Abstract
Gastrointestinal cancer is the most common malignancy in humans, often accompanied by poor prognosis. N6-methyladenosine (m6A) modification is widely present in eukaryotic cells as the most abundant RNA modification. It plays a crucial role in RNA splicing and processing, nuclear export, translation, and stability. Human AlkB homolog 5 (ALKBH5) is a type of RNA demethylase exhibiting abnormal expression in various gastrointestinal cancers.It is closely related to the tumorigenesis, proliferation, migration, and other biological functions of gastrointestinal cancer. However, recent studies indicated that the role and mechanism of ALKBH5 in gastrointestinal cancer are complicated and even controversial. Thus, this review summarizes recent advances in elucidating the role of ALKBH5 as a tumor suppressor or promoter in gastrointestinal cancer. It examines the biological functions of ALKBH5 and its potential as a therapeutic target, providing new perspectives and insights for gastrointestinal cancer research.
Similar content being viewed by others
Background
Gastrointestinal cancer is among the most common and deadly tumors worldwide, accounting for approximately one-fourth of the global cancer incidence and a mortality rate as high as one-third [1]. Although current conventional treatments, such as surgical resection, chemotherapy, and radiotherapy, are used for treating gastrointestinal cancer, the risks of cancer recurrence and drug resistance remain high. Precision targeting of tumors holds great promise in cancer therapy, leading to more in-depth research and analysis of cancer.
Epigenetic modifications are alterations independent of the DNA sequence [2], primarily regulating gene expression at the transcriptional level. Discoveries have been made in various aspects, including DNA and RNA methylation [3, 4], histone modifications [5], transcriptional control [6], chromatin remodeling [7], non-coding RNA [8], and cancer immunotherapy [9]. Recent research indicated that cancer cells are often regulated by relevant epigenetic proteins, which are essential for maintaining normal cell growth, inducing differentiation, and initiating, sustaining, and propagating disease and abnormal cell states [10]. There is growing evidence indicating that epigenetic modifications, especially RNA modifications play a crucial role in tumorigenesis [11, 12].
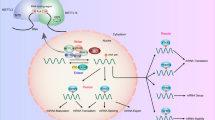
With the development of high-throughput sequencing technologies, over 170 RNA modifications have been identified, and m6A modification is one of the most prevalent types [13]. Transcriptome sequencing reveals that m6A binding sites are found within the RRACH sequence (R = A/G, H = A/C/U), predominantly enriched in the 3’ untranslated regions (UTRs) near the termination codon of mRNA exons [14, 15]. M6A modification participates in various RNA metabolism processes, including splicing, nuclear export, translation, decay, processing, and RNA-protein interactions. Additionally, it plays a crucial role in embryonic stem cell differentiation, meiosis, tissue development, circadian rhythm, and tumor occurrence[16,17,18,19,20]. M6A modification is a dynamic and reversible process, which is primarily led by methyltransferases (Writers), demethylases (Erasers), and identified and promoted by some specific RNA-binding proteins (Readers)[21](Fig. 1).
Up to now, methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), methyltransferase-like 16 (METTL16), Wilms tumor 1 associated protein (WTAP), zinc finger CCCH-type containing 13 (ZC3H13) proteins, RNA-binding motif protein 15 (RBM15), Vir-like m6 A methyltransferase-associated (VIRMA/KIAA1429), Cbl proto-oncogene like1 (CBLL1/Hakai), and Fl(2)d-associated complex component (Flacc) were regarded as Writers, and they can interact with each other to form a stable methyltransferase complex (MTC), or plays a supporting role in catalyzing the heterodimeric methyltransferase activity [22,23,24,25,26,27,28,29,30,31](Fig. 1). Erasers, including Fat mass and obesity-associated protein (FTO) and ALKBH5, are central to the removal of m6A modifications [32, 33]. They are part of the alpha-ketoglutarate-dependent dioxygenase family, mediating the reverse process of m6A methylation under the action of Fe(II) and α-ketoglutarate[34]. FTO is involved in the regulation of the cell cycle, cell differentiation, splicing, cancer development, immunotherapy, and various other biological functions [35,36,37,38]. Importantly, ALKBH5, as another member of the α-ketoglutarate-dependent dioxygenase family, was found that knocking out ALKBH5 not only increases the m6A levels of RNA inside cells but also enhances the export of these RNAs from the nucleus to the cytoplasm [33]. Readers mainly include the YT521-B homology (YTH) domain family, IGF2 mRNA binding protein (IGF2BPs), and heterogeneous nuclear ribonucleoproteins (HNRNPs)[39,40,41,42,43,44,45,46,47]. And they mainly play a role in post-transcriptional regulation by identifying and binding to m6A-targeted genes, regulating downstream functions (Fig. 1).
Molecular mechanism of m6A modification. M6A is mediated by writers, erasers and readers, the details are as follows. METTL14 binds to METTL3 and forms a stable MTC. WTAP recruits MTC and localizes it in the nuclear speckle, performing a function that aids in catalyzing the activity of methyltransferases. ZC3H13, RBM15, and VIRMA act on the MTC to regulate the occurrence of m6A methylation. Hakai serves as a core component of the m6A writer and interacts with other writers. METTL16, which is a conserved U6 snRNA methyltransferase, controls the homeostasis of S-adenosylmethionine (SAM) by post-transcriptionally regulating the expression of SAM synthase genes. FTO and ALKBH5 belong to the family of ketoglutarate-dependent dioxygenases, mediating the reverse process of m6A methylation under the action of Fe(II) and α-ketoglutarate. YTHDF2 is the first discovered m6A reader protein, regulating the degradation of mRNA. YTHDF1 can promote the translation of m6A-modified mRNAs by binding to m6A sites and regulating translation factors. YTHDF3 can act on YTHDF1 to enhance the translation of mRNAs, and it can also act on YTHDF2 to regulate the degradation of mRNAs. YTHDC1 regulates the splicing function of mRNAs by recruiting splicing factors, and it also mediates m6A-dependent nuclear export. YTHDC2, as an RNA helicase, can enhance the translation efficiency of mRNAs while reducing their RNA abundance. IGF2BPs recognize the GG(m6A)C sequence and promote the stability, translation, and storage of targeted mRNAs. HNRNPs regulate the processing, and maturation of miRNAs and the abundance and splicing of mRNAs in an m6A-dependent manner. (figure was created with Biorender.com)
Among these regulators, ALKBH5, which was first discovered in 2013 as a demethylase for m6A, is becoming a hub in the research of epigenetic regulation of the development of cancer cells. The complicated biological functions of ALKBH5 have been widely found to be involved in various gastrointestinal cancers, including gastric cancer (GC), colorectal cancer (CRC), liver cancer (LC), pancreatic cancer (PC), and esophageal squamous cell carcinoma (ESCC). This review will focus on the role of ALKBH5 in gastrointestinal cancer and discuss directions for future research and potential clinical application of ALKBH5 for gastrointestinal cancer.
