Abstract
Context
Diet is emerging as a modifiable component of lifestyle for influencing the incidence of liver cancer.
Objective
To investigate and quantify the potential relationship between food groups and liver cancer.
Data sources
PubMed and Web of Science were searched for eligible observational studies until 31st March, 2023.
Data extraction
The meta-analysis was conducted by pooling relative risk (RR), odds ratio (OR) or hazards ratio (HR) with 95% confidence intervals (CIs). Potential sources of heterogeneity were detected by subgroup analysis. Sensitivity analysis and publication bias test were also carried out.
Data analysis
Through stepwise screening, a total of 27 studies were included. The pooled estimates of liver cancer for whole grains and legumes intake were 0.66 (95% CI: 0.54–0.82; I2 = 25.3%) and 0.86 (95% CI: 0.75–0.99; I2 = 14.3%), respectively. However, there were null associations of nuts, poultry, egg and sweetened beverages consumption with liver cancer and the association between refined grains and liver cancer was inconclusive. In dose-response meta-analysis, the pooled estimates of liver cancer were 0.77 (95% CI: 0.65–0.91) for every 50 g/day increment in whole grains intake. Non-linear dose-response relationship (P = 0.031) was observed in the association between the intake of legumes and liver cancer, and the protective effect occurred with the dose ranging from 8 g/day to 40 g/day.
Conclusions
This meta-analysis shows that whole grains and legumes were inversely associated with liver cancer, whereas intake of nuts, poultry, egg and sweetened beverages may not be associated with liver cancer. Further quantitative research needs to be undertaken within a range of populations to investigate the relationship between food groups and liver cancer.
Systematic review registration
PROSPERO registration no. CRD42021246142
Similar content being viewed by others
Background
Liver cancer is the sixth most frequently diagnosed cancer and the third leading cause of cancer-related death worldwide in 2020 [1]. The global incidence and mortality of liver cancer have been on the rise for the past 10 years, with more than 900,000 new cases diagnosed and more than 800,000 cancer deaths annually [1]. Also, liver cancer is the most common cancer in 11 geographically diverse countries that located in Eastern Asia, South- Eastern Asia, and Northern and Western Africa [1]. Considering an overall increasing burden of liver cancer, thus, it is important to identify risk factors of liver cancer to provide ideas for its prevention. The well-established risk factors for liver cancer include chronic viral hepatitis, metabolic liver disease, alcohol drinking, smoking, obesity, and exposure to carcinogenic substances such as aflatoxins [2]. Increasing evidence suggested that diet as a modifiable component of lifestyle is suspected to influence the incidence of liver cancer [3].
Eating patterns assessed by hypothesis-driven approaches such as the Mediterranean diet score and the healthy eating index were reported to be associated with the risk of liver cancer [4,5,6]. However, concentrating on specific food groups may help to understand the role of dietary factors play in the risk of developing liver cancer, which could be more easily communicated to the public and could form the basis for dietary recommendations for the prevention of liver cancer. The World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) have recently released reports on the prevention of liver cancer through diet and physical activity [7]. There is limited but suggestive evidence that consuming fish, coffee, and dairy products may decrease the risk of liver cancer. The WCRF and AICR reports similarly suggested maintaining a healthy diet rich in fruits, vegetables, nuts and whole grains may reduce the risk of liver cancer. Despite the availability of these reports, up-to-date evidence about the association of liver cancer with consumption of grains, legumes, poultry, nuts, egg and sugar-sweetened beverages (SSBs) has not been synthesized. In addition, the results from different studies on the association of specific food groups and liver cancer are inconsistent [8]. Therefore, a thorough investigation regarding the impact of specific food groups on liver cancer is warranted to inform future dietary guidelines.
In the present study, we conducted a systematic review and meta-analysis of the association of specific food groups including grains (whole grains and refined grains), legumes, nuts, poultry, eggs and sweetened beverages with liver cancer to provide a better dietary instruction for the lay public.
