Abstract
Background and objective
The value of the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) assessment in the context of metabolic abnormalities is growing in importance. Nevertheless, the relationship between NHHR and hyperuricemia (HUA) is unknown. This study seeks to investigate the relationship between NHHR and HUA.
Methods
The data derived from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) included 7,876 adult participants. The multivariable logistic regression model, subgroup analysis and smooth fitting curve were utilized in order to investigate the association between NHHR and HUA.
Results
In the fully adjusted model 3, NHHR was significantly associated with HUA. Specifically, participants in the highest quartile of NHHR had 1.95 times higher odds of HUA prevalence compared to those in the lowest quartile [2.95 (2.39, 3.64), P < 0.0001]. Although the overall trend suggested a positive association, further analysis using smooth fitting curves and threshold effect analysis indicated that this association was nonlinear, with an inflection point at 5.8. The positive association persisted across different HUA definitions and after removing outliers. Subgroup analysis showed significant interactions between NHHR and HUA in different races and diabetes statuses. The odds of HUA prevalence were higher among non-diabetic participants [1.40 (1.32, 1.49), P < 0.0001] compared to diabetic participants [1.18 (1.06, 1.32), P = 0.0031]. Mexican Americans had the lowest odds of HUA prevalence [1.09 (0.92, 1.27), P = 0.2413] compared to other races.
Conclusions
There is a significant positive association between NHHR and HUA, indicating that NHHR may serve as a potential risk assessment maker for HUA, although further prospective studies are needed for validation.
Similar content being viewed by others
Background
Hyperuricemia (HUA) is a metabolic disorder which is characterized by an elevation in the levels of uric acid. The prevalence of HUA is 20% among women and 20.2% among men in the United States. The incidence of HUA has shown an increasing trend globally in recent years [1, 2]. The deposition of uric acid and urate in the human body can result in a number of complications, including as gout, kidney damage, and kidney stones [3, 4]. Increasing evidence suggests that HUA is linked to a wide range of diseases, including chronic kidney disease, metabolic syndrome, and cardiovascular diseases [5,6,7,8]. HUA poses a serious threat to human health and quality of life, imposing a substantial economic burden on global public health [9].
Lipid metabolism dysregulation in the body is linked to the onset and progression of various cardiovascular diseases. Moreover, the relationship between uric acid and metabolic syndrome is bidirectional and complex. This complex interplay suggests that uric acid is both a marker and a mediator of metabolic disturbances [10]. Compared to traditional assessments of body lipid indicators, the value of the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) assessment in the context of metabolic abnormalities is growing in importance. Studies have shown that NHHR holds significant importance in the research of cardiovascular diseases and metabolic syndrome [11, 12]. Elevated NHHR levels also have significant clinical value in the risk assessment of diseases in particular breast cancer, abdominal aortic aneurysm, depression, and kidney stones [13,14,15,16]. Obesity and insulin resistance are becoming global health issues and are also risk factors for HUA. These conditions are inextricably linked to dysregulation of lipid metabolism in the body [17,18,19]. Dysregulation of lipid metabolism can alter the body’s metabolic patterns and affect disease progression in patients with HUA [20, 21]. However, the relationship between NHHR, as a novel lipid indicator, and HUA remains unclear.
Therefore, we utilized sample data from the National Health and Nutrition Examination Survey (NHANES) of American adults to investigate the association between NHHR and HUA. This study may provide a novel insight into the pathogenesis of HUA for future research.
Methods
Study population
NHANES, a significant epidemiological survey project led by the National Center for Health Statistics (NCHS), is for the purpose of evaluating the health and nutrition of Americans, Providing critical data on the health status of Americans to policymakers, researchers, and the public [22]. From the 15,560 participants in NHANES 2017–2020, we excluded 6,373 participants who lacked serum uric acid data and were under 18 years of age, and 1,311 participants who lacked NHHR data. Ultimately, 7,876 participants who met the study criteria were included (Fig. 1).