The structure and role of ALKBH5
ALKBH5 is a member of the AlkB family, a 2-oxoglutarate and ferrous acid-dependent nucleic acid oxygenase. It is located on human chromosome 17p11.2. Human ALKBH5 has a full length of 394 amino acids and its catalytic core contains features of a double-stranded β-helix fold (DSBH). Aik W et al. suggested that the DSBH fold of ALKBH5 is composed of eight reverse parallel β-strands, with the major β-sheet consisting of β6, β8, β11, and β13, and the minor β-sheet being formed by β7, β9, β10, and β12 [48]. However, Feng C et al. suggested that the DSBH fold of ALKBH5 does not have the typical eight reverse parallel β-strands, where β4, β5, β8, and β9 form the major sheet while β6, β7, and a short α-helix (α7) plus a long loop (C1) form the minor sheet [49]. The unique structural feature of ALKBH5 that plays an important role in substrate recognition and catalysis is the nucleotide recognition caps called “Flip1” and “Flip2” outside the DSBH fold. In addition, a disulfide bond formed between Cys-230 and Cys-267 has been identified in ALKBH5, and this structure is believed to underlie the selectivity of ALKBH5 for single-stranded substrates [48, 49].
Normally, ALKBH5 is highly expressed in the testis, and it has been found that increased m6A expression in ALKBH5-deficient male mice affects apoptosis in mid-meiotic spermatocytes, leading to impaired fertility [33, 50]. In addition to the fact that ALKBH5 can affect the spermatogenesis process, Pollard PJ et al. discovered that ALKBH5 is directly regulated by hypoxia-inducible factor 1α (HIF-1α), an oxygenase dependensst on 2-oxoglutarate (2OG) that is induced under hypoxic conditions. [51]. It has also shown that ALKBH5 can affect osteogenesis in ligamentum flavum cells through the protein kinase B (AKT) signaling pathway [52], as well as the osteogenic process through the NF-κB (nuclear factor-κB) signaling pathway [53].
The role of ALKBH5 in gastrointestinal cancer
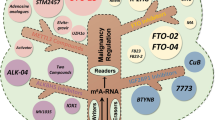
Increasing evidence suggests that the m6A demethylase ALKBH5 is aberrantly expressed in various gastrointestinal cancers, closely associated with tumorigenesis, tumor proliferation, migration, invasion, and more (Table 1), making it a potential novel target for cancer treatment (Fig. 2). In the following section, we discuss the expression of ALKBH5 in gastrointestinal cancer and the mechanisms involved.
Biological functions of ALKBH5 in gastrointestinal cancer. ALKBH5 regulates tumor cell proliferation, migration, and invasion. ALKBH5 also controls monocyte recruitment and M2 polarization, as well as cellular autophagy. ALKBH5 also plays a role in tumor immune infiltration, hot/cold tumor transition, and the enhancement or attenuation of tumor radiosensitivity. It can also regulate epithelial-mesenchymal transition, stemness maintenance, glycolysis, and RNA degradation. (figure was created with Biorender.com)
Colorectal cancer
CRC is the third deadliest cancer in the world, ranking consistently among the top three in both incidence and mortality rates among all cancer types. It accounts for approximately 10% of all cancer-related deaths each year [54]. Despite increasing research and treatment efforts dedicated to colorectal cancer each year, many molecular mechanisms remain unclear. There is growing evidence that m6A modification plays a crucial role in the molecular regulation of CRC (Fig. 3).
Bioinformatics results revealed a decreased expression of ALKBH5 in CRC. Its expression is strongly correlated with survival prognosis, staging, distant metastasis, and the American Joint Committee on Cancer (AJCC) stage, establishing it as one of the independent prognostic indicators for CRC. Immune checkpoint inhibitor therapy, as one of the mature approaches in current cancer treatment, is often less effective due to the low immunogenicity of cold tumors. ALKBH5, in collaboration with YTHDF1, can impact the immune environment, promoting the transformation of colon adenocarcinoma (COAD) patients from the cold tumor type to the hot tumor type [55, 56], greatly enhancing the effectiveness of immunotherapy. ALKBH5 can bind to the Wnt pathway inhibitor AXIN2, inducing its degradation, thereby activating Wnt/β-catenin and its associated protein Dickkopf-related protein 1 (DKK1). This process induces DKK1 to recruit inhibitory cells derived from the bone marrow, driving immune suppression in CRC [57]. Overexpression of ALKBH5 suppresses CRC cell proliferation, migration, and invasion. It alleviates the malignant progression of CRC by promoting CD8(+) T cell infiltration in the tumor microenvironment through the NF-κB (nuclear factor-κB)-CCL5(C-C motif chemokine ligand 5) axis [58]. Furthermore, related studies suggest that ALKBH5 is downregulated in CRC and is associated with poor prognosis in CRC patients. ALKBH5 can suppress the occurrence and development of CRC by removing the methylation modification of its downstream target gene plant homeodomain finger protein 20 (PHF20), thereby reducing the mRNA stability of PHF20 [59]. Wu and colleagues discovered that improving the identification and delivery system for CRC treatment can effectively alleviate the development of CRC. They confirmed the effectiveness of this approach in mitigating CRC development by synthesizing folate-modified exosome-liposome hybrid nanoparticles loaded with ALKBH5 mRNA and utilizing nano therapy to modulate the ALKBH5/JMXD8/PKM2 (Pyruvate kinase M2) axis and suppress glycolysis [60].
