Abstract
Background
Recent years have seen the emergence of numerous novel indicators for visceral obesity. This study investigates the potential correlation between the Chinese visceral adiposity index (CVAI) and hyperuricemia (HUA).
Methods
This research, derived from a 2011 cross-sectional analysis in Dalian, China, employed restricted cubic spline (RCS) plots to identify inflection points. Subsequently, one-way and multifactorial logistic regression models were utilized, with HUA as the outcome variable. Additionally, subgroup analyses and interaction tests were conducted. Eventually, receiver operating characteristic (ROC) curves were calculated to assess the effectiveness of CVAI and other body composition indices in predicting HUA.
Results
The study included 10,061 individuals, with a HUA prevalence of 14.25%. Significant relationships with HUA were observed for CVAI. RCS analysis revealed a J-shaped relationship between CVAI and HUA. Compared to those in the low CVAI category, HUA was notably associated with individuals in the high CVAI category in multifactorial logistic regression (OR = 2.661, 95% CI: 2.323, 3.047). Subgroup analyses demonstrated stronger relationships in women, participants without hypertension, and participants without diabetes. Additional modeling via ROC curves suggested that the CVAI may offer effective predictive value for HUA.
Conclusion
This study confirmed that an elevated CVAI elevates the risk of HUA in middle-aged and elderly populations in the Dalian community. The findings advance obesity prevention strategies that mitigate HUA risk and support healthcare initiatives for China's aging population.
Similar content being viewed by others
Introduction
Uric acid is the final substance produced through the metabolism of purines within the body's metabolic processes. When a large amount of purine-rich foods are consumed, or when the body's capacity to excrete uric acid is diminished, this will lead to an accumulation of uric acid. This condition, characterized by increased uric acid levels in the bloodstream, is referred to as hyperuricemia (HUA) [1]. HUA may precipitate a range of complications including gout, metabolic syndrome, renal disease, and cardiovascular and cerebrovascular diseases [2,3,4,5,6]. Recent lifestyle changes, particularly in diet, have contributed to a rising prevalence of HUA [7,8,9]. For instance, the prevalence among Chinese adults increased from 11.1% in 2015–16 to 14.0% in 2018–19, with higher rates observed in men than in women [10]. With China's aging population projected to grow, an annual increase in the incidence of HUA is expected among middle-aged and elderly populations. As a result, it is becoming more critical to emphasize the prevention and management of HUA within this aging demographic for the purposes of health maintenance and treatment.
Past research has shown a connection between HUA and obesity [11]. A recent study highlighted a steady rise in both abdominal and overall obesity among adults in their middle years, with more than half of the adult population in China being overweight or obese [12, 13]. While body mass index (BMI) is frequently employed as a metric to assess obesity, it has significant limitations. Specifically, BMI cannot differentiate between muscle mass and fat mass, nor can it accurately represent fat distribution. For instance, individuals who have substantial muscle mass and a low body fat percentage might be incorrectly categorized as overweight by BMI, whereas those with high fat mass and low muscle mass might appear normal or underweight [14, 15]. Given that Asians tend to have higher visceral fat levels than Westerners do at equivalent BMI levels, waist circumference (WC), the waist-to-hip ratio (WHpR), and the waist-to-height ratio (WHtR) have been consistently utilized to evaluate central obesity, while it also has limitations in accurately differentiating subcutaneous from visceral fat. Increased visceral adipose tissue can lead to dysfunctional obesity. Therefore, assessing visceral obesity is crucial for evaluating the risk of HUA among Chinese individuals [16, 17]. However, the routine use of CT and MRI for assessing visceral fat may not be practical for widespread clinical application. The relevant literature indicates that the lipid accumulation product (LAP) and a body shape index (ABSI) can be used as indicators to assess visceral obesity. In recent years, Xia et al. developed the Chinese visceral adiposity index (CVAI) as a tool to measure visceral obesity in Asian populations [18]. Previous studies have linked CVAI with several health conditions, including cardiovascular disease, diabetes, pancreatitis, and stroke [19,20,21,22,23]. Currently, just a single study has documented the relationship between CVAI and HUA. In a cohort study from a Chinese region, Liu et al. discovered that the CVAI had superior predictive power for HUA compared to other indicators, particularly in the female population [24]. However, there has been no comprehensive examination of this relationship. Therefore, in this cross-sectional analysis, we examined the relationship between CVAI and HUA in middle-aged and elderly populations, utilizing data collected in the Dalian area during 2011. It also evaluates the predictive ability of CVAI relative to other body composition indices (BMI, WHpR, WHtR, LAP, ABSI) for HUA. This study sought to provide a comprehensive understanding of how CVAI and HUA are related in this specific population subset.