Definition of outcome and exposure variables
The outcome variable in this study is HUA, which is diagnosed based on serum uric acid levels. HUA is diagnosed when a serum uric acid concentration reaches a level of ≥ 6 mg/dL in females and ≥ 7 mg/dL in males [23]. Sensitivity analysis was conducted using different definitions of HUA, including serum uric acid concentration ≥ 6.8 mg/dL [24], to ensure the robustness of the study findings. Serum uric acid levels were measured by professionals using the Roche Cobas 6000 analyzer (c501 module).
The exposure variable is NHHR. This is calculated by dividing non-high-density lipoprotein cholesterol (total cholesterol minus high-density lipoprotein cholesterol) by high-density lipoprotein cholesterol [25].
Covariables
To more accurately understand the relationship between HUA and NHHR, We included potential confounding covariables including demographic factors (gender, race, age, education level, family income-to-poverty ratio (PIR), marital status), lifestyle factors (physical activity status, drink status, smoking status), clinical measurements (glycated hemoglobin, total bilirubin, fasting blood glucose, serum creatinine, urinary albumin, serum urea nitrogen, aspartate aminotransferase, alanine aminotransferase, waist circumference, body mass index (BMI), systolic and diastolic blood pressure and medical history (diabetes, hyperlipidemia, high blood pressure, and kidney stones). The definitions of these covariables can be found in Supplementary Table 1. Missing values for covariables are replaced by medians for continuous variables and plurals for categorical variables.
Statistical analysis
All statistical analyses were performed using Empower software (www.empowerstats.com) and R version 4.1.3. The appropriate NHANES sampling weights, as prescribed in the guidelines established by the Centers for Disease Control and Prevention (CDC), were duly applied. In the case of continuous data variables, variance-weighted analysis was employed, while in the case of categorical variables, weighted chi-square tests were used to assess differences between NHHR quartiles. In constructing multivariable analyses, we employed three different models to evaluate the association between NHHR and HUA. Model 1 is not adjusted to account for any variables. Model 2 was adjusted for demographic factors (including race, age, and gender). Model 3 incorporated all covariable adjustments. To deal with potential outliers in the NHHR distribution, we examined the data for abnormal truncated tail values. Outliers were defined as values greater than three standard deviations from the mean. These outliers were removed and the analyses repeated to assess the robustness of the association between NHHR and HUA. We performed linear trend tests by converting NHHR levels into quartile categorical variables. To examine the nonlinear relationship and inflection points between NHHR and HUA, a smooth fitting curve and threshold effect analysis were utilised. Finally, subgroup analyses were performed to identify any statistically significant differences between the various subgroups, including race, gender, age, BMI, diabetes, high blood pressure and estimated glomerular filtration rate (eGFR). We decided that the p-value of less than 0.05 was statistically significant.
Results
Baseline characteristics
The weighted distributions of all clinical characteristics for participants are shown in Table 1 across the quartiles of the NHHR. This study included 7,876 adults with an mean age of 48.43 ± 17.32 years. The study population consisted of 48.29% male and 51.71% female participants. The mean NHHR was 2.72 ± 1.23, and the NHHR range for the 1st to 4th quartiles was 0.28–1.80, 1.80–2.50, 2.50–3.35, and 3.35–11.9, respectively. The overall prevalence of HUA was 17.28%, with quartile-specific prevalence rates of 10.96% in the 1st quartile, 12.75% in the 2nd quartile, 19.29% in the 3rd quartile, and 25.78% in the 4th quartile. Significant differences were observed across NHHR quartiles in terms of race, gender, age, drink status, glycated hemoglobin, systolic and diastolic blood pressure, fasting blood glucose, PIR, education level, marital status, smoking status, serum creatinine, urinary albumin, aspartate aminotransferase, total bilirubin, alanine aminotransferase, and the prevalence of diabetes, hyperlipidemia, high blood pressure, kidney stones, and HUA (p < 0.05).
