Abstract
Background
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) have represented an important change in the management of hypercholesterolemia, although, until now, they have barely been used. Without PCSK9i, many patients with atherosclerotic cardiovascular disease (CVD) or those at very high risk do not reach their therapeutic LDLc objectives.
Objective
The analysis aimed to examine the clinical and biochemical characteristics of subjects receiving PCSK9i treatment in the Dyslipidemia Registry of the Spanish Atherosclerosis Society.
Methods
All consecutive subjects aged ≥ 18 years from different Lipid Units included in the Dyslipidemia Registry of the SEA were analyzed. Inclusion criteria consisted of unrelated patients aged ≥ 18 at the time of inclusion with hypercholesterolemia (LDL-C ≥ 130 mg/dL or non-HDL-C ≥ 160 mg/dL after the exclusion of secondary causes) who were studied for at least two years after inclusion. Participants’ baseline and final visit clinical and biochemical characteristics were analyzed based on whether they were on primary or secondary prevention and whether they were taking PCSK9i at the end of follow-up.
Results
Eight hundred twenty-nine patients were analyzed, 7014 patients in primary prevention and 1281 in secondary prevention at baseline. 4127 subjects completed the required follow-up for the final analysis. The median follow-up duration was 7 years (IQR 3.0–10.0). Five hundred patients (12.1%) were taking PCSK9i at the end of the follow-up. The percentage of PCSK9i use reached 35.6% (n = 201) and 8.7% (n = 318) in subjects with and without CVD, respectively. Subjects on PCSK9i and oral lipid-lowering agents with and without CVD achieved LDLc reductions of 80.3% and 75.1%, respectively, concerning concentrations without lipid-lowering drugs. Factors associated with PCSK9i use included increasing age, LDLc without lipid-lowering drugs and the Dutch Lipid Clinic Network (DLCN) score. However, hypertension, diabetes, smoking, and LDLc after oral lipid-lowering drugs were not independent factors associated with PCSK9i prescription. In subjects with CVD, the use of PCSK9i was higher in men than in women (an odds ratio of 1.613, P = 0.048).
Conclusions
Approximately one-third of CVD patients received PCSK9i at the end of follow-up. The use of PCSK9i was more focused on baseline LDLc concentrations rather than on CVD risk. Women received less PCSK9i in secondary prevention compared to men.
Similar content being viewed by others
Introduction
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) have represented crucial advance in managing hypercholesterolemia. PCSK9 facilitates the degradation of the low-density lipoprotein (LDL) receptor by preventing its recycling. PCSK9i means more LDL receptors in the cell surface and an increase in the removal of LDL cholesterol (LDLc) from circulation. Studies have shown that PCSK9i can lower LDLc levels by up to 60%, making it a highly effective way of reducing the risk of cardiovascular events in patients with hypercholesterolemia [1, 2]. The effects of PCSK9i have been extensively studied in multiple randomized clinical trials, including populations with cardiovascular disease (CVD) [3], heterozygous [4] and homozygous familial hypercholesterolemia (FH) [5], combined dyslipidemia [6], diabetes [7], or statin intolerance [8]. Across these trials, PCSK9i has consistently demonstrated a marked reduction in low-density lipoprotein cholesterol (LDLc) levels with excellent tolerability [3, 8]. When used in combination with oral lipid-lowering drugs, the vast majority of patients achieve current LDLc goals [9]. Furthermore, in clinical trials focused on CVD reduction, PCSK9i has significantly decreased CVD events in both primary 10and secondary prevention [10,11,12], with some studies also showing a reduction in total mortality [12].
Without PCSK9i, many patients with atherosclerotic CVD, or those at very high risk, do not reach the therapeutic LDLc objectives [13, 14]. PCSK9i has been underutilized, and the percentage of CVD patients receiving these drugs is below 2% [13, 14]. The reasons for said underutilization are likely complex, with medication costs being a significant factor. Several studies have compared the cost-effectiveness of PCSK9i with other lipid-lowering therapies, such as statins. While PCSK9i has shown impressive results in terms of reducing CVD events and improving cardiovascular outcomes, its high cost remains a significant obstacle to its widespread utilization [15, 16]. In Spain, PCSK9i has been available on prescription since 2016, albeit with substantial restrictions on reimbursement from the National Health System. It is limited to individuals with FH or those in secondary CVD prevention with LDLc levels ≥ 100 mg/dL despite high-dose potent statin therapy. However, even within this subgroup, the use of PCSK9i varies widely.