Materials and methods
The current meta-analysis was conducted according to the standards and recommendations set by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [9], and was registered in the international prospective register of systematic reviews (PROSPERO: CRD42021246142). The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist for reporting the meta-analysis is shown in Supplementary Table S1.
Search strategy and selection criteria
PubMed and Web of Science databases were systematically searched from the inception to 31st, March 2023. The search terms listed in supplementary table S2 were employed to retrieve the relevant articles. In addition, references of related studies were also checked to identify additional publications of interest.
Studies meeting the following criteria were included: (1) study type was an observational study; (2) information about the association for at least one of the following 6 food groups: grains (whole grains and refined grains), legumes, nuts, poultry, egg and sugar sweetened beverages on liver cancer; (3) directly reported odds ratio (OR) or hazards ratio (HR) or relative risk (RR) with 95% confidence interval (CI), or indirectly provided relevant data for calculation; (4) if study populations overlapped, the one with larger sample size was included. The exclusion criteria were as follows: (1) food groups included in published meta-analyses related to liver cancer in recent three years (including fruits, vegetables, dairy products, total meat, red meat, white meat, processed meat and fish) [10,11,12]; (2) animal study, review, meta-analysis, letter or comment; (3) no access to full text; (4) duplicate studies retrieved from various databases. Two reviewers (Liu K and Chen W) independently performed study review and inclusion, and discrepancies were solved by a third reviewer (Ye D).
Data extraction and quality assessment
Data was extracted cross-checked by two researchers (Liu K and Xu L) independently from eligible studies. The extracted information included name of first author, publication year, data collection year, type of study design, country or region, sample size, dietary assessment method, type of food groups, type of liver cancer and variables adjusted or matched.
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of included studies with scores ranging from 0 to 9 points [13]. This scale evaluates studies on the following aspects: (I) selection of cases and controls (4 scores); (II) comparability of cases and controls (2 scores); (III) ascertainment of exposure and non-response rate (3 scores). Studies with a quality score of no less than 7 points were considered as high quality. Two reviewers (Liu K and Zhou Y) assessed the study quality, and discrepancies were resolved by consensus and discussion.
Statistical analysis
The multivariate-adjusted estimates were selected if they were reported in the original article; otherwise, the unadjusted estimates were calculated using the original data. In the categorical meta-analysis, ever intake of food groups was compared with non/occasional intake of food groups, which was defined by study-specific reference ranges. If the group of ever intake of food groups was set up into multiple categories, we combined the effect estimates into a single value in each study. For the food groups with less than 3 studies, no quantitative analysis was carried out.
The heterogeneity was evaluated by Q-statistic test and I-squared (I2) value [14, 15]. The random-effects model was used to pool the effect estimates, as the approach can be used whether or not there is heterogeneity [16]. Subgroup analyses were performed by year of publication, geographic location, quality score, sample size and study design. Furthermore, sensitivity analysis was used to check the stability of the pooled results by omitting one study at a time and combining the effect values of the remaining studies. Begg’s [17] test and Egger’s [18] test were used to evaluate publication bias.
For the significant association between specific food groups and liver cancer, we also pooled estimates comparing highest with lowest intake of food groups among the studies with equal or more than three different categories of intake of food groups. Furthermore, we conducted a two-stage dose-response meta-analysis [19]. Briefly, a restricted cubic splines model with four knots at fixed percentiles (5%, 35%, 65%, and 95% of exposure level) was used, which had negligible influence on the estimates [20]. If the original article provided exposure range but not the average or median, the midpoint of the upper limit and lower limit of the interval was regarded as the exposure level. When the highest category was open-ended, the width was assumed to be the same as that of the adjacent interval. In addition, we assigned zero as the lowest exposure dose when the lowest category was open-ended [20]. If the included studies used different units to assign the dose, we converted them into grams per day according to standard conversion from standard documents [21,22,23] (poultry: 1 serving = 100 g; nuts: 1 serving = 28 g; egg: 1 serving = 50 g).
All statistical analyses were performed using STATA version 11.0 and P < 0.05 was regarded as statistically significant.