It is worth noted that experimental results from some scholars indicate that ALKBH5 can act as an oncogene, promoting the occurrence and development of CRC. That suggests that ALKBH5 may have a dual regulatory role in CRC. Shen et al. discovered that ALKBH5 can function as an upstream target of a Rab GTPase family protein (RAB5A), and through m6A-YTHDF2-dependent mechanisms, reduce the mRNA degradation efficiency of RAB5A, increasing the expression of RAB5A, thereby promoting the progression of CRC [61]. The regulation of CRC by ALKBH5 in non-coding RNA has also been reported. The ALKBH5-LncRNA NEAT1 (lncRNA nuclear paraspeckle assembly transcript 1) axis may serve as a potential therapeutic target for CRC. NEAT1 is upregulated in CRC and is associated with poor prognosis, and ALKBH5 promotes the progression of COAD by reducing the methylation of lncRNA NEAT1 [62]. ALKBH5 can decrease the m6A modification of Forkhead box O3 (FOXO3), and enhance the RNA stability of FOXO3. Thus, it targets miR-21 through FOXO3 and increases sprouty2 (SPRY2) expression, forming the FOXO3/miR-21/SPRY2 axis to regulate the progression of CRC [63]. Research indicates that CircRNA AFF2 is highly expressed in radiation-sensitive colorectal cancer patients, and those with high expression have a better prognosis. Its regulation is closely associated with the ALKBH5/YTHDF2 m6A-dependent pathway. CircAFF2 can reverse the radiation sensitivity induced by ALKBH5 or YTHDF2 and may serve as a potential target for radiotherapy in CRC [64]. Luo et al. discovered that ALKBH5 is downregulated in CRC. ALKBH5 removes m6A modification on the mRNA of solute carrier family 7 members 11 (SLC7A11), reducing mRNA stability, thereby decreasing SLC7A11 transcription and expression, promoting ferroptosis in CRC cells [65].
In summary, these studies confirm the close association of ALKBH5 with the progression of CRC, suggesting that ALKBH5 may hold significant clinical significance as a target for drug therapy in CRC.
Liver cancer
LC is the sixth most common cancer globally, ranking fourth in mortality among all cancers [66]. Studies suggest that ALKBH5 is highly expressed in hepatocellular carcinoma (HCC) and correlates with poor prognosis in HCC patients. Tumor-associated macrophages (TAMs) play a critical role in establishing the tumor microenvironment. ALKBH5, through an m6A-dependent mechanism, regulates the expression of mitogen-activated protein kinase kinase kinase 8 (MAP3K8), mediating the activation of downstream c-Jun N-terminal kinase (JNK) and extracellular regulated kinase (ERK) pathways, and promoting HCC cell proliferation, migration, and the recruitment of programmed death-ligand 1 (PD-L1) + macrophages [67]. Related studies have reported interactions of ALKBH5 with PD-L1 mRNA in intrahepatic cholangiocarcinoma (ICC). Through the ALKBH5-PD-L1 axis, it maintains the expression of PD-L1 in tumor cells, suppressing T-cell proliferation and cytotoxicity, and regulating the occurrence of ICC [68]. Liver cancer stem cells (LCSCs) are closely associated with the treatment and recurrence of LC. ALKBH5 regulates the expression of SRY-related HMG box (SOX4) through demethylation, thereby activating the sonic hedgehog (SHH) signaling pathway and promoting the progression of LCSCs [69]. Extracellular vesicles (EVs) play a crucial role in the intercellular transfer of various bioactive substances that promote tumor proliferation, migration, invasion, and development. Han et al. discovered that bone-metastasized HCC-derived EVs (BM-EVs) can promote the progression of HCC by transferring miR-3190 targeting ALKBH5 [70]. Recently, Zhang et al. found that LINC02551 serves as a target of ALKBH5, disrupting the combination between DEAD-box RNA helicase (DDX24) and E3 ubiquitin ligase tripartite motif-containing 27 (TRIM27) to reduce the ubiquitination of DDX24 and subsequent degradation, ultimately promoting HCC growth and metastasis [71].
Some studies suggest that ALKBH5 is downregulated in HCC compared to normal liver cells and may act as a tumor suppressor to inhibit cancer development processes such as proliferation, migration, and invasion in HCC. Therefore, further exploration of the role of ALKBH5 in HCC is warranted. Wang et al. found that ALKBH5 is downregulated in HCC, through interaction with the m6A reader protein IGF2BP1, downregulates the expression of its target gene AdipoQ Receptor 4 (PAQR4) at the transcriptional and translational levels, thereby inhibiting the activation of the PI3K/AKT pathway and the growth of LC [72]. Research indicates that LY6/PLAUR Domain Containing 1 (LYPD1) can act as an oncogene to promote the occurrence and development of HCC. ALKBH5, through an m6A-dependent mechanism, diminishes the expression of LYPD1 and strengthens the inhibitory effect of ALKBH5 on LYPD1 under the recognition and stabilization of IGF2BP1[73]. Hepatic stellate cells (HSCs) can induce radiation-induced liver fibrosis (RILF) under radiotherapy for HCC. ALKBH5 can regulate the hepatic microenvironment and serve as a radiosensitization target for HCC, providing new insights into the radiotherapy and prognosis of HCC [74] (Fig. 3).
Overall, the role of ALKBH5 expression in LC is complex and diverse. It can either promote or inhibit LC progression, and these contradictory findings may be due to different pathways regulated by ALKBH5.