Elevated NHHR levels are linked to a higher odds of HUA Prevalence
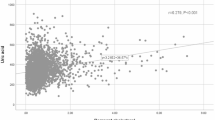
Table 2 shows a positive association between NHHR and HUA across all three models, all of which were statistically significant. When NHHR was treated as a quartile variable, the highest quartile of NHHR participants exhibited a 1.95 times greater odds of HUA prevalence compared to the lowest quartile [2.95 (2.39, 3.64), P < 0.0001]. When different definitions of HUA were used, this similar association has also been observed in this instance. The results of Supplementary Table 2 indicate that this positive association remained statistically significant even after the exclusion of the outliers. The findings of the smooth fitting curve showed a nonlinear relationship between NHHR and HUA (Fig. 2). Table 3 indicates that the inflection points for NHHR and HUA were 5.8.
Subgroup analysis
Table 4 shows the different associations between NHHR and HUA across various subgroups. A positive association between NHHR and HUA was identified in each subgroup. Additionally, we observed significant interactions between NHHR and HUA in the race and diabetes subgroups (p < 0.05). The odds of HUA prevalence were higher among non-diabetic participants [1.40 (1.32, 1.49), P < 0.0001] compared to diabetic participants [1.18 (1.06, 1.32), P = 0.0031]. Among the different races, Mexican Americans had the lowest odds of HUA prevalence [1.09 (0.92, 1.27), P = 0.2413] compared to other races.
Discussion
In this study of 7,876 U.S. adults, the results demonstrated the positive association between NHHR and the odds of HUA prevalence, which persisted across three different models. This remains the case even when different HUA definitions are employed and outliers are removed. The results of the smooth fitting curve indicated a nonlinear positive association between NHHR and HUA. The threshold effect analysis showed an inflection point at 5.8. Furthermore, subgroup analysis revealed significant interactions between different races, diabetes status, and NHHR levels, affecting the odds of HUA prevalence.
HUA is associated with the onset and development of various chronic diseases and has become a significant health issue that cannot be ignored, imposing a heavy burden on public health [1, 5, 26, 27]. The relationship between uric acid and metabolic syndrome is bidirectional and complex; elevated uric acid levels are not only a consequence of metabolic syndrome but can also exacerbate its components, such as insulin resistance, obesity, and lipid metabolism dysregulation. Conversely, insulin resistance can increase uric acid reabsorption in the kidneys, further elevating uric acid levels [10]. Therefore, identifying risk factors for HUA is crucial for guiding clinical prevention and treatment. Increasing research indicates that lipid metabolism is vital in the development of HUA. A study carried out in Northwest China showed that dyslipidemia increases the odds of HUA prevalence, with higher triglyceride levels and total cholesterol levels identified as risk factors for HUA [28]. A retrospective study involving 3,884 subjects found that triglycerides are risk factor for HUA. Individuals with hypertriglyceridemia have a 2.353 times higher odds of HUA prevalence compared to those with normal triglyceride levels [29]. A large-scale cohort study conducted in China by Liu et al [30], over a period of four years involving 15,198 subjects demonstrated that a higher triglyceride to high-density lipoprotein cholesterol ratio is positively correlated with the odds of HUA prevalence. Another study also showed a positive association between residual cholesterol and HUA in Americans, which is similar to what we found, but the association was stronger in those without diabetes [31].
NHHR, as a newly developed lipid indicator, has been shown to have significant predictive value for the risk of kidney stones and diabetes [15, 32]. An elevated NHHR indicates dysregulation of lipid metabolism. The pathogenesis between NHHR and HUA remains unclear. Our study results suggest that elevated NHHR levels are positively associated with the odds of HUA prevalence. Based on existing studies, we propose several possible theoretical explanations: (1) Elevated lipid metabolism levels are closely related to insulin resistance [33, 34]. Insulin resistance is associated with increased expression of Urate transporter 1, which promotes uric acid reabsorption and inhibits the function of adenosine triphosphate-binding cassette subfamily G member 2, reducing uric acid excretion [35], this consequently resulted in elevated serum uric acid levels. Anti-diabetic medications such as pioglitazone and metformin, which improve insulin resistance, can reduce serum uric acid levels [36, 37]. (2) Low serum high-density lipoprotein cholesterol (HDL-C) levels can lead to decreased glomerular filtration function, thereby increasing serum uric acid levels. Low serum HDL-C levels are key factors in atherosclerosis and are major causes of renal artery stenosis and decreased glomerular filtration function [38]. A body of evidence indicates that a low serum HDL-C level represents a risk factor associated with the advancement of chronic kidney disease [39]. (3) High triglyceride levels can lead to increased oxidation of free fatty acids, producing more acetyl-CoA. The liver utilizes free fatty acids to synthesize more purines, resulting in elevated uric acid levels [40]. (4) HDL-C has anti-inflammatory properties. Low levels of HDL-C can weaken antioxidant and anti-inflammatory effects [41]. Low levels of HDL-C trigger inflammatory responses and oxidative stress in the body, promoting purine metabolism and increasing uric acid production [40, 42]. Therefore, we have reason to infer that elevated NHHR levels are associated with HUA.