Results
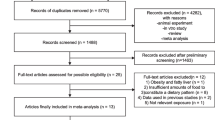
The flowchart of the literature search is presented in Fig. 1. By searching databases of PubMed and Web of Science, and manually searching relevant references, a total of 6466 studies were searched, and 27 eligible studies were finally included based on selection criteria. Among these eligible articles, 12 were from Asia [24,25,26,27,28,29,30,31,32,33,34,35], 7 from Europe [36,37,38,39,40,41,42], 7 from America [43,44,45,46,47,48,49], and one from Africa [50]. There were 15 articles with prospective study [24, 29, 30, 32, 35, 39, 40, 42,43,44,45,46,47,48,49], and 12 articles with retrospective study [25,26,27,28, 31, 33, 34, 36,37,38, 41, 50]. The median quality score of all included articles was 7, which resulted from 16 articles with a score of 7 or more and 11 articles with a score of less than 7. Detailed characteristics of the included studies are shown in Table 1.
Grains
For whole grains, a total of 2 prospective studies [46, 48] and 2 retrospective studies [36, 41] with the sample size of 612,624 (1414 cases) were included to identify the association between whole grains intake and liver cancer. The summary estimates were 0.66 (95% CI: 0.54–0.82; I2 = 25.3%; Fig. 2A) for ever versus non/occasional whole grains intake.
Sensitivity analysis showed that exclusion of each study did not significantly change the overall results (Supplementary Fig. S1). No publication bias was observed by Begg’s test (P = 0.734) or Egger’s test (P = 0.721) (Supplementary Fig. S2). In the subgroup analysis, the significant associations were found among the studies in Asian and American populations, assessed with higher quality, larger sample size and prospective study design. Subgroup analysis of publication year showed similar results with main analysis (Supplementary Table S3).
The inverse association between whole grains intake and liver cancer remained significant for highest versus lowest whole grains intake (OR = 0.58, 95% CI: 0.41–0.82; I2 = 54.7%; Fig. 2B). A linear dose-response relation between whole grains intake and the liver cancer was detected, as depicted in Fig. 2C. The pooled estimate of liver cancer was 0.77 (95% CI: 0.65–0.91; P = 0.002; n = 2) per 50 g/day increase in whole grains intake. However, no evidence of a non-linear association was observed (P = 0.102 for non-linearity).
Few large prospective studies have reported the association between refined grains and liver cancer. Pasta as a refined grain was investigated in two case-control studies for association with liver cancer [37, 41]. A multicenter case-control study conducted in Italy in 1999–2002 [37], including 185 liver cancer cases, showed that intake of pasta average 5.25 servings unit/week was associated with higher liver cancer (OR = 2.47, 95% CI: 1.09–5.63). However, no association was observed between pasta intake and liver cancer in the Cirrhosis and Risk of Hepatocellular Carcinoma in the East (CiRCE) study [41]. Overall, further studies on the association of refined grains and its types with liver cancer are warranted.
Legumes
A total of 5 prospective studies [24, 29, 30, 32, 35] and 3 retrospective studies [33, 38, 41] among 351,931 individuals with 2125 cases were included to identify the association between legumes consumption and liver cancer. The summary estimates of liver cancer were 0.86 (95% CI: 0.75–0.99; I2 = 14.3%; Fig. 3A) for ever versus non/occasional legumes consumption. However, this association is not robust in sensitivity analysis (Supplementary Fig. S3). There was no sign of asymmetry with a P value of 0.711 by Begg’s test and 0.629 by Egger’s test (Supplementary Fig. S4). In the subgroup analysis, the significant inverse associations were found only among the Asian populations and lower quality studies (Supplementary Table S4).
The association between legumes consumption and liver cancer was insignificant when comparing highest versus lowest legumes consumption (OR = 0.91, 95% CI: 0.71–1.18; I2 = 0.0%; Fig. 3B). The nonlinear dose-response relationship curve (P = 0.031 for non-linearity; n = 4) showed that decreased liver cancer was observed when legumes intake ranged from 8 g/day to 40 g/day, and the most protective effect (OR = 0.76, 95% CI: 0.56–0.96) was observed at the dose of 36 g/day legumes consumption, as depicted in Fig. 3C.
