Abstract
Background
Glutathione is a valuable tri-peptide that is industrially produced by fermentation using the yeast Saccharomyces cerevisiae, and is widely used in the pharmaceutical, food, and cosmetic industries. It has been reported that addition of l-serine (l-Ser) is effective at increasing the intracellular glutathione content because l-Ser is the common precursor of l-cysteine (l-Cys) and glycine (Gly) which are substrates for glutathione biosynthesis. Therefore, we tried to enhance the l-Ser biosynthetic pathway in S. cerevisiae for improved glutathione production.
Results
The volumetric glutathione production of recombinant strains individually overexpressing SER2, SER1, SER3, and SER33 involved in l-Ser biosynthesis at 48 h cultivation was increased 1.3, 1.4, 1.9, and 1.9-fold, respectively, compared with that of the host GCI strain, which overexpresses genes involved in glutathione biosynthesis. We further examined simultaneous overexpression of SHM2 and/or CYS4 genes involved in Gly and l-Cys biosynthesis, respectively, using recombinant GCI strain overexpressing SER3 and SER33 as hosts. As a result, GCI overexpressing SER3, SHM2, and CYS4 showed the highest volumetric glutathione production (64.0 ± 4.9 mg/L) at 48 h cultivation, and this value is about 2.5-fold higher than that of the control strain.
Conclusions
This study first revealed that engineering of l-Ser and Gly biosynthetic pathway are useful strategies for fermentative glutathione production by S. cerevisiase.
Similar content being viewed by others
Background
Glutathione is the most abundant non-protein thiol compound in all living organisms [1]. Because of its important physiological functions including its ability to act as an antioxidant, a detoxifier of xenobiotics, and an immune booster [2,3,4,5,6,7,8], glutathione has been widely used in the medical, food and cosmetic industries [9, 10]. Therefore, the demand for glutathione has increased in recent years.
At present, glutathione is produced mainly by fermentation using yeast. Glutathione biosynthesis is carried out by two consecutive adenosine triphosphate (ATP)-consuming reactions catalyzed by γ-glutamylcysteine (γ-GC) synthetase (GCS, EC 6.3.2.2), encoded by GSH1, and glutathione synthetase (GS, EC 6.3.2.3), encoded by GSH2, from three precursor amino acids, l-glutamate (l-Glu), l-cysteine (l-Cys), and glycine (Gly) (Fig. 1). GCS catalyzes the reaction to form γ-GC from l-Glu and l-Cys. GS catalyzes the reaction to form glutathione from γ-GC and Gly. Previously, we obtained a strain overexpressing multicopy of GSH1 and GSH2 genes (GCI strain) that showed increased glutathione production [11], and have used this GCI strain as a host [12,13,14,15].
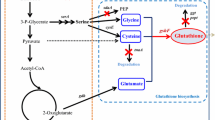
The metabolic pathway of the glutathione biosynthesis via l-Ser, l-Cys and Gly biosynthesis in S. cerevisiae. First, 3-phospho-glycerate (3PG) dehydrogenase encoded by SER3 and SER33 converts 3PG to 3-phospho-hydroxypyruvate (3PHP) using the oxidized form of nicotinamide adenine dinucleotide (NAD+) as a cofactor. Second, 3-phosphoserine aminotransferase encoded by SER1 transfers an amino group from l-Glu to the 2-oxo group of 3PHP and produces l-O-phosphoserine (LPS) and 2-oxoglutarate (2OG). Finally, phosphoserine phosphatase encoded by SER2 dephosphorylates LPS to l-Ser. The produced l-Ser is further metabolized to l-Cys and Gly by cystathionine β-synthase encoded by CYS4 and serine hydroxymethyltransferase encoded by SHM2, respectively. Other abbreviations are as follows, 5,10-CH2-THF: (6R)-5,10-methylenetetrahydrofolate; ADP: adenosine diphosphate; ATP: adenosine triphosphate; NADH: reduced form of nicotinamide adenine dinucleotide; Pi: phosphate; H2S: hydrogen sulfide; THF: tetrahydrofolate.
Some studies have reported that supplying several types of amino acids during glutathione fermentation increased glutathione production in Saccharomyces cerevisiae [16, 17]. The addition of l-Cys was especially effective at increasing glutathione production, and metabolic engineering of l-Cys biosynthesis was also effective in enhancing glutathione production [11]. It was also reported that addition of l-serine (l-Ser) was effective in increasing the intracellular glutathione content [17] because l-Ser is the common precursor of l-Cys and Gly (Fig. 1). This fact suggests that intracellular l-Ser supplementation by genetic engineering should improve glutathione production without external addition of l-Ser. As shown in Fig. 1, l-Ser biosynthesis is carried out by three consecutive reactions from 3-phospho-glycerate (3PG), which is an intermediate of glycolytic pathway [18].
In this study, we enhanced glutathione productivity by metabolic engineering the l-Ser biosynthetic pathway from 3PG using the GCI strain as a host. Furthermore, we attempted to combine engineering of the l-Cys and Gly biosynthetic pathways with the l-Ser biosynthetic pathway to further enhance glutathione production.