The objective of this study was to find out what factors influence the decision to prescribe PCKS9i among those subjects who are candidates for using it according to established therapeutic objectives, and who would be included in the financing conditions. This analysis examines the clinical and biochemical characteristics of subjects receiving PCSK9i treatment in the Lipid Units of the SEA registry, distinguishing between primary and secondary prevention. These findings reflect the actual use of PCSK9i in our society and the factors associated with the use of this treatment.
Patients and methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Design and participant selection
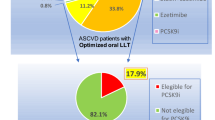
This study included all consecutive subjects aged ≥ 18 with a clinical diagnosis of hypercholesterolemia (LDLc ≥ 130 mg/dL or non-HDLc ≥ 160 mg/dL after the exclusion of secondary causes: hypothyroidism, nephrotic syndrome, pregnancy, cholestasis, immunosuppressants and antivirals) from the 65 different Lipid Units located in General Hospitals in Spain who had been followed for at least two years [17, 18]. All subjects provided written informed consent for a protocol approved by the Aragonese Ethical Committee of Clinical Research, Spain (#PI18/262). Participants’ clinical and biochemical characteristics, based on whether they were receiving PCSK9i during follow-up, were studied (Fig. 1).
Physical examination, cardiovascular outcomes, and lipid determinations
The physical examination baseline data included anthropometry measurements, clinical history, CVD risk factors (hypertension, diabetes, smoking), personal and family CVD history. Baseline lipid data were collected from a fasting blood sample and included total cholesterol, LDLc, HDLc, triglycerides (TG) and lipoprotein(a) (Lp(a)). The blood test was measured locally without lipid lowering medications whenever possible. LDLc was calculated using the Friedewald formula when TG were less than 400 mg/dL. The clinical diagnosis of FH was based on the Dutch Lipid Clinic Network (DLCN) score [19].
FH was diagnosed when DLCN points were ≥ 6. Three sets of lipid values are analyzed in the current report. Those without lipid drugs, in some cases historical values; the lipids with treatment at the beginning of follow-up which correspond to the data with the first lipid-lowering treatment once the patients are treated in the Unit; and the lipid values reported during the last visit of the follow-up.
The study was conducted in accordance with the Declaration of Helsinki for the protection of the rights and welfare of people participating in biomedical research.
Statistical analyses
Baseline characteristics
Continuous baseline variable values are presented as mean standard deviation (SD) or median and interquartile range (IQR) for non-normal distributed variables. Categorical variables were analyzed as absolute and relative frequencies and the chi-squared test (or Fisher’s exact test where appropriate) for differences among groups.
The lipid levels were assessed in screening and at the end of the follow-up and the differences among groups were analyzed using the ANOVA test for continuous variables. Changes mid-study to lipid levels were described as absolute and relative mean changes with 95% confidence intervals (CI). Intergroup comparisons were carried out using an independent-sample t-test or Mann–Whitney U test for continuous variables with normal and non-normal distributions, respectively, and a chi-squared test or Fisher’s exact test for categorical variables. Predictors for the prescription of PCSK9i during follow-up were calculated by logistic regression. The regression model was generated including the variables shown in Table 1. Afterwards, we implemented the new model, keeping all the variables that showed p-value < 0.2 and major CVD risk factors. All data analyses were performed using SPSS version 21.
Results
Baseline characteristics of participants
A total of 8295 patients with primary hypercholesterolemia were included in the study (Table 1). Among them, 7014 patients were recruited for primary prevention, while 1281 had experienced CVD at the time of inclusion in the registry. Table 1 details the baseline characteristics of the patients. In the primary prevention group, there were more women (51.2%) than men (48.8%), whereas men were predominant in the secondary prevention group (73.4%). Patients in the secondary prevention group were older and had a higher prevalence of obesity, hypertension, and diabetes. Current smokers were more common among patients without CVD history. The DLCN categories were similar in both groups, with a median DLCN score of 4.0 points. Untreated LDLc levels were lower among patients in the secondary prevention group compared to those without CVD (183 mg/dL vs. 199 mg/dL, respectively). Patients with CVD were more likely to be on PCSK9i (either as monotherapy or in combination with lipid-lowering drugs) compared to those in primary prevention (8.1% vs. 1.7%, respectively) at the start of the follow-up.