Pancreatic cancer
PC is a prevalent malignancy of the digestive tract, marked by challenging early diagnosis, concealed symptoms, and high mortality, with a 5-year survival rate of less than 10% [75]. Research suggests that ALKBH5 is downregulated in PC. Kaplan-Meier survival analysis demonstrates a significant correlation between low ALKBH5 expression and overall survival in PC patients. ALKBH5 interacts with the YTHDF2 reading protein to upregulate the expression of the period circadian regulator 1 (PER1) gene in an m6A-dependent manner. The upregulation of PER1 activates the P53-related signaling pathway, suppressing the growth of PC cells [76]. Antisense LncRNA is closely linked to tumor development. He et al. discovered that Potassium two pore domain channel subfamily K member 15 and WISP2 antisense RNA 1 (KCNK15-AS1) is downregulated in PC cells and tissues, leading to the suppression of migration and invasion in PC cells. Mechanistically, ALKBH5 enhances the expression of KCNK15-AS1 by demethylating m6A modification. It recruits the proto-oncogene mouse double minute 2 (MDM2) to facilitate the ubiquitination of RE1-silencing transcription factor (REST), leading to the transcriptional upregulation of phosphatase and tension homolog (PTEN) to deactivate the AKT signaling pathway [77, 78]. Iron metabolism plays a crucial role in multiple aspects of cancer cells, including DNA synthesis, mitochondrial respiration, cell proliferation, and the tumor microenvironment. In pancreatic ductal adenocarcinoma (PDAC), ALKBH5 regulates the stability of F-box and leucine-rich repeat protein 5 (FBXL5) RNA. Overexpression of ALKBH5 results in a marked decrease in intracellular iron levels, along with reduced cell migration and invasion capabilities. FBXL5, through the regulation of iron proteins such as iron regulatory protein 2 (IRP2), contributes to the control of PDAC occurrence and progression [79]. Tang and colleagues discovered that overexpression of ALKBH5 enhances the sensitivity of PDAC cells to chemotherapy. Reduced levels of ALKBH5 are linked to poor prognosis in PDAC and various other cancers. ALKBH5 can impact the Wnt signaling pathway, decrease RNA methylation of Wnt inhibitory factor 1 (WIF-1), and inhibit PC tumorigenesis [80]. Studies suggest that ALKBH5-mediated m6A modification results in the upregulation of DNA damage-inducible transcript 4 (DDIT4-AS1) expression in PDAC. DDIT-AS1, by stabilizing DDIT4 and activating the mechanistic target of the rapamycin (mTOR) pathway, enhances cancer stem cells and inhibits chemosensitivity to gemcitabine (GEM) [81]. M6A methylation is closely associated with the tumor hypoxic microenvironment. Methylated RNA immunoprecipitation sequencing (MeRIP-seq) results reveal that histone deacetylase type 4 (HDAC4) is an m6A-targeted gene in the tumor-hypoxic environment, and it modulates the tumor-hypoxic microenvironment through the ALKBH5/HDAC4 /HIF1α pathway [82] (Fig. 3). In summary, ALKBH5 is downregulated in PC, and could influence the growth, migration, invasion, and chemotherapy sensitivity of PC cells through multiple mechanisms.
Gastric cancer
GC is presently among the most common cancers, ranking fifth in the incidence of various cancers [54]. Significant attention should be given to the diagnosis and treatment of GC. Experimental evidence from in vivo and in vitro studies indicates that ALKBH5 is upregulated in GC and correlates with clinical poor prognosis and low survival rates. LINC00659 facilitates the binding and upregulation of ALKBH5 with Janus kinase 1 (JAK1) mRNA in a m6A-YTHDF2-dependent manner, thereby promoting the development of GC [83]. Wang and colleagues discovered that lncRNA NRON is highly expressed in GC and promotes the occurrence and development of GC by binding with demethylase ALKHB5 to mediate Nanog (homeobox domain transcription factor) mRNA decay. It is anticipated to be a prognostic factor and potential therapeutic target for GC patients [84]. Studies suggest a close association between lncRNA NEAT1 and ALKBH5. MeRIP experiments and rescue experiments confirm that ALKBH5 can bind to lncRNA NEAT1, mediating the demethylation process of NEAT1 in an m6A-dependent manner. This process influences the expression of EZH2 (a subunit of the polycomb repressive complex) and contributes to the invasion and metastasis of GC [85]. Bioinformatics results indicate that ALKBH5 acts as an upstream target of Protein kinase, membrane-associated tyrosine/threonine 1 (PKMYT1), negatively regulating PKMYT1 expression. In collaboration with the reading protein IGF2BP3, PKMYT1’s mRNA stability is increased. Depletion of ALKBH5 results in the upregulation of PKMYT1 expression, consequently promoting the invasion and migration of GC [86](Fig. 3). In conclusion, the expression of ALKBH5 is upregulated in GC. ALKBH5 promotes the invasion and metastasis of GC cells through various mechanisms, and is associated with poor prognosis and low survival rates of GC patients.
Esophageal squamous cell carcinoma
ESCC is the seventh most common cancer globally, and it stands as the sixth most common cause of cancer-related deaths. Notably, ESCC exhibits high recurrence rates, leading to an unfavorable prognosis over the long term [87]. Xiao et al. discovered reduced expression of ALKBH5 in ESCC. The overexpression of ALKBH5 suppresses the proliferation, migration, and invasion of ESCC cells. Simultaneously, it induces a certain degree of G1 phase arrest in ESCC cells, suggesting that the deficiency in ALKBH5 expression is one of the contributing factors to the malignancy of ESCC tumors. In vivo, experiments confirm that the loss of ALKBH5 significantly inhibits the tumor growth of ESCC cells transplanted subcutaneously in BALB/c nude mice. ALKBH5 acts as an independent prognostic factor for patient survival and is correlated with poor prognosis in ESCC patients [88].
Currently, research on ALKBH5 in ESCC is relatively scarce, and more substantial research is needed for making the role and mechanism clearer.