Results
Effect of external l-Ser supplementation on glutathione production by GCI strain
l-Ser is a common precursor of both l-Cys and Gly, which are both substrates for glutathione biosynthesis (Fig. 1). To confirm the effect of external l-Ser supplementation on glutathione production in the GCI host strain, we measured biomass concentration (g/L), intracellular glutathione content (%), and volumetric glutathione production (mg/L) every 24 h for 72 h of fermentation with and without 300 mg/L l-Ser addition (Fig. 2). As shown in Fig. 2A, the biomass concentration was increased by addition of l-Ser (1.65 ± 0.05 g/L at 72 h) compared with no l-Ser addition (1.42 ± 0.03 g/L at 72 h). Furthermore, as shown in Fig. 2b, addition of l-Ser also increased the intracellular glutathione content (2.2 ± 0.1% at 72 h) compared with no l-Ser addition (1.5 ± 0.1% at 72 h) by about 1.5-fold. Because of the synergetic effect of the increased biomass concentration and intracellular glutathione content, the volumetric glutathione production was consistent for more than 72 h with l-Ser addition, whereas it was decreased when no l-Ser was added (Fig. 2C). However, the maximum yield of glutathione production was not enhanced by l-Ser addition at 24 h.
Time-dependent glutathione production of the GCI strain with or without external l-Ser supplementation. A Biomass concentration (g/L). B Intracellular glutathione content (%). C Volumetric glutathione production (mg/L). Blue and red filled circles represent the GCI strain grown without l-Ser and with 300 mg/L l-Ser, respectively. Values are the mean, and error bars show the standard deviation (n = 3)
Glutathione production by recombinant GCI strains engineered in l-Ser biosynthetic pathway
L-Ser biosynthesis occurred through the l-Ser biosynthetic pathway from its precursor of 3PG, consistent with continuous reactions catalyzed by Ser2, Ser1, Ser3, and Ser33 (Fig. 1). To increase glutathione biosynthesis through enhancement of the l-Ser biosynthetic pathway, recombinant GCI strains overexpressing each gene (SER2, SER1, SER3, and SER33) and simultaneously overexpressing strains (SER3/SER33 and SER2/SER1/SER3/SER33) were constructed, as well as a vector control GCI strain (Table 2). Figure 3 shows the biomass concentration, intracellular glutathione content, volumetric glutathione production, and GSSG ratio of these strains at 24 and 48 h. The biomass concentration of GCI/SER2 (1.22 ± 0.03 g/L), GCI/SER1 (1.57 ± 0.01 g/L), GCI/SER3 (1.64 ± 0.05 g/L), GCI/SER33 (1.62 ± 0.04 g/L), and GCI/SER3/SER33 (1.67 ± 0.08 g/L) at 48 h were higher than that of the control GCI/Vector strain (1.12 ± 0.02 g/L), respectively (Fig. 3A). On the other hand, the biomass concentration of GCI/SERs (0.94 ± 0.04 g/L), which overexpresses SER2, SER1, SER3, and SER33 at 48 h was decreased compared to that of the GCI/Vector strain. The intracellular glutathione content of the GCI/SER2 (2.8 ± 0.8%), GCI/SER1 (2.3 ± 0.2%), GCI/SER3 (3.2 ± 0.2%), GCI/SER33 (2.9 ± 0.1%), GCI/SER3/SER33 (2.5 ± 0.2%), and GCI/SERs (2.5 ± 0.2%) at 48 h were higher than that of the vector control GCI strain (1.6 ± 0.1%) (Fig. 3B). Because of the increased biomass concentration and intracellular glutathione content in the most of the recombinant strains, all constructed strains also showed improved volumetric glutathione production at 48 h compared to the GCI/Vector strain (17.9 ± 0.8 mg/L) (Fig. 3C). The volumetric glutathione production of the GCI/SER2 (33.9 ± 3.9 mg/L), GCI/SER1 (36.8 ± 1.3 mg/L), GCI/SER3 (47.0 ± 3.2 mg/L), GCI/SER33 (47.7 ± 1.4 mg/L), and GCI/SER3/SER33 (41.8 ± 4.5 mg/L) at 48 h were apparently higher than that of the vector control GCI strain (Fig. 3C). The GSSG ratio of GCI/SER2 (26.1 ± 0.8%), GCI/SER3 (32.0 ± 3.3%), and GCI/SERs (37.0 ± 5.6%) at 48 h were higher than that of the GCI/Vector strain (22.1 ± 0.6%). The GSSG ratio of GCI/SER1 (20.7 ± 0.2%), GCI/SER33 (23.6 ± 1.5%), and GCI/SER3/SER33 (22.6 ± 0.9%) at 48 h were almost same to that of the GCI/Vector stain.