Nuts, poultry, egg and sweetened beverages
No significant associations of nuts, poultry, egg and sweetened beverages with liver cancer were observed. For poultry, there was a significant association found only in the American population. For egg, the significant associations were found among the studies with European population, higher quality and larger sample size (Supplementary Table S7). Subgroup analysis for nuts and sweetened beverages showed that the association remained insignificant when stratified by confounding factors, which are shown in Supplementary Table S5 and S8.
Discussion
In this systematic review and meta-analysis, we systematically assessed the associations between six priori defined food groups and liver cancer. Our results showed that whole grains and legumes food were inversely related with liver cancer, of which the marginal association of legumes should be interpreted with caution. However, we have not found significant associations of nuts, poultry, egg and sweetened beverages intake with liver cancer, and the association between refined grains and liver cancer was inconclusive.
For whole grains, there was a negative association with liver cancer. The linear dose-response analysis showed that each additional daily 50 g whole grains intake was associated with a 23% decreased liver cancer. Even though the analysis was based on a small number of studies (4 studies in the pooled analysis and 2 studies in the dose-response analysis), subgroup analysis suggested that the protective effect of whole grains on liver cancer were consistently found in high-quality and large-scaled studies. Increased intake of whole grains and their component bran has been reported to have beneficial effects on diseases related to liver disease and liver cancer, including glycemia, insulin sensitivity, metabolic regulation, and reduced inflammation, etc [51,52,53,54,55]. Therefore, increasing intake of whole grain and bran may protect against liver cancer by mitigating the carcinogenic effect of hyperinsulinemia and inflammation. Also, experimental studies showed that whole grain may exert its potential antitumor (including cancers of colorectum and liver) activity through improvement of gut integrity and alteration of gut microbiota composition [56,57,58]. The biologic mechanisms for the inverse associations of whole grains with the liver disease remain to be fully elucidated.
Interestingly, we found that a reverse association of legumes and liver cancer with a low heterogeneity. Furthermore, the dose-response relationship meta-analysis suggested that there was a statistically significant association between legumes consumption and liver cancer at a certain dose range of 8-40 g/day. However, given the lack of robustness of the result and the fact that most studies focused on Asian populations, further evidence is needed in larger cross-population cohort studies in future. Regarding the potential mechanism, legumes contain a variety of phytochemicals, such as phytoestrogens, mostly isoflavone (genistein and daidzein) and lignans as well as saponins and phytosterols [59]. Anticancer effect of long-term genistein intake has been linked to suppressing hepatocellular carcinoma initiation and development through AMPK-mediated anti-inflammation and pro-apoptosis [60]. Besides genistein, legumes saponins may produce the anticancer effect through inhibition of hepatocellular carcinoma cells growth, direct cytotoxity, induction of apoptosis, antiestrogenic activity, etc [61,62,63,64]. These mechanisms might support the notion that legumes food intake was negatively associated with liver cancer incidence.
For nuts, most of tree nuts contain multiple hydrophilia compounds (quercetin, resveratrol, and ellagic acid) as well as lipophilic components (tocopherols, tocotrienols, omega-3, and omega-6 fatty acids), which have been shown to exert indirect anticancer activities via their anti-inflammatory and antioxidant actions [65]. A recent study showed that high consumption of nuts was significantly associated with decreased risk of overall cancer, especially apparent against cancers of the digestive system [66]. However, our study did not support the association between total nuts consumption and liver cancer. In terms of poultry intake, poultry as white meat is considered to provide suitable terrestrial sources of n-3 long chain polyunsaturated fatty acids (n-3 PUFA) [67], which has the properties of anti-inflammatory and anti-carcinogenic [68,69,70]. Although previous two prospective studies found the inverse association between poultry intake and liver cancer [43, 44], the possibility that residual confounders are responsible for the observed inverse association simply cannot be excluded because a high intake of poultry often clusters with a healthier overall eating pattern and lifestyle [71]. Additionally, we did not observe significant associations of egg and sweetened beverages with liver cancer based on a small number of studies. The varying results on these food groups can be attributed to the amount and type of food consumed, the different study sample sizes, demographics. Because of these inconsistencies further research will provide important evidence.