Methods
Study design
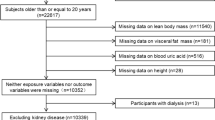
The REACTION (Risk Evaluation of Cancers in Chinese Diabetic Individuals: A Longitudinal Study) was a comprehensive nationwide investigation that focused on a broad population of individuals aged 40 years or older residing in the community. It included 25 centers throughout mainland China, encompassing a total of 259,657 participants [25,26,27]. Data from the Dalian Center, which were collected between August and December 2011, were analyzed in the present study. Individuals with incomplete data, those on medications affecting serum uric acid (SUA) levels, those with a history of gout, and anyone under 40 years of age were excluded. Ultimately, 10,061 subjects meeting these criteria were enrolled, and the screening process is illustrated in Fig. 1. This study received ethical approval from the Institutional Review Board of Ruijin Hospital at the Medical College of Shanghai Jiao Tong University (RUIJIN-2011–14), and all participants provided written informed consent.
Strategies for research
The participants involved in this survey included medical and nursing staff from the Department of Endocrinology at Dalian Central Hospital, all trained by Shanghai Ruijin Hospital prior to conducting this epidemiological survey. A questionnaire was utilized to gather personal information and data on demographic characteristics (age, sex, height, weight, WC), lifestyle habits (smoking and drinking alcohol), and past chronic diseases. Detailed information on chronic diseases, smoking, and alcohol consumption can be found in Table S1. Trained inspectors collected fasting venous blood samples to measure blood lipids, SUA, and fasting plasma glucose (FPG). The RGZ-120 device was used to measure patients' weight and height, with patients being required to remove their clothes and shoes for accuracy; height was measured to the nearest tenth of a centimeter. Blood pressure readings were obtained under controlled conditions while the participants were seated. The researchers also ensured accuracy by recording the average of three blood pressure readings.
Variable definition
HUA: SUA levels ≥ 420 µmol/L in men or ≥ 360 µmol/L in women [28].
Figure 2 displays the methods used to calculate CVAI and several body composition indices.
Statistical analysis
Categorical variables were summarized by frequency and constitutive ratio (n%). Chi-square tests were utilized for comparisons between the two groups. Normality tests were conducted on all continuous numerical variables using the Kolmogorov–Smirnov test to assess normality. For variables not adhering to a normal distribution, the median (represented as Q25, Q75) was calculated. These median values were then log-transformed prior to conducting t-tests. Missing data were handled through multiple interpolations. A 3-knot restricted cubic spline (RCS) plot was utilized to explore the nonlinear relationship between CVAI and HUA. The aim was to establish the cutoff point using the RCS curve, followed by conducting a multivariable logistic regression analysis anchored on this threshold. This analysis led to the construction of three models: Model 1 was not adjusted; Model 2 included adjustments for age and sex; and Model 3 was further adjusted for a history of hypertension, coronary heart disease (CHD), diabetes, and alcohol consumption. Subsequently, subgroup analyses were performed to assess whether potential covariates (age, sex, BMI, history of hypertension, and diabetes) altered the connection between CVAI and HUA, with P < 0.05 indicating significant interactions. Furthermore, the receiver operating characteristic (ROC) curves were used to establish a basic model incorporating age, sex, hypertension, diabetes, CHD, alcohol consumption, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and FPG. Six traditional and novel body composition indices were sequentially added to the model. The incremental predictive ability of these indices for HUA was observed and compared based on the area under the curve (AUC). All statistical tests were conducted with R software (version 4.3.2). When analyzing statistical significance, it is important to consider two-sided P-values of less than 0.05.