ALKBH5 promotes or inhibits the progression of gastrointestinal cancer by targeting related molecules in concert with reader proteins. (A) ALKBH5 regulates the molecular mechanism of CRC. (B) ALKBH5 regulates the molecular mechanism of HCC, ICC, and LCSCs. (C) ALKBH5 regulates the molecular mechanism of PC and PDAC. (D) ALKBH5 regulates the molecular mechanism of GC. (figure was created with Biorender.com)
Future perspectives
In recent years, with the confirmed demethylase activity of ALKBH5 and the rapid development of high-throughput sequencing for m6A methylation, research on the demethylase ALKBH5 has steadily advanced worldwide. The dysregulation of m6A demethylase ALKBH5 is observed in various gastrointestinal cancers and can directly or indirectly function as a regulatory gene in multiple cancers, regulating processes such as tumor cell proliferation, migration, invasion, metastasis, and drug resistance, thereby influencing the progression of cancer. However, it is worth noting that ALKBH5 plays a dual role in inhibiting or promoting cancer development in certain digestive tract tumors. For example, in CRC, ALKBH5 inhibits CRC development by reducing the mRNA stability of PHF20 [54], while lncRNA NEAT1 promotes CRC progression under the demethylation effect of ALKBH5 [57]. This may be related to the heterogeneity of tumors, differences in the clinical samples collected by researchers, differences in research models, and so on. Therefore, further in-depth research and analysis of the genes regulated by ALKBH5 in specific cancers are needed. Additionally, multiple studies indicate that in the process of regulating the occurrence and development of cancer through downstream target genes, reader proteins often play an auxiliary modifying role. Reader proteins enhance the binding ability between ALKBH5 and target genes by regulating the stability of downstream gene mRNA, mediating the demethylation process of target genes. This provides a new direction for the research on the regulatory mechanism of ALKBH5 in cancer and the development of targeted therapeutic drugs that affect tumor progression through the m6A-dependent regulation of related genes.
Conclusions
ALKBH5 has emerged as an important regulator and promising therapeutic target for the treatment of gastrointestinal cancer. However, the current research on the mechanism of ALKBH5 in gastrointestinal cancer is still in the preliminary stage, and there is a significant gap that needs to be filled in understanding the mechanisms of ALKBH5 in regulating metabolism, angiogenesis, and related signaling pathways, which may be the causes of the dual or controversial role of ALKBH5 in gastrointestinal cancer. More efforts in advanced studies will hold great potential and promote our understanding of the role of ALKBH5 in gastrointestinal cancer as well as lead to the development of more effective and personalized treatments for patients with gastrointestinal cancer.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- m6A:
-
N6-methyladenosine
- UTRs:
-
Untranslated regions
- ALKBH5:
-
AlkB homolog 5
- METTL3:
-
Methyltransferase-like 3
- METTL14:
-
Methyltransferase-like 14
- METTL16:
-
Methyltransferase-like 16
- WTAP:
-
Wilms tumor 1 associated protein
- ZC3H13:
-
Zinc finger CCCH-type containing 13
- RBM15:
-
RNA-binding motif protein 15
- VIRMA:
-
Vir-like m6 A methyltransferase-associated
- CBLL1:
-
Cbl proto-oncogene like1
- Flacc:
-
Fl(2)d-associated complex component
- FTO:
-
Fat mass and obesity-associated protein
- YTH:
-
YT521-B homology
- YTHDF1/2/3:
-
YTH N6-methyladenosine RNA binding protein 1/2/3
- YTHDC1/2:
-
YT521-B homology-domain-containing protein 1/2
- IGF2BPs:
-
IGF2 mRNA binding protein
- IGF2BP1/2:
-
Insulin-like growth factor 2 mRNA binding protein 1/2
- HNRNPs:
-
Heterogeneous nuclear ribonucleoproteins
- GC:
-
Gastric cancer
- CRC:
-
Colorectal cancer
- LC:
-
Liver cancer
- PC:
-
Pancreatic cancer
- PDAC:
-
Pancreatic ductal adenocarcinoma
- COAD:
-
Colon adenocarcinoma
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Intrahepatic cholangiocarcinoma
- LCSCs:
-
Liver cancer stem cells
- HSCs:
-
Hepatic stellate cells
- ESCC:
-
Esophageal squamous cell carcinoma
- DSBH:
-
Double-stranded β-helix fold
- HIF-1α:
-
Hypoxia-inducible factor 1α
- AJCC:
-
American Joint Committee on Cancer
- AKT:
-
Protein kinase B
- NF-κB:
-
Nuclear factor-κB
- DKK1:
-
Dickkopf-related protein 1
- CCL5:
-
C-C motif chemokine ligand 5
- PHF20:
-
Plant homeodomain finger protein 20
- PKM2:
-
Pyruvate kinase M2
- RAB5A:
-
A Rab GTPase family protein
- NEAT1:
-
Nuclear paraspeckle assembly transcript 1
- FOXO3:
-
Forkhead box O3
- SPRY2:
-
Sprouty2
- SLC7A11:
-
Solute carrier family 7 members 11
- TAMs:
-
Tumor-associated macrophages
- MAP3K8:
-
Mitogen-activated protein kinase kinase kinase 8
- JNK:
-
C-Jun N-terminal kinase
- ERK:
-
Extracellular regulated kinase
- PD-L1:
-
Programmed death-ligand 1
- SOX4:
-
SRY-related HMG box
- SHH:
-
Sonic hedgehog
- EVs:
-
Extracellular vesicles
- BM-EVs:
-
Bone-metastasized HCC-derived EVs
- DDX24:
-
DEAD-box RNA helicase
- TRIM27:
-
E3 ubiquitin ligase tripartite motif-containing 27
- PAQR4:
-
AdipoQ Receptor 4
- PI3K:
-
Phosphatidylinositol 3-kinase
- LYPD1:
-
LY6/PLAUR Domain Containing 1
- RILF:
-
Radiation-induced liver fibrosis
- PER1:
-
Period circadian regulator 1
- KCNK15-AS1:
-
K member 15 and WISP2 antisense RNA 1
- MDM2:
-
Mouse double minute 2
- REST:
-
RE1-silencing transcription factor
- PTEN:
-
Phosphatase and tension homolog
- FBXL5:
-
F-box and leucine-rich repeat protein 5
- IRP2:
-
Iron regulatory protein 2
- WIF-1:
-
Wnt inhibitory factor 1
- DDIT4-AS1:
-
DNA damage-inducible transcript 4
- mTOR:
-
Mechanistic target of rapamycin
- GEM:
-
Gemcitabine
- MeRIP-seq:
-
Methylated RNA immunoprecipitation sequencing
- HDAC4:
-
Histone deacetylase type 4
- JAK1:
-
Janus kinase 1
- PKMYT1:
-
Protein kinase, membrane associated tyrosine/threonine 1
References
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F: Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159(1):335–349.e315.