The present study also has several limitations. First, it is worth noting that residual components may influence the association of these food groups with liver cancer. For example, fermented beans than unfermented beans, sugar sweetened beverages than artificially sweetened beverages. Similarly, limited studies have provided information on the histopathological type of liver cancer, and therefore, we could not perform subgroup analyses or conduct the analysis separately according to these factors since preliminary studies reported limited or unclear results for food composition and liver cancer type. Secondly, meta-analysis across ethnic populations often leads to heterogeneity of findings because they have different dietary backgrounds, and statistically significant heterogeneity among studies was observed. However, the use of random-effects model was allowed to take the heterogeneity among studies into account. Thirdly, relatively few studies on some food groups are available and insufficient for further quantitative analysis, and there is no detailed data for dose-response analysis of the association between poultry consumption and liver cancer. Fourthly, the included studies are at risk of selection bias for cases and controls and the potential misclassification introduced by the lack of specificity in exposure definition. Moreover, insufficient adjustment for potential confounders (e.g., HBV/HCV status, total energy intake etc.) is also an important consideration on account of the nature of observational studies. Finally, the cutoff value of distinguishing between high and low consumption of food groups were diversiform in the included studies, which might contribute to the heterogeneity among the studies.
Conclusion
In summary, our results demonstrated that whole grains, and legumes were inversely related with liver cancer. A null association was noted between nuts, poultry, egg and sweetened beverages consumption and liver cancer. Therefore, further well-designed cohort or clinical studies are needed to confirm the association.
Data Availability
The datasets used and/or analyzed during the current study are publicly available and accessible.
Abbreviations
- BCAA:
-
Branched-chain amino acid
- CI:
-
Confidence interval
- HCC:
-
Hepatocellular Carcinoma
- HR:
-
Hazards ratio
- NOS:
-
Newcastle-Ottawa Scale
- OR:
-
Odds ratio
- RR:
-
Relative risk
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA-CANCER J CLIN. 2021;71(3):209–49.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. NAT REV GASTRO HEPAT. 2019;16(10):589–604.
Wan-Shui Y, Xu-Fen Z, Zhi-Ning L, Qi-Hong Z, Yu-Ting T, Jing G, Hong-Lan L, Yong-Bing X. Diet and liver cancer risk: a narrative review of epidemiological evidence. BRIT J NUTR 2020, 124(3).
Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, Chong D, VoPham T, Meyerhardt JA, Wen D et al. Dietary Patterns and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. HEPATOLOGY 2019, 70(2):577–586.
Federica T, Dimitrios T, Jerry P, Francesca B, Marta R, Renato T, Silvia F, Maurizio M, Antonia T, Carlo LV et al. Mediterranean diet and hepatocellular carcinoma. J HEPATOL 2014, 60(3).
Li WQ, Park Y, McGlynn KA, Hollenbeck AR, Taylor PR, Goldstein AM, Freedman ND. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. 2014;60(2):588–97.
Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: impact and future directions. J NUTR. 2020;150(4):663–71.
George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. NUTRIENTS 2021, 13(1).
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ-BRIT MED J. 2009;339:b2700.
Guo XF, Shao XF, Li JM, Li S, Li KL, Li D. Fruit and vegetable intake and liver cancer risk: a meta-analysis of prospective cohort studies. FOOD FUNCT. 2019;10(8):4478–85.
Yu J, Liu Z, Liang D, Li J, Ma S, Wang G, Chen W. Meat intake and the risk of Hepatocellular Carcinoma: a Meta-analysis of Observational Studies. Nutr Cancer 2022, 74(9).
Zhao Q, He Y, Wang K, Wang C, Wu H, Gao L, Hu A, Yang W, Wang S. Dairy consumption and Liver Cancer risk: a systematic review and dose-response Meta-analysis of Observational Studies. NUTR CANCER. 2021;73(11–12):2821–31.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. EUR J EPIDEMIOL. 2010;25(9):603–5.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ-BRIT MED J. 2003;327(7414):557–60.
Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. SYST REV-LONDON. 2017;6(1):243.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J EPIDEMIOL. 2005;15(6):235–43.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. AM J EPIDEMIOL. 2012;175(1):66–73.
Su Q, Sun X, Zhu L, Yan Q, Zheng P, Mao Y, Ye D. Breastfeeding and the risk of childhood cancer: a systematic review and dose-response meta-analysis. BMC MED. 2021;19(1):90.
Bao Y, Hu FB, Giovannucci EL, Wolpin BM, Stampfer MJ, Willett WC, Fuchs CS. Nut consumption and risk of pancreatic cancer in women. BRIT J CANCER. 2013;109(11):2911–6.
Keum N, Lee DH, Marchand N, Oh H, Liu H, Aune D, Greenwood DC, Giovannucci EL. Egg intake and cancers of the breast, ovary and prostate: a dose-response meta-analysis of prospective observational studies. BRIT J NUTR. 2015;114(7):1099–107.
Mohammadi H, Jayedi A, Ghaedi E, Golbidi D, Shab-Bidar S. Dietary poultry intake and the risk of stroke: a dose-response meta-analysis of prospective cohort studies. CLIN NUTR ESPEN. 2018;23:25–33.
Hirayama T. A large-scale cohort study on risk factors for primary liver cancer, with special reference to the role of cigarette smoking. CANCER CHEMOTH PHARM. 1989;23(Suppl):114–S117.
Srivatanakul P, Parkin DM, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L’Abbe KA, Wild CP. Liver cancer in Thailand. II. A case-control study of hepatocellular carcinoma. INT J CANCER. 1991;48(3):329–32.
Fukuda K, Shibata A, Hirohata I, Tanikawa K, Yamaguchi G, Ishii M. A hospital-based case-control study on hepatocellular carcinoma in Fukuoka and Saga prefectures, northern Kyushu, Japan. Jpn J Cancer Res. 1993;84(7):708–14.
Zhang JY, Wang X, Han SG, Zhuang H. A case-control study of risk factors for hepatocellular carcinoma in Henan, China. AM J TROP MED HYG. 1998;59(6):947–51.
Yu SZ, Huang XE, Koide T, Cheng G, Chen GC, Harada K, Ueno Y, Sueoka E, Oda H, Tashiro F, et al. Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res. 2002;93(12):1287–92.
Sharp GB, Lagarde F, Mizuno T, Sauvaget C, Fukuhara T, Allen N, Suzuki G, Tokuoka S. Relationship of hepatocellular carcinoma to soya food consumption: a cohort-based, case-control study in Japan. INT J CANCER. 2005;115(2):290–5.
Zhang W, Xiang YB, Li HL, Yang G, Cai H, Ji BT, Gao YT, Zheng W, Shu XO. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women’s and men’s health studies. CANCER SCI. 2013;104(10):1353–61.
Shawon MA, Yousuf M, Raheem E, Ahmed S, Dipti TT, Hoque MR, Taniguchi H, Karim MR. Epidemiology, clinical features, and impact of food habits on the risk of hepatocellular carcinoma: a case-control study in Bangladesh. PLoS ONE. 2020;15(4):e232121.
Abe SK, Sawada N, Ishihara J, Takachi R, Mori N, Yamaji T, Shimazu T, Goto A, Iwasaki M, Inoue M, et al. Comparison between the impact of fermented and unfermented soy intake on the risk of liver cancer: the JPHC Study. EUR J NUTR. 2021;60(3):1389–401.
Lam KC, Yu MC, Leung JW, Henderson BE. Hepatitis B virus and cigarette smoking: risk factors for hepatocellular carcinoma in Hong Kong. CANCER RES. 1982;42(12):5246–8.
Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF, Chang WY, Sheen MC, Lin TM. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13(3):398–406.