Glutathione production by GCI strains overexpressing l-Ser biosynthetic genes. A Biomass concentration (g/L). B Intracellular glutathione content (%). C Volumetric glutathione production (mg/L). D GSSG ratio (%). Blue and red filled bars represent the values after growth for 24 and 48 h, respectively. The GSSG ratio was defined as the ratio of the g-GSSG in cells to the g-total glutathione (GSH and GSSG) in cells. Values are the mean, and error bars show the standard deviation (n = 3)
Glutathione production by GCI/SER3 and GCI/SER33 strains engineered in Gly and/or l-Cys biosynthetic pathways
Enhancing l-Cys and Gly biosynthesis from l-Ser with enhancing l-Ser biosynthesis would be expected to further improve glutathione production by S. cerevisiae. l-Cys and a part of Gly are synthesized from l-Ser through reactions catalyzed by cystathionine γ-synthase (Cys4) and cytosolic serine hydroxymethyltransferase (Shm2), respectively (Fig. 1). In l-Ser biosynthetic genes, overexpression of SER3 or SER33 was effective to improve volumetric glutathione production (Fig. 3). Therefore, we constructed SHM2 and/or CYS4 overexpressing strains using GCI/Vector, GCI/SER3, and GCI/SER33 as platform strains (Fig. 4). Unlike the case of the glutathione production by l-Ser biosynthetic gene overexpressing strains, the results of the strains overexpressing SHM2 and/or CYS4 using GCI/Vector, GCI/SER3, and GCI/SER33 as platform strains were complicated. The biomass concentration, intracellular glutathione content, and volumetric glutathione production of GCI/SHM2, GCI/CYS4, and GCI/SHM2/CYS4 at 48 h were apparently higher than those of the GCI/Vector (Fig. 4A–C; Additional file 1: Table S4). On the other hand, the GSSG ratio of the GCI/SHM2 (29.6 ± 1.7%) and GCI/CYS4 (30.4 ± 2.4%) at 48 h were somewhat higher than the that of the GCI/Vector (Fig. 4D), and the GSSG ratio of the GCI/SHM2/CYS4 (26.1 ± 2.5%) at 48 h was almost same to the that of the GCI/Vector. These results indicate that overexpression of the SHM2 and/or CYS4 is effective for glutathione production by S. cerevisiae even when l-Ser biosynthetic pathway of the platform strain was not engineered. The intracellular glutathione content and volumetric glutathione production of the GCI/SER3/SHM2, GCI/SER3/CYS4, and GCI/SER3/SHM2/CYS4 at 48 h were also higher than those of the platform strain GCI/SER3 (Fig. 4B, C; Additional file 1: Table S4). However, the biomass concentration of the GCI/SER3/SHM2 (1.73 ± 0.16 g/L) and GCI/SER3/CYS4 (1.75 ± 0.06 g/L) at 48 h were slightly higher than that of the GCI/SER3 (Fig. 4A), and that of the GCI/SER3/SHM2/CYS4 (1.58 ± 0.11 g/L) was apparently decreased. Unlike using the GCI/Vector as a platform, the GSSG ratio of the GCI/SER3/SHM2 (41.6 ± 0.2%), GCI/SER3/CYS4 (41.0 ± 0.4%), and GCI/SER3/SHM2/CYS4 (40.5 ± 2.0%) at 48 h were higher than that of the GCI/SER3 (Fig. 4D). The biomass concentration, intracellular glutathione content, and volumetric glutathione production of the GCI/SER33/SHM2 and GCI/SER33/CYS4 at 48 h were obviously decreased compared to those of the platform strain GCI/SER33, whereas those of the GCI/SER33/SHM2/CYS4 were higher than those of the GCI/SER33 (Fig. 4A–C; Additional file 1: Table S4). Meanwhile, the GSSG ratio of the GCI/SER33/SHM2 (25.8 ± 1.6%), GCI/SER33/CYS4 (27.0 ± 0.7%), and GCI/SER33/SHM2/CYS4 (27.9 ± 0.4%) at 48 h were slightly higher than that of the GCI/SER3 (Fig. 4D).