Dawson MA, Kouzarides T: Cancer epigenetics: from mechanism to therapy. Cell 2012, 150(1):12–27.
Mattei AL, Bailly N, Meissner A: DNA methylation: a historical perspective. Trends in Genetics : TIG 2022, 38(7):676–707.
Pan Y, Ma P, Liu Y, Li W, Shu Y: Multiple functions of m(6)A RNA methylation in cancer. Journal of Hematology & Oncology 2018, 11(1):48.
Yano S, Ishiuchi T, Abe S, Namekawa SH, Huang G, Ogawa Y, Sasaki H: Histone H3K36me2 and H3K36me3 form a chromatin platform essential for DNMT3A-dependent DNA methylation in mouse oocytes. Nature Communications 2022, 13(1):4440.
Li J, Yuan S, Norgard RJ, Yan F, Sun YH, Kim IK, Merrell AJ, Sela Y, Jiang Y, Bhanu NV et al: Epigenetic and Transcriptional Control of the Epidermal Growth Factor Receptor Regulates the Tumor Immune Microenvironment in Pancreatic Cancer. Cancer Discovery 2021, 11(3):736–753.
Uddin MS, Mamun AA, Alghamdi BS, Tewari D, Jeandet P, Sarwar MS, Ashraf GM: Epigenetics of glioblastoma multiforme: From molecular mechanisms to therapeutic approaches. Seminars in Cancer Biology 2022, 83:100–120.
Mercer TR, Dinger ME, Mattick JS: Long non-coding RNAs: insights into functions. Nature Reviews Genetics 2009, 10(3):155–159.
Cao J, Yan Q: Cancer Epigenetics, Tumor Immunity, and Immunotherapy. Trends in Cancer 2020, 6(7):580–592.
Wimalasena VK, Wang T, Sigua LH, Durbin AD, Qi J: Using Chemical Epigenetics to Target Cancer. Molecular Cell 2020, 78(6):1086–1095.
Lin YT, Wu KJ: Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-β signaling. Journal of Biomedical Science 2020, 27(1):39.
Li S, Kuo HD, Yin R, Wu R, Liu X, Wang L, Hudlikar R, Peter RM, Kong AN: Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochemical Pharmacology 2020, 175:113890.
Delaunay S, Frye M: RNA modifications regulating cell fate in cancer. Nature Cell Biology 2019, 21(5):552–559.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C: YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell research 2017, 27(3):315–328.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M et al: Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485(7397):201–206.
He PC, He C: m(6) A RNA methylation: from mechanisms to therapeutic potential. The EMBO Journal 2021, 40(3):e105977.
Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS et al: Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science (New York, NY) 2015, 347(6225):1002–1006.
Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y et al: m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 2017, 548(7667):338–342.
Kane SE, Beemon K: Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Molecular and Cellular Biology 1985, 5(9):2298–2306.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR: Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012, 149(7):1635–1646.
Meyer KD, Jaffrey SR: Rethinking m(6)A Readers, Writers, and Erasers. Annual Review of Cell and Developmental Biology 2017, 33:319–342.
Chen XY, Zhang J, Zhu JS: The role of m(6)A RNA methylation in human cancer. Molecular Cancer 2019, 18(1):103.
Guo T, Duan H, Chen J, Liu J, Othmane B, Hu J, Li H, Zu X: N6-Methyladenosine Writer Gene ZC3H13 Predicts Immune Phenotype and Therapeutic Opportunities in Kidney Renal Clear Cell Carcinoma. Frontiers in Oncology 2021, 11:718644.
Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, Liu T: The Critical Role of RNA m(6)A Methylation in Cancer. Cancer Research 2019, 79(7):1285–1292.
Wang P, Doxtader KA, Nam Y: Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Molecular Cell 2016, 63(2):306–317.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS et al: Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Research 2014, 24(2):177–189.
Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L et al: Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Molecular Cell 2018, 69(6):1028–1038.e1026.
Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D et al: Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes & development 2018, 32(5–6):415–429.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H et al: VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discovery 2018, 4:10.
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK: The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169(5):824–835.e814.
Wang Y, Zhang L, Ren H, Ma L, Guo J, Mao D, Lu Z, Lu L, Yan D: Role of Hakai in m(6)A modification pathway in Drosophila. Nature Communications 2021, 12(1):2159.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG et al: N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nature Chemical Biology 2011, 7(12):885–887.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH et al: ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Molecular Cell 2013, 49(1):18–29.
Wang T, Kong S, Tao M, Ju S: The potential role of RNA N6-methyladenosine in Cancer progression. Molecular Cancer 2020, 19(1):88.
Wu R, Liu Y, Yao Y, Zhao Y, Bi Z, Jiang Q, Liu Q, Cai M, Wang F, Wang Y et al: FTO regulates adipogenesis by controlling cell cycle progression via m(6)A-YTHDF2 dependent mechanism. Biochimica ET Biophysica Acta-molecular And Cell Biology Of Lipids 2018, 1863(10):1323–1330.
Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C et al: R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell 2018, 172(1–2):90–105.e123.
Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ et al: FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Research 2014, 24(12):1403–1419.
Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C et al: m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nature Communications 2019, 10(1):2782.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G et al: N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505(7481):117–120.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L: YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nature Communications 2016, 7:12626.
Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY et al: Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Molecular Cell 2016, 61(4):507–519.
Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P et al: YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. eLife 2017, 6.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J et al: Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Research 2017, 27(9):1115–1127.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL et al: Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nature Cell Biology 2018, 20(3):285–295.
Zhao BS, Roundtree IA, He C: Post-transcriptional gene regulation by mRNA modifications. Nature Reviews Molecular Cell Biology 2017, 18(1):31–42.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF: HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 2015, 162(6):1299–1308.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T: N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518(7540):560–564.