Kurahashi N, Inoue M, Iwasaki M, Tanaka Y, Mizokami M, Tsugane S. Isoflavone consumption and subsequent risk of hepatocellular carcinoma in a population-based prospective cohort of japanese men and women. INT J CANCER. 2009;124(7):1644–9.
La Vecchia C, Negri E, Decarli A, D’Avanzo B, Franceschi S. Risk factors for hepatocellular carcinoma in northern Italy. INT J CANCER. 1988;42(6):872–6.
Talamini R, Polesel J, Montella M, Dal Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C, Franceschi S. Food groups and risk of hepatocellular carcinoma: a multicenter case-control study in Italy. INT J CANCER. 2006;119(12):2916–21.
Kanazir M, Boricic I, Delic D, Tepavcevic DK, Knezevic A, Jovanovic T, Pekmezovic T. Risk factors for hepatocellular carcinoma: a case-control study in Belgrade (Serbia). TUMORI J. 2010;96(6):911–7.
Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, Nothlings U, Lukanova A, Lagiou P, Boffetta P, et al. Consumption of fish and meats and risk of hepatocellular carcinoma: the european prospective investigation into Cancer and Nutrition (EPIC). ANN ONCOL. 2013;24(8):2166–73.
Stepien M, Duarte-Salles T, Fedirko V, Trichopoulou A, Lagiou P, Bamia C, Overvad K, Tjonneland A, Hansen L, Boutron-Ruault MC, et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a european cohort. EUR J NUTR. 2016;55(1):7–20.
Rizk M, Guilloteau A, Mouillot T, Thiefin G, Bronowicki JP, Richou C, Doffoel M, Diab AM, Hillon P, Cottet V. Dietary components modulate the risk of hepatocellular carcinoma in cirrhotic patients. NUTR RES. 2019;61:82–94.
Guo W, Ge X, Lu J, Xu X, Gao J, Wang Q, Song C, Zhang Q, Yu C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. NUTRIENTS 2022, 14(24).
Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. CANCER PREV RES. 2011;4(11):1903–11.
Ma Y, Yang W, Li T, Liu Y, Simon TG, Sui J, Wu K, Giovannucci EL, Chan AT, Zhang X. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. INT J EPIDEMIOL. 2019;48(6):1863–71.
Sui J, Yang W, Ma Y, Li TY, Simon TG, Meyerhardt JA, Liang G, Giovannucci EL, Chan AT, Zhang X. A prospective study of nut consumption and risk of primary Hepatocellular Carcinoma in the U.S. women and men. CANCER PREV RES. 2019;12(6):367–74.
Yang W, Ma Y, Liu Y, Smith-Warner SA, Simon TG, Chong DQ, Qi Q, Meyerhardt JA, Giovannucci EL, Chan AT, et al. Association of Intake of whole grains and Dietary Fiber with risk of Hepatocellular Carcinoma in US adults. JAMA ONCOL. 2019;5(6):879–86.
Luo X, Sui J, Yang W, Sun Q, Ma Y, Simon TG, Liang G, Meyerhardt JA, Chan AT, Giovannucci EL, et al. Type 2 diabetes Prevention Diet and Hepatocellular Carcinoma Risk in US Men and Women. AM J GASTROENTEROL. 2019;114(12):1870–7.
Liu X, Yang W, Petrick JL, Liao LM, Wang W, He N, Campbell PT, Zhang ZF, Giovannucci E, McGlynn KA, et al. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. NAT COMMUN. 2021;12(1):6388.
Jones GS, Graubard BI, Ramirez Y, Liao LM, Huang WY, Alvarez CS, Yang W, Zhang X, Petrick JL, McGlynn KA. Sweetened beverage consumption and risk of liver cancer by diabetes status: a pooled analysis. CANCER EPIDEMIOL. 2022;79:102201.
Soliman AS, Hung CW, Tsodikov A, Seifeldin IA, Ramadan M, Al-Gamal D, Schiefelbein EL, Thummalapally P, Dey S, Ismail K. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. HEPATOL INT. 2010;4(4):681–90.
Weickert MO, Pfeiffer A. Impact of Dietary Fiber consumption on insulin resistance and the Prevention of type 2 diabetes. J NUTR. 2018;148(1):7–12.