Glutathione production by GCI/Vector, GCI/SER3, and GCI/SER33 strains overexpressing SHM2 and/or CYS4 genes. A Biomass concentration (g/L). B Intracellular glutathione content (%). C Volumetric glutathione production (mg/L). D GSSG ratio (%). Blue and red filled bars represent the values after growth for 24 and 48 h, respectively. Values are the mean, and error bars show the standard deviation (n = 3)
Glutathione production by GCI/SER3/SER33 and GCI/SERs strains engineered in Gly and/or l-Cys biosynthetic pathways
As mentioned above, the intracellular glutathione content and volumetric glutathione production of the GCI/SER3/SER33 and GCI/SERs were lower than those of the GCI/SER3 and GCI/SER33. However, because we thought the cause of these low glutathione yields was excess l-Ser accumulation in cells by simultaneous overexpression of l-Ser biosynthetic genes, we constructed SHM2 and/or CYS4 overexpressing strains using the GCI/SER3/SER33 and GCI/SERs as the platform strains and examined glutathione production of these strains (Fig. 5). The biomass concentration, intracellular glutathione content, and volumetric glutathione production of the GCI/SER3/SER33/SHM2 and GCI/SER3/SER33/CYS4 (Fig. 5A–C; Additional file 1: Table S5) were decreased or almost same than those of the platform GCI/SER3/SER33 strain. On the other hand, although the GCI/SER3/SER33/SHM2/CYS4 showed almost the same biomass concentration at 48 h (1.66 ± 0.06 g/L) (Fig. 5A) compared to that of the GCI/SER3/SER33, this strain showed higher intracellular glutathione content and volumetric glutathione production at 48 h (Fig. 5B, C; Additional file 1: Table S5). The GSSG ratio of the GCI/SER3/SER33/SHM2, GCI/SER3/SER33/CYS4, and GCI/SER3/SER33/SHM2/CYS4 at 48 h (Fig. 5D; Additional file 1: Table S5) were higher than that of the GCI/SER3/SER33 strain. The biomass concentration of the GCI/SERs/SHM2 (1.37 ± 0.10 g/L), GCI/SERs/CYS4 (1.54 ± 0.03 g/L), and GCI/SERs/SHM2/CYS4 (1.66 ± 0.06 g/L) at 48 h were improved compared to that of the platform strain GCI/SERs. However, the intracellular glutathione content of the GCI/SERs/SHM2 (0.8 ± 0.3%), GCI/SERs/CYS4 (1.9 ± 0.1%), and GCI/SERs/SHM2/CYS4 (1.8 ± 0.3%) at 48 h were lower than that of the GCI/SERs. Consequently, the volumetric glutathione production of the GCI/SERs/SHM2 (10.9 ± 4.5 mg/L) and GCI/SERs/SHM2/CYS4 (23.4 ± 7.2 mg/L) at 48 h were decreased, and the volumetric glutathione production of the GCI/SERs/CYS4 (30.8 ± 1.4 mg/L) at 48 h was increased compared to that of the GCI/SERs. The GSSG ratio of the GCI/SERs/SHM2 (29.9 ± 2.1%), GCI/SERs/CYS4 (25.8 ± 0.6%), and GCI/SERs/SHM2/CYS4 (28.3 ± 2.0%) at 48 h were lower than that of the GCI/SERs.
Glutathione production by GCI/SER3/SER33 and GCI/SERs strains overexpressing SHM2 and/or CYS4 genes. A Biomass concentration (g/L). B Intracellular glutathione content (%). C Volumetric glutathione production (mg/L). D GSSG ratio (%). Blue and red filled bars represent the values after growth for 24 and 48 h, respectively. Values are the mean, and error bars show the standard deviation (n = 3)
Discussion
l-Ser biosynthesis from 3PG in S. cerevisiae occurs via three consecutive reactions catalyzed by SER2, SER1, and SER3/SER33. The intracellular glutathione content of the recombinant strains individually overexpressing SER2, SER1, SER3, and SER33 were 1.4- to 1.8-fold higher than that of the control strain at 48 h (Fig. 3B). Albers et al. showed that transcription levels of SER2, SER3, and SER33 were less than that of SER1 [18]. The result that overexpression of SER2, SER3, and SER33 increased intracellular glutathione content compared with that of SER1 is likely primarily influenced by transcription levels of each gene. Furthermore, the result that the overexpression of SER3 and SER33 were the most effective to enhance the glutathione production (Fig. 3B, C) suggested that SER3 and SER33 were the most critical step in the l-Ser biosynthetic pathway, and this fact is consistent with the previous study which reported deletion of SER3 and SER33 equally influenced metabolite production [18]. Thus, we selected the GCI/SER3 and GCI/SER33 strains as the platform strain for further improvement of glutathione biosynthesis. However, simultaneous overexpression of SER3 and SER33 resulted in decreased intracellular glutathione content and volumetric glutathione production compared to those of the GCI/SER3 and GCI/SER33 strains (Fig. 3B, C). Furthermore, simultaneous overexpression of SER2, SER1, SER3, and SER33 lead to poor growth and consecutive low glutathione production (Fig. 3A–C). These results may imply that surplus l-Ser production or decrease of 3PG by enhanced l-Ser biosynthesis exerts unfavorable effects on growth and glutathione production of host cells. The increase of GSSG ratio in the GCI/SERs strain also showed that the strain was under strong stress because GSSG ratio showed index of oxidative stress of cells [12,13,14]. However, the strains overexpressing SER3 also showed high GSSG ratio compared with the control strain. In some cases, production of GSSG help to avoid a negative feedback regulation by Gsh1, and consequently increase total glutathione production [12,13,14]. The overexpression of SER3 may lead modest stress of the cells for increased glutathione production. On the other hand, the strains overexpressing SER33 showed slightly lower GSSG ratio than that of the control strains. These different effects of overexpression of SER3 and SER33 on GSSG ratios may show different mechanisms of improved glutathione production.