Results
Baseline characteristics
The baseline characteristics of the HUA group were analyzed in comparison to those of the control group. Table 1 shows that of the subjects, 8,627 (85.75%) were classified into the control group, whereas 1,434 (14.25%) were classified into the HUA group. The prevalence of HUA was 14.25%. All variables showed notable differences between the HUA and control groups, except for smoking. The HUA group presented higher values for age; SBP, DBP, CVAI, BMI, WC, WHpR, WHtR, LAP, SUA, creatinine, LDL, TG, ALT, AST, FPG, and insulin levels. Conversely, the ABSI and HDL levels were lower. Additionally, the HUA group presented a greater male ratio; a more frequent occurrence of hypertension, diabetes, CHD, and regular alcohol consumption; and lower education levels than did the control group.
Dose–response relationship between the CVAI and HUA
In addition, a 3-knot RCS was employed to model the relationship between CVAI and elevated HUA, as depicted in Fig. 3. The CVAI was plotted as the horizontal coordinate and the odds ratio (OR) was plotted as the vertical coordinate. The results, after adjusting for potential covariates, indicated that higher levels of CVAI corresponded with an increasing risk of HUA, displaying an approximately J-shaped curve (nonlinear P < 0.001). The corresponding inflection point value was 141.22.
The dose–response relationship between CVAI and HUA. We employed a 3-knot RCS to fit the dose–response relationship between CVAI and HUA. We adjusted the model fully for age, sex, history of hypertension, CHD, diabetes, and alcohol consumption. The red solid line represents the curve fitting between variables, and the shaded area indicates the 95% CI of the fit. Abbreviation: RCS, restricted cubic spline; CVAI, Chinese visceral adiposity index; CHD, coronary heart disease; CI, confidence interval
Relationship between CVAI and HUA
The relationship between CVAI and HUA is detailed in Table 2. After accounting for potential confounders in Model 3, the findings indicated a notable positive relationship with an OR of 1.018 (95% CI: 1.016, 1.020). The RCS was applied to examine the nonlinear relationship between CVAI and HUA. An inflection point value of 141.22 was derived, which was then used as a cutoff point to classify the CVAI as a dichotomous variable for multifactor logistic regression. In Model 1, the OR for the upper category in comparison with the lower category was 3.1 (95% CI: 2.761, 3.481). In Model 2, the OR for the higher category increased to 2.891 (95% CI: 2.532, 3.302). Even after further adjustments in Model 2 for additional covariates, the OR was 2.661 (95% CI: 2.323, 3.047), indicating that the relationship between higher CVAI and HUA remained statistically significant. The trend analysis confirmed a consistent positive correlation between the two categories (P for trend < 0.001). The study indicates a significant link between CVAI and HUA.
Subgroup analyses
Subgroup analyses and interaction tests were further carried out, stratifying the subgroups by age, sex, BMI, hypertension, diabetes, and CHD. As presented in Fig. 4, these analyses revealed that these factors influenced the relationship between the CVAI and HUA. The link between CVAI and HUA appeared stronger within specific subgroups: women, individuals without hypertension, and individuals without diabetes (P for interaction < 0.05). Additionally, age, BMI, and CHD appeared to have no substantial impact (P for interaction > 0.05). The test for interaction between subgroups indicated that the relationship between CVAI and HUA varies significantly across different subgroups.
