherwani S, Ketema EB, Sun Q, Lopaschuk GD. Concurrent diabetes and heart failure: interplay and novel therapeutic approaches. Cardiovasc Res. 2022;118(3):686–715.
Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501–25.
Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–6.
Jorgensen PG, Jensen MT, Mogelvang R, Fritz-Hansen T, Galatius S, Biering-Sorensen T, Storgaard H, Vilsboll T, Rossing P, Jensen JS. Impact of type 2 diabetes and duration of type 2 diabetes on cardiac structure and function. Int J Cardiol. 2016;221:114–21.
Yan WF, Xu HY, Jiang L, Zhang L, Guo YK, Li Y, Shen LT, Min CY, Yang ZG. Early longitudinal changes in left ventricular function and morphology in diabetic pigs: evaluation by 3.0T magnetic resonance imaging. Cardiovasc Diabetol. 2023;22(1):6.
Li Z, Xiong J, Guo Y, Tang H, Guo B, Wang B, Gao D, Dong Z, Tu Y. Effects of diabetes mellitus and glycemic traits on cardiovascular morpho-functional phenotypes. Cardiovasc Diabetol. 2023;22(1):336.
Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503–626.
Xu J, Yang W, Zhao S, Lu M. State-of-the-art myocardial strain by CMR feature tracking: clinical applications and future perspectives. Eur Radiol. 2022;32(8):5424–35.
Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the management of hypertrophic cardiomyopathy: A report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation 2024, 149(23):e1239-e1311.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Shi K, Huang S, Li X, Xu HY, Yang MX, Li Y, Guo YK, Yang ZG. Effect of obesity on left ventricular remodeling and clinical outcome in Chinese patients with hypertrophic cardiomyopathy: assessed by cardiac MRI. J Magn Reson Imaging. 2023;57(3):800–9.
Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg-Buchwald C, et al. Quantification of left atrial strain and strain rate using cardiovascular magnetic resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014;16(1):60.
Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 2020;22(1):1.
Ai S, Wang X, Wang S, Zhao Y, Guo S, Li G, Chen Z, Lin F, Guo S, Li Y, et al. Effects of glycemic traits on left ventricular structure and function: a mendelian randomization study. Cardiovasc Diabetol. 2022;21(1):109.
Jex N, Chowdhary A, Thirunavukarasu S, Procter H, Sengupta A, Natarajan P, Kotha S, Poenar AM, Swoboda P, Xue H, et al. Coexistent diabetes is associated with the presence of adverse phenotypic features in patients with hypertrophic cardiomyopathy. Diabetes Care. 2022;45(8):1852–62.
Wang S, Zhang K, He M, Guo H, Cui H, Wang S, Lai Y. Effect of type 2 diabetes on cardiac arrhythmias in patients with obstructive hypertrophic cardiomyopathy. Diabetes Metab Syndr. 2024;18(3):102992.
Lee HJ, Kim HK, Rhee TM, Choi YJ, Hwang IC, Yoon YE, Park JB, Lee SP, Kim YJ, Cho GY. Left atrial reservoir strain-based left ventricular diastolic function grading and incident heart failure in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2022;15(4):e013556.
Raphael CE, Liew AC, Mitchell F, Kanaganayagam GS, Di Pietro E, Newsome S, Owen R, Gregson J, Cooper R, Amin FR, et al. Predictors and mechanisms of atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2020;136:140–8.
Jiang L, Yan WF, Zhang L, Xu HY, Guo YK, Li ZL, Liu KL, Zeng LM, Li Y, Yang ZG. Early left ventricular microvascular dysfunction in diabetic pigs: a longitudinal quantitative myocardial perfusion CMR study. Cardiovasc Diabetol. 2024;23(1):9.
Liu X, Gao Y, Guo YK, Xia CC, Shi R, Jiang L, Shen MT, Xie LJ, Peng WL, Qian WL, et al. Cardiac magnetic resonance T1 mapping for evaluating myocardial fibrosis in patients with type 2 diabetes mellitus: correlation with left ventricular longitudinal diastolic dysfunction. Eur Radiol. 2022;32(11):7647–56.
Lee MMY, McMurray JJV, Lorenzo-Almoros A, Kristensen SL, Sattar N, Jhund PS, Petrie MC. Diabetic cardiomyopathy. Heart. 2019;105(4):337–45.
Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging. 2015;8(12):1444–60.
Van Ryckeghem L, Keytsman C, Verboven K, Verbaanderd E, Frederix I, Bakelants E, Petit T, Jogani S, Stroobants S, Dendale P, et al. Exercise capacity is related to attenuated responses in oxygen extraction and left ventricular longitudinal strain in asymptomatic type 2 diabetes patients. Eur J Prev Cardiol. 2022;28(16):1756–66.
Gao Y, Yang ZG, Ren Y, Liu X, Jiang L, Xie LJ, Hu BY, Shen MT, Xu HY, Li ZL, et al. Evaluation of myocardial fibrosis in diabetes with cardiac magnetic resonance T1-mapping: correlation with the high-level hemoglobin A1c. Diabetes Res Clin Pract. 2019;150:72–80.
Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53(3):284–91.
Acknowledgements
Not applicable.
Funding
The study was supported by the Postdoctoral Fellowship Program of CPSF (grant number: GZC20241154), Science and Technology Support Program of Sichuan Province (grant number: 2024NSFSC1795), the Postdoctor Research Fund of West China Hospital, Sichuan University (grant number: 2024HXBH162), National Natural Science Foundation of China (grant number: 82202104) and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (grant number: ZYGD23019).
Author information
Authors and Affiliations
Contributions
SQY, conception and design of study, analyzed images, collection and interpretation of data, drafted the manuscript. KS, conception and design of study, analyzed images, critical revision of the manuscript. JW, YG, RS and WFY, collection and interpretation of data, critically reviewed the manuscript. HYX, YL and YKG, critical revision of the manuscript. ZGY, conception and design of study, critically reviewed the manuscript, supervised the overall study. All authors read and approved the final version of submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Biomedical Research Ethics Committee of our hospital approved this study (No. 2019 − 767). Written informed consent have been waived owing to the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, SQ., Shi, K., Li, Y. et al. The impact of diabetes mellitus on cardiac function assessed by magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Cardiovasc Diabetol 23, 293 (2024). https://doi.org/10.1186/s12933-024-02384-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02384-y