In the diabetes subgroup of this study, we found that among participants with different NHHR levels, non-diabetic participants had a higher odds of HUA prevalence compared to diabetic participants. This may be because non-diabetic participants might have higher insulin resistance, whereas diabetic patients receive medication to control blood glucose and insulin levels and are often treated with lipid-lowering drugs. Some glucose-controlling and lipid-lowering medications have been shown to reduce serum uric acid levels [36, 37, 43]. Recent studies have highlighted Sodium-Glucose Co-Transporter 2 inhibitors facilitate increased renal excretion of uric acid by inhibiting its reabsorption in the renal proximal tubules [44, 45]. We also found that among participants with different NHHR levels, a lower odds of HUA prevalence was observed in Mexican Americans compared to other racial groups. This may be due to genetic factors, dietary habits, and lifestyle differences [46].
The strengths of our study include utilizing NHANES data, overseen by the CDC, which ensures the rigour and authenticity of the data through a nationally representative survey methodology. Admittedly, our study has certain limitations. Since our study design is cross-sectional, we cannot accurately infer the causal relationship between NHHR and HUA. Although we included many confounding factors that influence NHHR and HUA, there are still some potential confounders that we could not include, such as genetic factors, the use of glucose-controlling and lipid-lowering medications, and the consumption of high-purine foods.
Conclusion
The study results indicate that higher NHHR levels are positively associated with the odds of HUA prevalence, especially when NHHR levels are below the inflection point of 5.8. Early intervention and treatment for individuals with specific NHHR levels may reduce the occurrence of HUA and related complications. Based on these findings, NHHR is expected to become a new indicator for assessing lipid levels in HUA, providing new insights into the link between lipid metabolism disorders and the formation of HUA. Consequently, to substantiate these findings, large-scale prospective studies are required.
Data availability
The survey data are publicly available on the internet for data users and researchers throughout the world ( www.cdc.gov/nchs/nhanes/ ).
Abbreviations
- HUA:
-
Hyperuricemia
- NHHR:
-
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- CDC:
-
Centers for Disease Control and Prevention
- PIR:
-
Family income-to-poverty ratio
- BMI:
-
Body Mass Index
- HDL-C:
-
High-density lipoprotein cholesterol
- eGFR:
-
Estimated glomerular filtration rate
References
Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and Hyperuricemia in the United States and Decadal trends: the National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019;71:991–9.
Liu R, Han C, Wu D, Xia X, Gu J, Guan H, Shan Z, Teng W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res Int 2015, 2015:762820.
Xu Z, Yao X, Duan C, Liu H, Xu H. Metabolic changes in kidney stone disease. Front Immunol. 2023;14:1142207.
Su HY, Yang C, Liang D, Liu HF. Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. Biomed Res Int 2020, 2020:5817348.
Shu J, Zhao R, Xu H, Liu X, Guo H, Lu C. Hyperuricemia is associated with metabolic syndrome: a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES). Prev Med Rep. 2023;36:102520.
Han Y, Cao Y, Han X, Di H, Yin Y, Wu J, Zhang Y, Zeng X. Hyperuricemia and gout increased the risk of long-term mortality in patients with heart failure: insights from the National Health and Nutrition Examination Survey. J Transl Med. 2023;21:463.
Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular Biological and Clinical understanding of the pathophysiology and treatments of Hyperuricemia and its Association with Metabolic Syndrome, Cardiovascular diseases and chronic kidney disease. Int J Mol Sci 2021, 22.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62:170–80.
Jeong YJ, Park S, Yon DK, Lee SW, Tizaoui K, Koyanagi A, Jacob L, Kostev K, Dragioti E, Radua J, et al. Global burden of gout in 1990–2019: a systematic analysis of the Global Burden of Disease study 2019. Eur J Clin Invest. 2023;53:e13937.
Borghi C, Fogacci F, Piani F. Not all the eggs and the chickens are the same: the case of uric acid and metabolic syndrome. Eur J Intern Med. 2022;103:36–7.
Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC, Kim SW, Chung JH, Min YK, Lee MS, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168:2678–83.
Zhu L, Lu Z, Zhu L, Ouyang X, Yang Y, He W, Feng Y, Yi F, Song Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. 2015;73:931–8.
Luo X, Ye J, Xiao T, Yi T. Exploration of the association of a lipid-related biomarker, the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR), and the risk of breast cancer in American women aged 20 years and older. Int J Surg 2024.
Lin W, Luo S, Li W, Liu J, Zhou T, Yang F, Zhou D, Liu Y, Huang W, Feng Y, Luo J. Association between the non-HDL-cholesterol to HDL- cholesterol ratio and abdominal aortic aneurysm from a Chinese screening program. Lipids Health Dis. 2023;22:187.
Hong H, He Y, Gong Z, Feng J, Qu Y. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and kidney stones: a cross-sectional study. Lipids Health Dis. 2024;23:102.
Qi X, Wang S, Huang Q, Chen X, Qiu L, Ouyang K, Chen Y. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: a cross-sectional NHANES study. J Affect Disord. 2024;344:451–7.
Kuwabara M, Kuwabara R, Niwa K, Hisatome I, Smits G, Roncal-Jimenez CA, MacLean PS, Yracheta JM, Ohno M, Lanaspa MA et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10.
Han Y, Zhou Z, Zhang Y, Zhao G, Xu B. The Association of Surrogates of Insulin Resistance with hyperuricemia among middle-aged and older individuals: a Population-based Nationwide Cohort Study. Nutrients 2023, 15.
Peng TC, Wang CC, Kao TW, Chan JY, Yang YH, Chang YW, Chen WL. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int 2015, 2015:127596.
Ma L, Wang J, Ma L, Ge Y, Wang XM. The effect of lipid metabolism disorder on patients with hyperuricemia using Multi-omics analysis. Sci Rep. 2023;13:18211.
Kvasnička A, Friedecký D, Brumarová R, Pavlíková M, Pavelcová K, Mašínová J, Hasíková L, Závada J, Pavelka K, Ješina P, Stibůrková B. Alterations in lipidome profiles distinguish early-onset hyperuricemia, gout, and the effect of urate-lowering treatment. Arthritis Res Ther. 2023;25:234.
Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2 2013:1–23.
Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, et al. Hyperuricemia, Acute and chronic kidney Disease, Hypertension, and Cardiovascular Disease: report of a Scientific Workshop Organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71:851–65.
Bjornstad P, Laffel L, Lynch J, El Ghormli L, Weinstock RS, Tollefsen SE, Nadeau KJ. Elevated serum uric acid is Associated with Greater Risk for Hypertension and Diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the Treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42:1120–8.
Wang A, Li Y, Zhou L, Liu K, Li S, Zong C, Song B, Gao Y, Li Y, Tian C, et al. Non-HDL-C/HDL-C ratio is associated with carotid plaque stability in general population: a cross-sectional study. Front Neurol. 2022;13:875134.
Yin Y, Zhou E, Wu J. Association between hyperuricemia and long-term mortality in patients with hypertension: results from the NHANES 2001–2018. Front Cardiovasc Med. 2024;11:1306026.