To further improve glutathione production by S. cerevisiae, we additionally overexpressed SHM2 and/or CYS4 genes encoding cytosolic serine hydroxymethyltransferase and cystathionine β-synthase, respectively (Fig. 1). Generally, cystathionine β-synthase encoded by CYS4 catalyzes condensation of l-Ser and l-homocysteine to produce l-cystathionine and H2O. Consecutively, l-cystathionine is broken down into l-Cys, 2-oxobutanoic acid, and ammonia by cystathionine γ-lyase (CYS3). However, we didn’t adopt overexpression of CYS3 in this study because Cys4 of S. cerevisiae can directly catalyze l-Ser and H2S to l-Cys and H2O [19] as shown in Fig. 1.
Unexpectedly, overexpression of SHM2 and/or CYS4 effectively enhanced biomass concentration, intracellular glutathione content, and consecutive volumetric glutathione production even when the GCI strain was used as a platform.
l-Cys is known as a late-limiting precursor for glutathione biosynthesis, and therefore a lot of studies have employed external addition of l-Cys to the culture medium for enhancing glutathione production [16, 17, 20,21,22,23]. Enhancing l-Cys biosynthesis with or without enhancing l-Ser biosynthesis also improved glutathione production of S. cerevisiae not only in this study, but also in previous study [11]. However, the result that enhancing Gly biosynthesis by genetic engineering on glutathione production has not been reported so far. According to the report of Wang et al., l-Glu abundantly exists in S. cerevisiae cells during fermentative glutathione production (185.9 ± 10 μmol/g-dry cell) [24] and intracellular Gly concentration (0.44 ± 0.15 μmol/g-dry cell) was almost the same as that of l-Cys (0.42 ± 0.2 μmol/g-dry cell) without addition of these precursors to the medium. In other cases, l-Cys concentration was lower than that of Gly; however, Gly concentration was much lower than that of l-Glu [25, 26]. Thus, there is a possibility that enhancing Gly biosynthesis promotes glutathione biosynthesis. In fact, addition of Gly to the medium improved glutathione production in a previous study [24]. On the other hand, Alfafara et al. reported that supplementation of Gly in the medium had no effect on the glutathione production of S. cerevisiae [16]. This disagreement may be caused by various factors of fermentation such as genotype of host S. cerevisiae, preculture conditions, initial cell concentration, medium composition, and so on. Indeed, even in this study, overexpression of SHM2 in GCI/SER33, GCI/SER3/SER33, and GCI/SERs didn’t improve glutathione production (Fig. 4B, C).
Among the GCI/SER3/SER33 and GCI/SERs strains overexpressing SHM2 and/or CYS4, only GCI/SER3/SER33/SHM2/CYS4 and GCI/SERs/CYS4 showed modestly increased volumetric glutathione production compared to the platform strains (Fig. 5C). If the cause of poor glutathione production by GCI/SER3/SER33 and GCI/SERs were surplus l-Ser biosynthesis, this disadvantage could be solved by overexpression of SHM2 and/or CYS4 regardless of the combination. However, the recovery of the cell growth by overexpression of SHM2 and/or CYS4 in GCI/SERs seemed to be the result that surplus biosynthesized l-Ser was metabolizing to Gly and l-Cys (Fig. 5A, D). However, overexpression of SHM2 and/or CYS4 had no effect or decreased cell growth when GCI/SER3/SER33 was used as a platform (Fig. 5A). These results imply that there may be exquisite and complicated balance between supplies of l-Ser, l-Cys, and Gly, in enhanced glutathione production of S. cerevisiae. In this study, GCI/SER3/SHM2/CYS4 showed the highest volumetric glutathione production at 48 h (64.0 ± 4.9 mg/L) among all strains constructed, and this yield is about 2.5-fold higher than that of the GCI/Vector strain. In other reports about fermentative glutathione production using yeasts, feeding of various precursors and carbon sources, and optimization of growth conditions have been often employed [23, 27,28,29,30]. In many cases adopting these strategies, the fermentation was carried out in nutrient rich condition with high cell density using glutathione high-producing strains and leached about 100–2500 mg/L of glutathione [31]. For example, Wen et al. reported 2190 mg/L of glutathione production in nutrient rich medium supplemented with l-Cys, l-Glu, and Gly using glutathione high-producing S. cerevisiae T65 strain under high cell density and glucose feeding conditions [28]. Comparing with glutathione production of these reports, the maximum glutathione production of this study (64.0 ± 4.9 mg/L) is very low. The S. cerevisiae strains in this study were grown in the nutrient limited minimal medium with low initial cell densities. These growth conditions may lead poor glutathione production compared with those of other reports. However, our objective of this study is to provide a new strategy that modification of l-Ser, l-Cys, and Gly biosynthetic genes improves glutathione production of S. cerevisiae. Indeed, glutathione production studies focused of genetic engineering reported about 10–300 mg/L of glutathione production [32] and the reason for these low yields may be the not optimized culture conditions. Furthermore, in many of these studies, genes directly involved in glutathione synthesis such as GSH1 [33], gshF encoding bifunctional glutathione synthase [34], and PRO1 encoding γ-glutamyl kinase [35] are overexpressed. In such situations, we focused on l-Ser biosynthetic genes and first revealed that engineering of l-Ser and Gly biosynthetic pathway are useful strategies for fermentative glutathione production by S. cerevisiase. These our findings may be applicable for other glutathione high-producing yeast strains to further improve glutathione production.