ROC curves
A baseline model was developed incorporating age, sex, hypertension, diabetes, CHD, alcohol consumption, creatinine, ALT, AST, and FPG. The sequential addition of six traditional and novel body composition indices, as detailed in Fig. 5 and Table 3, altered the model's AUC. Initially, the AUC was 0.703 (95% CI: 0.689, 0.717). It increased to 0.749 (95% CI: 0.735, 0.762) with the inclusion of the CVAI, to 0.736 (95% CI: 0.723, 0.750) with the BMI, to 0.705 (95% CI: 0.691, 0.719) with the WHpR, to 0.719 (95% CI: 0.705, 0.732) with the WHtR, to 0.750 (95% CI: 0.736, 0.763) with the LAP, and to 0.705 (95% CI: 0.691, 0.719) with the ABSI. The CVAI, BMI, WHtR, and LAP significantly improved the model's predictive accuracy (P < 0.05), particularly the LAP, which provided the highest incremental predictive value, followed by the CVAI, BMI, and WHtR. Conversely, the WHpR and ABSI did not significantly enhance the model's accuracy (P > 0.05). These results indicate that CVAI is a potentially effective marker for predicting HUA.
The AUC curves of CVAI and several body composition indices to identify HUA. The AUC curves of CVAI and several body composition indices to identify HUA. In the ROC curves, the blue line represents the baseline model, whereas the red line signifies the enhanced model that sequentially incorporates various obesity indices. Abbreviation: CVAI, Chinese visceral adiposity index; BMI, body mass index; WHpR, waist-to-hip ratio; WHtR, waist-to-height ratio; LAP, lipid accumulation product; ABSI, a body shape index
Discussion
The objective of this study was to investigate the relationship between the CVAI and HUA among middle-aged and elderly community-dwelling populations in Dalian, China. This cross-sectional analysis of 10,061 participants revealed a J-shaped correlation between elevated CVAI levels and HUA. Subgroup analyses further demonstrated that this correlation was particularly pronounced in women, individuals without hypertension, and individuals without diabetes. Additionally, the application of ROC curves indicated that the CVAI might serve as an effective predictor of HUA. Importantly, this study marks the initial discovery of a J-shaped correlation between CVAI and HUA among the Chinese population.
Han et al. reported an upward correlation involving BMI, WC, and SUA levels [29]. Research involving 14,624 adults in the United States demonstrated a dose-dependent relationship between BMI and HUA, attributing a population-attributable risk of 44% to this association [11]. Xiao et al. noted that low muscle mass significantly linked to a heightened risk of HUA, a finding consistent across different age and sex subgroups [30]. Further studies in the Xinjiang and Korean regions respectively found heightened risks of HUA in individuals with overweight, obesity, or abdominal obesity compared with their normal-weight counterparts [31], and in those with concurrently elevated serum TG levels and WC [32]. Moreover, other emerging indicators have been explored for their relationship with obesity and HUA. A three-year follow-up study indicated that neck circumference, unlike WC, stood as a standalone predictor of subsequent HUA in women [33]. Another study highlighted that the WHtR proved superior to BMI and WC in forecasting HUA [34]. A US cross-sectional study also reported a nonlinear relationship between the triglyceride glucose index and HUA [35].
The mechanisms underlying these findings can be elucidated as follows: Firstly, CVAI measures the accumulation of visceral fat, which increases free fatty acids. These acids flow into the liver, inducing de novo synthesis of purines and consequently elevating SUA levels [36, 37]. Secondly, the CVAI consists of HDL, reflecting lipid metabolism. Dysregulation in this system can impair renal blood flow. Additionally, increased adipose tissue may compress the kidneys, further diminishing renal blood flow and reducing uric acid excretion, thus elevating uric acid [38, 39]. Thirdly, visceral adipose deposition may disrupt lipid metabolism, influencing the body's inflammatory response and insulin resistance. This disturbance can adversely affect renal function, leading to increased SUA levels [40,41,42]. Lastly, enhanced accumulation of belly fat stimulates the release of inflammatory cytokines such as interleukin-6. These cytokines trigger inflammatory reactions and oxidative stress, which impair vascular endothelial function and contribute to metabolic abnormalities, further elevating uric acid levels [43, 44].