Aik W, Scotti JS, Choi H, Gong L, Demetriades M, Schofield CJ, McDonough MA: Structure of human RNA N⁶-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Research 2014, 42(7):4741–4754.
Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu W, Tong Y, Cheng C, Chen Z: Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. The Journal of Biological Chemistry 2014, 289(17):11571–11583.
Tang C, Klukovich R, Peng H, Wang Z, Yu T, Zhang Y, Zheng H, Klungland A, Yan W: ALKBH5-dependent m6A demethylation controls splicing and stability of long 3’-UTR mRNAs in male germ cells. Proceedings of the National Academy of Sciences of the United States of America 2018, 115(2):E325-e333.
Thalhammer A, Bencokova Z, Poole R, Loenarz C, Adam J, O’Flaherty L, Schödel J, Mole D, Giaslakiotis K, Schofield CJ et al: Human AlkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1α (HIF-1α). PloS One 2011, 6(1):e16210.
Wang HF, Kuang MJ, Han SJ, Wang AB, Qiu J, Wang F, Tan BY, Wang DC: BMP2 Modified by the m(6)A Demethylation Enzyme ALKBH5 in the Ossification of the Ligamentum Flavum Through the AKT Signaling Pathway. Calcified Tissue International 2020, 106(5):486–493.
Yu J, Shen L, Liu Y, Ming H, Zhu X, Chu M, Lin J: The m6A methyltransferase METTL3 cooperates with demethylase ALKBH5 to regulate osteogenic differentiation through NF-κB signaling. Molecular and Cellular Biochemistry 2020, 463(1–2):203–210.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71(3):209–249.
Yang P, Wang Q, Liu A, Zhu J, Feng J: ALKBH5 Holds Prognostic Values and Inhibits the Metastasis of Colon Cancer. Pathology Oncology Research : POR 2020, 26(3):1615–1623.
Yan G, An Y, Xu B, Wang N, Sun X, Sun M: Potential Impact of ALKBH5 and YTHDF1 on Tumor Immunity in Colon Adenocarcinoma. Frontiers in Oncology 2021, 11:670490.
Zhai J, Chen H, Wong CC, Peng Y, Gou H, Zhang J, Pan Y, Chen D, Lin Y, Wang S et al: ALKBH5 Drives Immune Suppression Via Targeting AXIN2 to Promote Colorectal Cancer and Is a Target for Boosting Immunotherapy. Gastroenterology 2023, 165(2):445–462.
Ge J, Liu SL, Zheng JX, Shi Y, Shao Y, Duan YJ, Huang R, Yang LJ, Yang T: RNA demethylase ALKBH5 suppresses tumorigenesis via inhibiting proliferation and invasion and promoting CD8(+) T cell infiltration in colorectal cancer. Translational Oncology 2023, 34:101683.
Zhang Z, Wang L, Zhao L, Wang Q, Yang C, Zhang M, Wang B, Jiang K, Ye Y, Wang S et al: N6-methyladenosine demethylase ALKBH5 suppresses colorectal cancer progression potentially by decreasing PHF20 mRNA methylation. Clinical and Translational Medicine 2022, 12(8):e940.
Wu S, Yun J, Tang W, Familiari G, Relucenti M, Wu J, Li X, Chen H, Chen R: Therapeutic m(6)A Eraser ALKBH5 mRNA-Loaded Exosome-Liposome Hybrid Nanoparticles Inhibit Progression of Colorectal Cancer in Preclinical Tumor Models. ACS Nano 2023, 17(12):11838–11854.
Shen D, Lin J, Xie Y, Zhuang Z, Xu G, Peng S, Tang G, Bai L, Zhu M, Zhang Y et al: RNA demethylase ALKBH5 promotes colorectal cancer progression by posttranscriptional activation of RAB5A in an m6A-YTHDF2-dependent manner. Clinical and Translational Medicine 2023, 13(5):e1279.
Guo T, Liu DF, Peng SH, Xu AM: ALKBH5 promotes colon cancer progression by decreasing methylation of the lncRNA NEAT1. American Journal Of Translational Research 2020, 12(8):4542–4549.
Wu X, Dai M, Li J, Cai J, Zuo Z, Ni S, Zhang Q, Zhou Z: m(6)A demethylase ALKBH5 inhibits cell proliferation and the metastasis of colorectal cancer by regulating the FOXO3/miR-21/SPRY2 axis. American Journal Of Translational Research 2021, 13(10):11209–11222.
Shao Y, Liu Z, Song X, Sun R, Zhou Y, Zhang D, Sun H, Huang J, Wu C, Gu W et al: ALKBH5/YTHDF2-mediated m6A modification of circAFF2 enhances radiosensitivity of colorectal cancer by inhibiting Cullin neddylation. Clinical and Translational Medicine 2023, 13(7):e1318.
Luo J, Yu H, Yuan Z, Ye T, Hu B: ALKBH5 decreases SLC7A11 expression by erasing m6A modification and promotes the ferroptosis of colorectal cancer cells. Clinical & translational Oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2023, 25(7):2265–2276.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS: Hepatocellular carcinoma. Nature Reviews Disease Primers 2021, 7(1):6.
You Y, Wen D, Zeng L, Lu J, Xiao X, Chen Y, Song H, Liu Z: ALKBH5/MAP3K8 axis regulates PD-L1 + macrophage infiltration and promotes hepatocellular carcinoma progression. International Journal of Biological Sciences 2022, 18(13):5001–5018.
Qiu X, Yang S, Wang S, Wu J, Zheng B, Wang K, Shen S, Jeong S, Li Z, Zhu Y et al: M(6)A Demethylase ALKBH5 Regulates PD-L1 Expression and Tumor Immunoenvironment in Intrahepatic Cholangiocarcinoma. Cancer Research 2021, 81(18):4778–4793.
Yang Q, Liang Y, Shi Y, Shang J, Huang X: The ALKBH5/SOX4 axis promotes liver cancer stem cell properties via activating the SHH signaling pathway. Journal of Cancer Research and Clinical Oncology 2023, 149(17):15499–15510.