Methods
Strains and media
Escherichia coli NovaBlue strain (Novagen, Madison, WI, USA) was used as the host strain for recombinant DNA manipulation. S. cerevisiae GCI [MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1], which was previously constructed from a YPH499 strain by expressing multiple copies of GSH1 and GSH2 genes by the δ-integration method [36], was used as the parental strain for additional overexpression of genes in this study. E. coli strains were grown in LB medium (10 g/L tryptone, 5 g/L yeast extract, and 5 g/L sodium chloride) supplemented with ampicillin (100 mg/L). Recombinant S. cerevisiae GCI strains were grown in SD (6.7 g/L yeast nitrogen base w/o amino acids, and 20 g/L glucose) medium. Adenine (20 mg/L), histidine (20 mg/L), tryptophan (40 mg/L), leucine (100 mg/L), uracil (20 mg/L) and aureobasidin A (0.5 mg/L) were supplemented as necessary.
Plasmid construction and yeast transformation
Each gene involved in the l-Ser biosynthetic pathway (SER2, SER1, SER3, and SER33) were amplified by polymerase chain reaction (PCR) method from S. cerevisiae chromosomal deoxyribonucleic acid (DNA) using primer sets of SER2F1/R1, SER1F1/R1, SER3F1/R1, and SER33F1/R1, respectively (Additional file 1: Table S1). The amplified DNA fragments from the SER2, SER1, SER3, and SER33 genes were inserted into the NotI site between PTDH3 and TTDH3 of pATP405 [37] to construct pATP405-SER2, pATP405-SER1, pATP405-SER3, and pATP405-SER33, respectively (Table 1). These constructed plasmids were then digested with EcoRV or AflII and transformed into S. cerevisiae GCI strain (Table 2).
The genes involved in the Gly biosynthetic pathway (SHM2) and l-Cys biosynthetic pathway (CYS4) were amplified by PCR method from S. cerevisiae chromosomal DNA using primer sets of SHM2F/R, and CYS4F/R, respectively (Additional file 1: Table S1). The amplified DNA fragment from the SHM2 gene was inserted into the NotI site of pATP406 [37] to construct pATP406-SHM2 (Table 1). The amplified fragment from the CYS4 gene was inserted into the PmeI site between PADH1 and TADH1 of pATP406 and pATP406-SHM2 to construct pATP406-CYS4 and pATP406-SHM2/CYS4, respectively (Table 1). These constructed plasmids were digested with NcoI, and transformed into appropriate S. cerevisiae host strains (Table 2).
To obtain recombinant strains simultaneously overexpressing all genes involved in the l-Ser, l-Cys and Gly biosynthetic pathways, SER2, SER1, and SER3 genes were amplified by PCR method from S. cerevisiae chromosomal DNA using primer sets of SER2F2/R2, SER1F2/R2, and SER3F2/R2, respectively (Additional file 1: Table S1). The amplified DNA fragment from SER2 was inserted into the PmeI site of pATP405-SER33 to construct pATP405-SER33/SER2 (Table 1). The amplified fragment from SER1 was inserted into the AscI site between PPGK1 and TPGK1 of pATP405-SER33/SER2 to construct pATP405-SER33/SER2/SER1 (Table 1). The amplified fragment from SER3 was inserted into the AscI site of pATP406, pATP406-SHM2, pATP406-CYS4, and pATP406-SHM2/CYS4 to construct pATP406-SER3, pATP406-SHM2/SER3, pATP406-CYS4/SER3, and pATP406-SHM2/CYS4/SER3, respectively (Table 1). These plasmids were digested with EcoRI or NcoI, and transformed into appropriate S. cerevisiae host strains (Table 2).
Transformation of S. cerevisiae was carried out using the lithium acetate method as described previously [38, 39]. Transformants were selected by leucine (pATP405) and uracil (pATP406) auxotrophies. The insertion of the target gene in each transformant was confirmed by PCR method using appropriate primers.
Glutathione production using S. cerevisiae mutant strains
Glycerol stocks of recombinant S. cerevisiae strains stored at −80 °C were streaked on solid SD medium and aerobically grown for 72 h. Grown cells on solid media were inoculated into 5 mL of the liquid SD medium and aerobically grown at 30 °C with agitation (200 rpm) for 24 h. An adequate volume of each cell culture was inoculated into 20 mL of the same medium in 200 mL of baffled erlenmeyer flask with or without 300 mg/L l-Ser to achieve an initial OD600 value of 0.15. Cells were then grown at 30 °C with agitation (200 rpm) for up to 72 h.