Baseline characteristics of participants taking PCSK9 inhibitors at the end of the follow-up
Out of 4127 subjects who completed the follow-up (3655 subjects without CVD and 565 with CVD at baseline), 137 and 519 patients were taking PCSK9i at baseline and the end of the follow-up, respectively. The median follow-up period was 7 years (IQR 3.0–10.0). The percentage of PCSK9i use increased to 35.6% (n = 201) and 8.7% (n = 318) in subjects with and without CVD, respectively (Table 2). Subjects on PCSK9i at the end of the follow-up had higher total cholesterol and LDLc levels compared to subjects not taking PCSK9i, in both those with and without CVD at baseline (Table 2). The DLCN score was also three to four times higher in subjects taking PCSK9i.
Baseline factors associated with the prescription of PCSK9 inhibitors
In subjects without CVD at baseline, increasing age, LDLc without lipid-lowering drugs, and DLCN scores were independent conditions associated with the prescription of PCSK9i. In subjects with CVD at baseline, increasing age, male gender, and DLCN scores were associated factors. Hypertension, diabetes, smoking, and LDLc after oral lipid-lowering drugs were not independent factors associated with prescription. In subjects with CVD, the use of PCSK9i was higher in men than in women (an odds ratio of 1.613, P = 0.048) (Table 3).
Biochemical characteristics at the end of the follow-up
LDLc concentrations at the end of the follow-up were 48.6 mg/dL and 71.0 mg/dL in subjects with and without CVD, respectively. These concentrations were significantly lower than those in subjects not taking PCSK9i (p-value < 0.001). TG concentrations were also lower in subjects treated with PCSK9i (Table 4). The evolution of LDLc concentrations without lipid-lowering drugs, after oral treatment, and at the end of the follow-up during evolution is depicted in Fig. 2. Subjects on PCSK9i at the end of the follow-up, with and without CVD, achieved LDLc reductions of 80.3% and 75.1%, respectively, compared to concentrations without lipid-lowering treatment (Fig. 1).
Discussion
In this study, the use of PCSK9i in a large cohort of patients with dyslipidemia over an average of 7 years follow-up at different lipid units in Spain has been analyzed. The clinical settings, patient numbers, and follow-up duration provide an insight into managing primary hypercholesterolemia in specialized centers within our country, explicitly identifying the patient populations for whom PCSK9i is utilized.
Several conclusions can be drawn from this study. Firstly, the use of PCSK9i in this population is notably higher than reported in other populations, particularly in secondary prevention. In the SEA registry, up to 32% of patients with a history of CVD received treatment involving PCSK9i, in contrast to 2% in the European population [13, 14]. This discrepancy may be attributed to the specialized nature of lipid metabolism centers treating severe hypercholesterolemia, including FH, where clinicians are knowledgeable and experienced in managing these drugs. Furthermore, it also reflects the increasing use of PCSK9i in current practice.
PCSK9i is employed as a third-line lipid-lowering treatment following potent statins and ezetimibe. Among subjects receiving PCSK9i, two-thirds in primary prevention and three-quarters in secondary prevention are concurrently treated with dual oral lipid-lowering therapy. This adherence to stepwise treatment guidelines suggests that clinicians know and follow recommended treatment protocols [19, 20] although the use of ezetimibe before initiating PCSK9i therapy is not mandatory in our country. It is noteworthy that not only the use of PCSK9i has increased during follow-up, but also the use of ezetimibe, which was little used at the beginning when patients were referred to lipid units and which increased at the end of follow-up, up to 73.1% in subjects in secondary prevention and treatment with PCSK9i. This is compatible with the low past use of Ezetimibe, especially in primary prevention, in our setting [21] that has recently increased [13].
Secondly, it is worth nothing that approximately 13% of subjects in both primary and secondary prevention, who are using PCSK9i, appear to be statin intolerant, as they do not report statin use. While this percentage is relatively high, it aligns with previously reported frequencies of statin intolerance within the population [22], and may be inflated in lipid clinics where such patients are frequently referred. Bempedoic acid could potentially serve as an alternative in these cases [23]; although, at the time of this report, this drug was not available in our country. The alternative prescription of bempedoic acid or its association with iPCSK9i is an option that has been little explored and could have a role in these subjects [24]. Statin intolerance in clinical practice is an important issue and PCSK9i is a useful tool to reach LDLc goals in this group of patients.