Subgroup analyses indicated a more pronounced correlation between CVAI and HUA in women and those without hypertension or diabetes. For the sex subgroups, intrinsic differences in adipose distribution and adipocyte characteristics between men and women may explain these findings. Firstly, men typically have more visceral adipose tissue (fat in the abdominopelvic cavity) compared to women, who generally have more subcutaneous adipose tissue (fat beneath the dermis) [45, 46]. Although women possess lower amounts of visceral fat, this may result in heightened sensitivity of CVAI to HUA. Additionally, studies have shown that the intraperitoneal adipocyte ratio is lower in women than in men and that fat cell size influences insulin resistance and the expression of proinflammatory adipokines. These differences might lead to a more pronounced relationship between visceral fat mass and SUA levels in women. Lastly, women differ from men in terms of metabolic rates and the metabolism of fats and carbohydrates. These differences may enhance the efficacy of the CVAI as a predictive marker for HUA risk in women. Regarding the subgroups without hypertension and diabetes, these conditions are associated with metabolic disorders that can mask or influence the relationship between visceral fat mass and SUA. In populations without hypertension and diabetes, changes in CVAI and SUA levels may better reflect the physiological interaction between visceral fat and SUA levels.
Advantages and drawbacks
The study presents several advantages when compared to previous research conducted in this specific field. Firstly, the study began by analyzing a large group of middle-aged and elderly populations from Dalian, China, to explore the connection between CVAI and HUA, which is applicable to the Chinese population. The homogeneity of the sample reduces sample bias and enhances representativeness. Secondly, the study conducted rigorous screening of the included population, excluding individuals with previous gout and those taking medications that affect SUA levels, thereby improving the dependability of the findings. Thirdly, the absence of some covariates could lead to inaccurate analysis results, thereby potentially affecting the conclusions. To minimize the impact of missing data, we employed multiple imputation techniques. However, this study has several limitations: Firstly, as Dalian is a coastal city in northern China, its residents consume more purine-rich foods such as seafood. The study was unable to exclude the potential interference of diet, exercise, and other lifestyle factors from the findings. Secondly, the sample was derived from the same region and consisted solely of community residents aged 40 and above; therefore, the study lacks generalizability and is not applicable to children, young adults, or populations in other countries and regions. Lastly, this cross-sectional study precludes establishing a causative link between CVAI and HUA.
Conclusion
This study revealed a J-shaped relationship between CVAI and HUA. The CVAI could serve as a potential marker for detecting HUA and preventing disease progression in middle-aged and elderly populations. This study's findings offer crucial tools for HUA's early detection, especially in women, without hypertension, and without diabetes. This study provides significant value for the early detection and targeted prevention of HUA. Such insights facilitate personalized treatment and monitoring, enhancing the precision management of this condition.
Availability of data and materials
The data analyzed in this article are not disclosed to the public but are available from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- HUA:
-
Hyperuricemia
- SUA:
-
Serum uric acid
- BMI:
-
Body mass index
- WHpR:
-
Waist-to-hip ratio
- WHtR:
-
Waist-to-height ratio
- CVAI:
-
Chinese visceral adiposity index
- WC:
-
Waist circumference
- SBP:
-
Systolic blood pressure
- ABSI:
-
A body shape index
- DBP:
-
Diastolic blood pressure
- LAP:
-
Lipid accumulation product
- CHD:
-
Coronary heart disease
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- FPG:
-
Fast plasma glucose
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- OR:
-
Odds ratio
References
Dhokte S, Czaja K. Visceral Adipose tissue: the hidden culprit for type 2 diabetes. Nutrients. 2024;16:1015.
Copur S, Demiray A, Kanbay M. Uric acid in metabolic syndrome: does uric acid have a definitive role? Eur J Intern Med. 2022;103:4–12.
Tanaka A, Toyoda S, Kato T, Yoshida H, Hamasaki S, Watarai M, Ishizu T, Ueda S, Inoue T, Node K. Association between serum urate level and carotid atherosclerosis: an insight from a post hoc analysis of the PRIZE randomised clinical trial. RMD Open. 2022;8:8.