Glutathione analysis
Reduced glutathione (GSH) and oxidized glutathione (GSSG) in recombinant S. cerevisiae cells were determined as described in our previous report [40]. Cell cultures (1 mL) grown for glutathione production were sampled every 24 h, and the OD600 of the culture samples were measured using a UVmini-1240 Spectrometer (Shimadzu, Kyoto, Japan). The cells were pelleted by centrifugation (16,000×g, 1 min) and rinsed with Milli-Q water twice. The rinsed cell pellets were re-suspended in Milli-Q water and incubated at 95 °C for 3 min, then cooled immediately on ice for 3 min and the supernatant was separated by centrifugation (16,000×g, 1 min). GSH and GSSG concentrations in the supernatant were determined by HPLC (Shimadzu) equipped with a YMC-Pack ODS-A column (YMC, Kyoto, Japan). The operating conditions were 30 °C with 50 mM potassium dihydrogen phosphate buffer (pH 2.8) and 10 mM sodium 1-heptanesulfonate as the mobile phase at a flow rate of 1.0 mL/min, and detection was performed with an ultraviolet detector SPD-20A (Shimadzu) at 210 nm. Volumetric glutathione production (mg/L-broth) and intracellular glutathione content (%) were calculated from OD600 and used to determine glutathione concentration, as previously described [40].
Availability of data and materials
All data of figures used in this study are included in Additional files of this article.
Abbreviations
- 2OG:
-
2-Oxoglutarate
- 3PG:
-
3-Phospho-glycerate
- 3PHP:
-
3-Phospho-hydroxypyruvate
- 5,10-CH2-THF:
-
(6R)-5,10-methylenetetrahydrofolate
- ADP:
-
Adenosine diphosphate
- ATP:
-
Adenosine triphosphate
- DNA:
-
Deoxyribonucleic acid
- γ-GC:
-
γ-Glutamylcysteine
- GCS:
-
Glutamylcysteine synthetase encoded
- Gly:
-
Glycine
- GS:
-
Glutathione synthetase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- H2S:
-
Hydrogen sulfide
- l-Cys:
-
L-cysteine
- l-Glu:
-
L-glutamine
- LPS:
-
L-O-phosphoserine
- l-Ser:
-
L-serine
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADH:
-
Reduced form of nicotinamide adenine dinucleotide
- PCR:
-
Polymerase chain reaction
- Pi:
-
Phosphate
- THF:
-
Tetrahydrofolate
References
Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60.
Flohé L. The glutathione peroxidase reaction: molecular basis of the antioxidant function of selenium in mammals. Curr Top Cell Regul. 1985;27:473–8.
Dröge W, Breitkreutz R. Glutathione and immune function. Proc Nutr Soc. 2000;59:595–600.
Penninckx MJ. A short review on the role of glutathione in the response of yeasts to nutritional, environmental, and oxidative stresses. Enzyme Microb Technol. 2000;26:737–42.
Vartanyan LS, Gurevich S, Kozachenko AI, Nagler LG, Lozovskaya EL, Burlakova EB. Changes in superoxide production rate and in superoxide dismutase and glutathione peroxidase activities in subcellular organelles in mouse liver under exposure to low doses of low-intensity radiation. Biochem (Mosc). 2000;65:442–6.
Ray S, Watkins DN, Misso NL, Thompson PJ. Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy. 2002;32:571–7.
Rolseth V, Djurhuus R, Svardal AM. Additive toxicity of limonene and 50% oxygen and the role of glutathione in detoxification in human lung cells. Toxicology. 2002;170:75–88.
Singh RJ. Glutathione: a marker and antioxidant for aging. J Lab Clin Med. 2002;140:380–1.
Li Y, Wei G, Chen J. Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol. 2004;66:233–42.
Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012;23:97–102.
Hara KY, Kiriyama K, Inagaki A, Nakayama H, Kondo A. Improvement of glutathione production by metabolic engineering the sulfate assimilation pathway of Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2011;94:1313–9.
Kiriyama K, Hara KY, Kondo A. Oxidized glutathione fermentation using Saccharomyces cerevisiae engineered for glutathione metabolism. Appl Microbiol Biotechnol. 2013;97:7399–404.
Hara KY, Aoki N, Kobayashi J, Kiriyama K, Nishida K, Araki M, Kondo A. Improvement of oxidized glutathione fermentation by thiol redox metabolism engineering in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2015;99:9771–8.
Kobayashi J, Sasaki D, Hara KY, Hasunuma T, Kondo A. Enzymatic improvement of mitochondrial thiol oxidase Erv1 for oxidized glutathione fermentation by Saccharomyces cerevisiae. Microb Cell Fact. 2017;16:44.
Kobayashi J, Sasaki D, Bamba T, Hasunuma T, Kondo A. Sustainable production of glutathione from lignocellulose-derived sugars using engineered Saccharomyces cerevisiae. Appl microbio biotechnol. 2019;103:1243–54.
Alfafara CG, Kanda A, Shioi T, Shimizu H, Shioya S, Suga K. Effect of amino acids on glutathione production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1992;36:538–40.
Wen S, Zhang T, Tan T. Utilization of amino acids to enhance glutathione production in Saccharomyces cerevisiae. Enz Microbial Technol. 2004;35:501–7.
Albers E, Laize V, Blomberg A, Hohmann S, Gustafsson L. Ser3p (Yer081wp) and Ser33p (Yil074cp) are phosphoglycerate dehydrogenases in Saccharomyces cerevisiae. J Biol Chem. 2003;278:10264–72.