Han S, Xue L, Wei Y, Yong T, Jia W, Qi Y, Luo Y, Liang J, Wen J, Bie N et al: Bone Lesion-Derived Extracellular Vesicles Fuel Prometastatic Cascades in Hepatocellular Carcinoma by Transferring ALKBH5-Targeting miR-3190-5p. Advanced Science (Weinheim, Baden-Wurttemberg, Germany) 2023, 10(17):e2207080.
Zhang H, Liu Y, Wang W, Liu F, Wang W, Su C, Zhu H, Liao Z, Zhang B, Chen X: ALKBH5-mediated m(6)A modification of lincRNA LINC02551 enhances the stability of DDX24 to promote hepatocellular carcinoma growth and metastasis. Cell Death & Disease 2022, 13(11):926.
Wang W, Huang Q, Liao Z, Zhang H, Liu Y, Liu F, Chen X, Zhang B, Chen Y, Zhu P: ALKBH5 prevents hepatocellular carcinoma progression by post-transcriptional inhibition of PAQR4 in an m6A dependent manner. Experimental Hematology & Oncology 2023, 12(1):1.
Chen Y, Zhao Y, Chen J, Peng C, Zhang Y, Tong R, Cheng Q, Yang B, Feng X, Lu Y et al: ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Molecular Cancer 2020, 19(1):123.
Chen Y, Zhou P, Deng Y, Cai X, Sun M, Sun Y, Wu D: ALKBH5-mediated m(6) A demethylation of TIRAP mRNA promotes radiation-induced liver fibrosis and decreases radiosensitivity of hepatocellular carcinoma. Clinical and Translational Medicine 2023, 13(2):e1198.
Kamisawa T, Wood LD, Itoi T, Takaori K: Pancreatic cancer. Lancet (London, England) 2016, 388(10039):73–85.
Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang Y, Feng Y, Pan Q, Wan R: RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Molecular Cancer 2020, 19(1):91.
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P, Liu D, Tian L, Yin J, Jiang K et al: ALKBH5 Inhibits Pancreatic Cancer Motility by Decreasing Long Non-Coding RNA KCNK15-AS1 Methylation. Cellular Physiology and Biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2018, 48(2):838–846.
He Y, Yue H, Cheng Y, Ding Z, Xu Z, Lv C, Wang Z, Wang J, Yin C, Hao H et al: ALKBH5-mediated m(6)A demethylation of KCNK15-AS1 inhibits pancreatic cancer progression via regulating KCNK15 and PTEN/AKT signaling. Cell Death & Disease 2021, 12(12):1121.
Huang R, Yang L, Zhang Z, Liu X, Fei Y, Tong WM, Niu Y, Liang Z: RNA m(6)A Demethylase ALKBH5 Protects Against Pancreatic Ductal Adenocarcinoma via Targeting Regulators of Iron Metabolism. Frontiers in Cell and Developmental Biology 2021, 9:724282.
Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi Y, He S, Shimamoto F: m(6)A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation and mediating Wnt signaling. Molecular Cancer 2020, 19(1):3.
Zhang Y, Liu X, Wang Y, Lai S, Wang Z, Yang Y, Liu W, Wang H, Tang B: The m(6)A demethylase ALKBH5-mediated upregulation of DDIT4-AS1 maintains pancreatic cancer stemness and suppresses chemosensitivity by activating the mTOR pathway. Molecular Cancer 2022, 21(1):174.
Liu X, Feng M, Hao X, Gao Z, Wu Z, Wang Y, Du L, Wang C: m6A methylation regulates hypoxia-induced pancreatic cancer glycolytic metabolism through ALKBH5-HDAC4-HIF1α positive feedback loop. Oncogene 2023, 42(25):2047–2060.
Fang Y, Wu X, Gu Y, Shi R, Yu T, Pan Y, Zhang J, Jing X, Ma P, Shu Y: LINC00659 cooperated with ALKBH5 to accelerate gastric cancer progression by stabilising JAK1 mRNA in an m(6) A-YTHDF2-dependent manner. Clinical and Translational Medicine 2023, 13(3):e1205.
Wang S, Wang Y, Zhang Z, Zhu C, Wang C, Yu F, Zhao E: Long Non-Coding RNA NRON promotes Tumor Proliferation by regulating ALKBH5 and Nanog in Gastric Cancer. Journal of Cancer 2021, 12(22):6861–6872.
Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, Zhao Y: ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. Journal Of Physiology And Biochemistry 2019, 75(3):379–389.
Hu Y, Gong C, Li Z, Liu J, Chen Y, Huang Y, Luo Q, Wang S, Hou Y, Yang S et al: Demethylase ALKBH5 suppresses invasion of gastric cancer via PKMYT1 m6A modification. Molecular Cancer 2022, 21(1):34.
Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC: Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 2022, 33(10):992–1004.
Xiao D, Fang TX, Lei Y, Xiao SJ, Xia JW, Lin TY, Li YL, Zhai JX, Li XY, Huang SH et al: m(6)A demethylase ALKBH5 suppression contributes to esophageal squamous cell carcinoma progression. Aging 2021, 13(17):21497–21512.
Acknowledgements
Not applicable.
Funding
High-level Talent Research Funding Program of the First Dongguan Affiliated Hospital of Guangdong Medical University (GCC2023004); Doctoral Initial Funding of Guangdong Medical University (4SG23190G, GDMU2022030); Dongguan Social Development Science and Technology Project (20231800940642); Discipline Construction Project of Guangdong Medical University (2051K20220006); GuangDong Basic and Applied Basic Research Foundation (2023A1515140148).
Author information
Authors and Affiliations
Contributions
XG and JD contributed to the background, future prospects, conclusion, and the finalization of the manuscript. SW, HQ and LH were responsible for part of the role of ALKBH5 in gastrointestinal cancers. YL, CR, ZS, CK, HW, LL and CM provided revisions to the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors who have contributed to this manuscript have provided their collective agreement for its publication in this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shu, W., Huang, Q., Chen, R. et al. Complicated role of ALKBH5 in gastrointestinal cancer: an updated review. Cancer Cell Int 24, 298 (2024). https://doi.org/10.1186/s12935-024-03480-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-024-03480-5