Zhou Z, Liang Y, Lin J, Zhang X, Qu H, Xu J, Zhao C, Zhao M. Serum uric acid concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Atherosclerosis. 2018;275:352–8.
Joosten LAB, Crişan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol. 2020;16:75–86.
Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, Redon J, Stack AG, Tsioufis KP. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. 2020;80:1–11.
Raja S, Kumar A, Aahooja RD, Thakuria U, Ochani S, Shaukat F. Frequency of hyperuricemia and its risk factors in the adult population. Cureus. 2019;11: e4198.
Zhang T, Gu Y, Meng G, Zhang Q, Liu L, Wu H, Zhang S, Wang X, Zhang J, Sun S, et al. Genetic risk, adherence to a healthy lifestyle, and hyperuricemia: the TCLSIH cohort study. Am J Med. 2023;136:476-483.e475.
Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60:1–10.
Zhang M, Zhu X, Wu J, Huang Z, Zhao Z, Zhang X, Xue Y, Wan W, Li C, Zhang W, et al. Prevalence of hyperuricemia among Chinese adults: findings from two nationally representative cross-sectional surveys in 2015–16 and 2018–19. Front Immunol. 2021;12: 791983.
Choi HK, McCormick N, Lu N, Rai SK, Yokose C, Zhang Y. Population impact attributable to modifiable risk factors for hyperuricemia. Arthritis Rheumatol. 2020;72:157–65.
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373–92.
Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, Yan Y, Mi J. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev. 2012;13:287–96.
Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–33.
Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46.
Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am J Clin Nutr. 2012;96:714–26.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6.
Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6: 38214.
Ren Y, Hu Q, Li Z, Zhang X, Yang L, Kong L. Dose-response association between Chinese visceral adiposity index and cardiovascular disease: a national prospective cohort study. Front Endocrinol (Lausanne). 2024;15:1284144.
Zhang X, Wang Y, Li Y, Gui J, Mei Y, Yang X, Liu H, Guo LL, Li J, Lei Y, et al. Optimal obesity- and lipid-related indices for predicting type 2 diabetes in middle-aged and elderly Chinese. Sci Rep. 2024;14:10901.
Zhu Y, Huang Y, Sun H, Chen L, Yu H, Shi L, Xia W, Sun X, Yang Y, Huang H. Novel anthropometric indicators of visceral obesity predict the severity of hyperlipidemic acute pancreatitis. Lipids Health Dis. 2024;23:120.
Ye X, Zhang G, Han C, Wang P, Lu J, Zhang M. The association between Chinese visceral adiposity index and cardiometabolic multimorbidity among Chinese middle-aged and older adults: a national cohort study. Front Endocrinol (Lausanne). 2024;15:1381949.
Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between Chinese visceral adiposity index and risk of stroke incidence in middle-aged and elderly Chinese population: evidence from a large national cohort study. J Transl Med. 2023;21:518.
Liu Z, Zhou Q, Tang Y, Li J, Chen Q, Yang H, Zhou S. Sex-specific differences in the associations between adiposity indices and incident hyperuricemia among middle-aged and older adults: a nationwide longitudinal study. Front Endocrinol (Lausanne). 2024;15:1336471.
Ning G. Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4:172–3.
Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6:147–57.
Wang T, Lu J, Su Q, Chen Y, Bi Y, Mu Y, Chen L, Hu R, Tang X, Yu X, et al. Ideal cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. 2019;4:874–83.
Piao W, Zhao L, Yang Y, Fang H, Ju L, Cai S, Yu D. The prevalence of hyperuricemia and its correlates among adults in China: results from CNHS 2015–2017. Nutrients. 2022;14:4095.
Han T, Meng X, Shan R, Zi T, Li Y, Ma H, Zhao Y, Shi D, Qu R, Guo X, et al. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int J Obes (Lond). 2018;42:1336–44.