Majtan T, Pey AL, Fernández R, Fernández JA, Martínez-Cruz LA, Kraus JP. Domain organization, catalysis and regulation of eukaryotic cystathionine beta-synthases. PLoS ONE. 2014;9: e105290.
Cha J, Park J, Jeon B, Lee Y, Cho Y. Optimal fermentation conditions for enhanced glutathione production by Saccharomyces cerevisiae FF-8. J Microbiol. 2004;42:51–5.
Shang F, Wang Z, Tan T. High-cell-density cultivation for co-production of ergosterol and reduced glutathione by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2008;77:1233–40.
Nisamedtinov I, Kevvai K, Orumets K, Rautio JJ, Paalme T. Glutathione accumulation in ethanol-stat fed-batch culture of Saccharomyces cerevisiae with a switch to cysteine feeding. Appl Microbiol Biotechnol. 2010;87:175–83.
Schmacht M, Lorenz E, Stahl U, Senz M. Medium optimization based on yeast’s elemental composition for glutathione production in Saccharomyces cerevisiae. J Biosci Bioeng. 2017;123:555–61.
Wang M, Sun J, Xue F, Shang F, Wang Z, Tan T. The effect of intracellular amino acids on GSH production by high-cell-density cultivation of Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2012;168:198–205.
Hans MA, Heinzle E, Wittmann C. Quantification of intracellular amino acids in batch cultures of Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2001;56:776–9.
Bu X, Sun L, Shang F, Yan G. Comparative metabolomics profiling of engineered Saccharomyces cerevisiae lead to a strategy that improving β-carotene production by acetate supplementation. PLoS ONE. 2017;12: e0188385.
Wen S, Zhang T, Tan T. Optimization of the amino acid composition in glutathione fermentation. Proc Biochem. 2005;40:3474–9.
Wen S, Zhang T, Tan T. Maximizing production of glutathione by amino acid modulation and high-cell-density fed-batch culture of Saccharomyces cerevisiae. Proc Biochem. 2006;41:2424–8.
Wang Z, Tan T, Song J. Effect of amino acids addition and feedback control strategies on the high-cell-density cultivation of Saccharomyces cerevisiae for glutathione production. Proc Biochem. 2007;42:108–11.
Lorenz E, Schmacht M, Stahl U, Senz M. Enhanced incorporation yield of cysteine for glutathione overproduction by fedbatch fermentation of Saccharomyces cerevisiae. J Biotechnol. 2015;216:131–9.
Schmacht M, Lorenz E, Senz M. Microbial production of glutathione. World J Microbiol Biotechnol. 2017;33:106.
Kobayashi J, Sasaki D, Hara KY, Kondo A. Genetic engineering of microorganisms for high glutathione production. In: Perjési P, editor. Glutathione: biosynthesis, functions and biological implications. Nova Science Publishers; 2019. p. 83–103.
Suzuki T, Yokoyama A, Tsuji T, Ikeshima E, Nakashima K, Ikushima S, Kobayashi C, Yoshida S. Identification and characterization of genes involved in glutathione production in yeast. J Biosci Bioeng. 2011;112:107–13.
Qiu Z, Deng Z, Tan H, Zhou S, Cao L. Engineering the robustness of Saccharomyces cerevisiae by introducing bifunctional glutathione synthase gene. J Ind Microbiol Biotechnol. 2015;42:537–42.
Tang L, Wang W, Zhou W, Cheng K, Yang Y, Liu M, Cheng K, Wang W. Three-pathway combination for glutathione biosynthesis in Saccharomyces cerevisiae. Microb Cell Fact. 2015;14:139.
Yamada R, Taniguchi N, Tanaka T, Ogino C, Fukuda H, Kondo A. Cocktail delta-integration: a novel method to construct cellulolytic enzyme expression ratio-optimized yeast strains. Microb Cell Fact. 2010;14:32.
Ishii J, Kondo T, Makino H, Ogura A, Matsuda F, Kondo A. Three gene expression vector sets for concurrently expressing multiple genes in Saccharomyces cerevisiae. FEMS Yeast Res. 2014;14:399–411.
Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–8.
Chen DC, Yang BC, Kuo TT. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–4.
Kobayashi J, Sasaki D, Kondo A. A procedure for precise determination of glutathione produced by Saccharomyces cerevisiae. Bio Protoc. 2018;8: e2887.
Acknowledgements
We are grateful to Dr. J. Ishii (Graduate School of Science, Technology and Innovation, Kobe University) for providing us with the pGK plasmid series.
Funding
This study was funded by a Special Coordination Fund for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction, Kobe) from the Ministry of Education, Culture, Sports, and Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Contributions
KYH and DS conceived the study and designed the experiments. JK performed the experiments and wrote the manuscript. TH and AK designed and guided the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kobayashi, J., Sasaki, D., Hara, K.Y. et al. Metabolic engineering of the l-serine biosynthetic pathway improves glutathione production in Saccharomyces cerevisiae. Microb Cell Fact 21, 153 (2022). https://doi.org/10.1186/s12934-022-01880-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01880-8