Jiang J, Zhang T, Liu Y, Chang Q, Zhao Y, Guo C, Xia Y. Prevalence of diabetes in patients with hyperuricemia and gout: a systematic review and Meta-analysis. Curr Diab Rep. 2023;23:103–17.
Liu F, Du GL, Song N, Ma YT, Li XM, Gao XM, Yang YN. Hyperuricemia and its association with adiposity and dyslipidemia in Northwest China: results from cardiovascular risk survey in Xinjiang (CRS 2008–2012). Lipids Health Dis. 2020;19:58.
Hou YL, Yang XL, Wang CX, Zhi LX, Yang MJ, You CG. Hypertriglyceridemia and hyperuricemia: a retrospective study of urban residents. Lipids Health Dis. 2019;18:81.
Liu XY, Wu QY, Chen ZH, Yan GY, Lu Y, Dai HJ, Li Y, Yang PT, Yuan H. Elevated triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio increased risk of hyperuricemia: a 4-year cohort study in China. Endocrine. 2020;68:71–80.
Zhou X, Weng X, Xu J, Wang W. Correlation between remnant cholesterol and hyperuricemia in American adults. Lipids Health Dis. 2024;23:176.
Tan MY, Weng L, Yang ZH, Zhu SX, Wu S, Su JH. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with type 2 diabetes mellitus: recent findings from NHANES 2007–2018. Lipids Health Dis. 2024;23:151.
Iqbal J, Al Qarni A, Hawwari A, Alghanem AF, Ahmed G. Metabolic syndrome, Dyslipidemia and Regulation of Lipoprotein Metabolism. Curr Diabetes Rev. 2018;14:427–33.
Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep. 2002;2:216–22.
Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, Uchida S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Ren Physiol. 2017;313:F826–34.
Tsunoda S, Kamide K, Minami J, Kawano Y. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: effect of a low-energy diet and an insulin-sensitizing agent. Am J Hypertens. 2002;15:697–701.
Yuan H, Hu Y, Zhu Y, Zhang Y, Luo C, Li Z, Wen T, Zhuang W, Zou J, Hong L, et al. Metformin ameliorates high uric acid-induced insulin resistance in skeletal muscle cells. Mol Cell Endocrinol. 2017;443:138–45.
Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int. 2013;83:28–40.
Kawachi K, Kataoka H, Manabe S, Mochizuki T, Nitta K. Low HDL cholesterol as a predictor of chronic kidney disease progression: a cross-classification approach and matched cohort analysis. Heart Vessels. 2019;34:1440–55.
de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4:12.
Bauer R, Brüne B, Schmid T. Cholesterol metabolism in the regulation of inflammatory responses. Front Pharmacol. 2023;14:1121819.
Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–9.
Derosa G, Maffioli P, Reiner Ž, Simental-Mendía LE, Sahebkar A. Impact of statin therapy on plasma uric acid concentrations: a systematic review and Meta-analysis. Drugs. 2016;76:947–56.
Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, Ferreira JP, Salsali A, Kaempfer C, Brueckmann M, Pocock SJ, et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. Eur Heart J. 2022;43:3435–46.
Bragagni A, Piani F, Borghi C. Surprises in cardiology: efficacy of gliflozines in heart failure even in the absence of diabetes. Eur Heart J Suppl. 2021;23:E40–4.
Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. 2020;12:3167–81.
Acknowledgements
We would like to thank all participants in this study.
Funding
This study was supported by The Science and Technology Promotion Program of the Chinese People’s Liberation Army Air Force Medical Center (2022ZTYB14) and Beijing Natural Science Foundation (7232174).
Author information
Authors and Affiliations
Contributions
ZJ, XZ, and FS designed the research. ZJ, XZ, HJ, XW, and DZ collected, analyzed the data, and drafted the manuscript. ZJ and FS revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The portions of this study involving human participants, human materials, or human data were conducted in accordance with the Declaration of Helsinki and were approved by the NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, Z., Zhu, X., Zhao, D. et al. Associations between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and hyperuricemia: a cross-sectional study. Lipids Health Dis 23, 280 (2024). https://doi.org/10.1186/s12944-024-02269-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02269-9