Xiao H, Hu L, Xie M, Du Y, Liao D. The agreement of low lean mass with obesity using different definitions and its correlation with hyperuricemia. Front Nutr. 2024;11: 1382254.
Wu X, Zhou M, Bu J, Chang Y, Mahan Y, Lu H, Zhang Y, Zhang X, Wang Z, Zhou L. Prevalence and risk factors of hyperuricemia in the urban health checkup population in Xinjiang, China: a cross-sectional study. Risk Manag Healthc Policy. 2023;16:1531–44.
Oh JE, Yu SR, Yoo JY, Shin KA. Relationship between asymptomatic hyperuricemia and the hypertriglyceridemic-waist phenotype in Korean adults: a cross-sectional study. Diabetes Metab Syndr Obes. 2024;17:1727–38.
An P, Chen K, Wang A, Jin X, Chen Y, Gu W, Yan W, Zang L, Dou J, Mu Y, Lv Z. Neck circumference is an independent risk factor for hyperuricemia within 3 years in women: a longitudinal study. Clin Rheumatol. 2020;39:3757–67.
Huang ZP, Huang BX, Zhang H, Zhu MF, Zhu HL. Waist-to-height ratio is a better predictor of hyperuricemia than body mass index and waist circumference in Chinese. Ann Nutr Metab. 2019;75:187–94.
Qiu L, Ren Y, Li J, Li M, Li W, Qin L, Ning C, Zhang J, Gao F. Nonlinear association of triglyceride-glucose index with hyperuricemia in US adults: a cross-sectional study. Lipids Health Dis. 2024;23:145.
Fabregat I, Revilla E, Machado A. Short-term control of the pentose phosphate cycle by insulin could be modulated by the NADPH/NADP ratio in rat adipocytes and hepatocytes. Biochem Biophys Res Commun. 1987;146:920–5.
Nakanishi N, Yoshida H, Nakamura K, Suzuki K, Tatara K. Predictors for development of hyperuricemia: an 8-year longitudinal study in middle-aged Japanese men. Metabolism. 2001;50:621–6.
Lee WC, Wu PY, Huang JC, Tsai YC, Chiu YW, Chen SC, Chang JM, Chen HC. Sex difference in the associations among obesity-related indices with incident hypertension in a large Taiwanese population follow-up study. J Pers Med. 2022;12:972.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–85.
Hossain MM, Mukheem A, Kamarul T. The prevention and treatment of hypoadiponectinemia-associated human diseases by up-regulation of plasma adiponectin. Life Sci. 2015;135:55–67.
Luo L, Liu M. Adiponectin: friend or foe in obesity and inflammation. Med Rev. 2021;2022(2):349–62.
McCormick N, O’Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, Choi HK. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization. Arthritis Rheumatol. 2021;73:2096–104.
Wang HP, Xu YY, Xu BL, Lu J, Xia J, Shen T, Fang J, Lei T. Correlation between abdominal fat distribution and serum uric acid in patients recently diagnosed with type 2 diabetes. Diabetes Metab Syndr Obes. 2023;16:3751–62.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61.
Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7.
Acknowledgements
The authors thank all participants in the study. The authors thank the Guang Ning Team at Ruijin hospital, Shanghai Jiaotong University School of Medicine, for helping establish the data set in the communities.
Funding
The study was supported by grants from the National Key R&D Program of China (2018YFC1311800) and the Dalian Science and Technology Bureau (project 2022RG11).
Author information
Authors and Affiliations
Contributions
Study design: ZN-G and YT-L. Data collection and analysis: L-L. Drafting and revising the manuscript: YT-L and L-L. All authors have read and agreed to the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Ethical Review Committee of Ruijin Hospital (RUIJIN-2011–14), and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Luo, L. & Gao, Z. J-shaped relationship between Chinese visceral adiposity index and hyperuricemia: a cross-sectional study. Lipids Health Dis 23, 267 (2024). https://doi.org/10.1186/s12944-024-02247-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02247-1