Ross AB, Godin JP, Minehira K, Kirwan JP. Increasing whole grain intake as part of prevention and treatment of nonalcoholic fatty liver disease. INT J ENDOCRINOL. 2013;2013:585876.
Chen JP, Chen GC, Wang XP, Qin L, Bai Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. NUTRIENTS 2017, 10(1).
Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29(2):207–11.
Steffen LM, Jacobs DJ, Murtaugh MA, Moran A, Steinberger J, Hong CP, Sinaiko AR. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. AM J EPIDEMIOL. 2003;158(3):243–50.
Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, Gibson GR, Tuohy KM. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. BRIT J NUTR. 2008;99(1):110–20.
Langkamp-Henken B, Nieves CJ, Culpepper T, Radford A, Girard SA, Hughes C, Christman MC, Mai V, Dahl WJ, Boileau T, et al. Fecal lactic acid bacteria increased in adolescents randomized to whole-grain but not refined-grain foods, whereas inflammatory cytokine production decreased equally with both interventions. J NUTR. 2012;142(11):2025–32.
Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J HEPATOL. 2020;72(3):558–77.
Elshemy H. Soybean and Nutrition. InTech; 2011.
Lee SR, Kwon SW, Lee YH, Kaya P, Kim JM, Ahn C, Jung EM, Lee GS, An BS, Jeung EB, et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer. 2019;19(1):6.
Kerwin SM. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr Med Chem Anticancer Agents. 2004;4(3):263–72.
Hwang ES. Antioxidant and quinone reductase activity of Soyasaponins in Hepa1c1c7 Mouse Hepatocarcinoma cells. Prev Nutr Food Sci. 2017;22(4):300–5.
Rao AV, Sung MK. Saponins as anticarcinogens. J NUTR. 1995;125(3 Suppl):717S–24.
Kang J, Badger TM, Ronis MJ, Wu X. Non-isoflavone phytochemicals in soy and their health effects. J AGR FOOD CHEM. 2010;58(14):8119–33.
Rusu ME, Mocan A, Ferreira I, Popa DS. Health benefits of nut consumption in Middle-Aged and Elderly Population. ANTIOXIDANTS-BASEL 2019, 8(8).
Long J, Ji Z, Yuan P, Long T, Liu K, Li J, Cheng L. Nut consumption and risk of Cancer: a Meta-analysis of prospective studies. CANCER EPIDEM BIOMAR. 2020;29(3):565–73.
Cartoni MA, Mattioli S, Twining C, Dal Bosco A, Donoghue AM, Arsi K, Angelucci E, Chiattelli D, Castellini C. Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition. NUTRIENTS 2022, 14(9).
Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. NEW ENGL J MED. 1989;320(5):265–71.
Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. MOL CANCER THER. 2009;8(11):3046–55.
Fauser JK, Prisciandaro LD, Cummins AG, Howarth GS. Fatty acids as potential adjunctive colorectal chemotherapeutic agents. CANCER BIOL THER. 2011;11(8):724–31.
Flood A, Rastogi T, Wirfalt E, Mitrou PN, Reedy J, Subar AF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann M, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. AM J CLIN NUTR. 2008;88(1):176–84.
Acknowledgements
The authors sincerely thank the researchers and participants of the original articles for their collection and management of data resources.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82103936), Natural Science Foundation of Zhejiang Province (LQ20H260008, LQ21H260001), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020KY195), Zhejiang Chinese Medical University Foundation (2020ZG16).
Author information
Authors and Affiliations
Contributions
DY conceived and designed the study. KL and WWC performed literature search. KL and YZ assessed the quality of included studies according NOS. DY resolved the argument between KL and YZ. KL, WWC, LHX and YZ performed data analysis. KL drafted the manuscript and DY revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, K., Chen, W., Zhou, Y. et al. Associations between food groups and liver cancer: a systematic review and meta-analysis of observational studies. Nutr J 22, 30 (2023). https://doi.org/10.1186/s12937-023-00858-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00858-5