Thirdly, this study analyzes the factors associated to PCSK9i prescription, and the results underscore that the diagnosis of FH influences the prescription of PCSK9i, with the DLCN score being the most strongly associated factor related to prescription. While PCSK9i effectively reduces LDLc levels, individuals with FH typically present with elevated LDLc, thus warranting treatment. However, other significant risk factors such as diabetes, hypertension or smoking, which contribute to increased cardiovascular risk, do not appear to influence prescription rates. PCSK9i mitigates cardiovascular risk, emphasizing the importance of prioritizing its use in patients with the highest risk, rather than solely focusing on LDLc concentrations [25]. Administrative factors may overinfluence prescription patterns, prioritizing LDLc thresholds over comprehensive risk reduction and potential CVD benefits. Consistent with previous findings [26], gender emerged as an independent factor in PCSK9i use, favoring men over women. This highlights the prevalent underuse of specific therapeutic measures based on misconceptions regarding risk in secondary CVD prevention in women [27]. It is recommended that when authorizing the prescription of PCSK9i, not only the diagnosis of a certain condition but also the individual risk of the subject is taken into account since the clinical benefit of PCSK9i may be closely linked to the global risk and not only to the LDLc level. Furthermore, prescription authorization should ensure impartial use of the treatment, avoiding unjustifiable gender disparities.
Finally, these results showed that patients with very high LDLc concentrations could achieve recommended LDLc levels and therapeutic goals with a mean LDLc reduction exceeding 80% by using the current therapeutic arsenal available. This figure is similar to those reported in other registries [28, 29]. However, the use of PCSK9 inhibitors remains low, given that the population in this study is mostly at very high cardiovascular risk. The price of PCSK9 inhibitors is the fundamental limitation for their prescription and their cost-effectiveness, even in secondary prevention, has been questioned [30, 31]. It is a pity that more patients cannot benefit from these potent, safe and effective drugs in cardiovascular prevention and a possible price reduction of more than 50% of the current price would help to increase their prescription [31, 32].
This suggests that future drugs that reduce LDLc should focus on easier administration, fewer side effects, or lower cost rather than enhancing lipid-lowering potency. It also underscores the need for specialized centers that focus on managing patients with lipid diseases within the different healthcare systems to achieve optimal outcomes in therapeutic goal attainment [33].
Strengths and limitations
The present study has several limitations. Firstly, it is an observational study in which patient management may vary throughout the follow-up period, and results are obtained during the final visit to the lipid clinic. Additionally, approximately only half of the patients in the registry had a follow-up duration sufficient for inclusion in this study. Furthermore, patients attending a lipid clinic exhibit specific characteristics, including more severe hypercholesterolemia and a higher prevalence of statin intolerance, potentially influencing the ease of PCSK9i prescriptions compared to other clinical settings.
Conclusion
The use of PCSK9i is present in almost one-third of subjects in secondary prevention treated in specialized units. The majority of these patients achieve LDLc concentrations < 55 mg/dL. The use of PCSK9i is more focused on LDLc concentrations rather than on CVD risk. Women receive less PCSK9i in secondary prevention compared to men. These results highlight the enormous potential of PCSK9 inhibition regarding the control of LDLc in high-risk patients but authorization for their prescription should consider the global risk of patients and should also ensure impartial use of the treatment, avoiding gender disparities.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DLCN:
-
Dutch Lipid Clinic network
- FH:
-
Familial hypercholesterolemia
- HDLc:
-
High-density lipoprotein cholesterol
- LDLc:
-
Low-density lipoprotein cholesterol
- Lp(a):
-
Lipoprotein(a)
- PCSK9i:
-
Pro-protein convertase subtilisin/kexin 9 inhibitor
- SEA:
-
Spanish Atherosclerosis Society
- TG:
-
Triglycerides
References
Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the Next Breakthrough in the Cardiovascular Field? J Am Coll Cardiol. 2015;65:2638–51.
Brandts J, Müller-Wieland D. PCSK9 Inhibition: New Treatment Options and Perspectives to Lower Atherogenic Lipoprotein Particles and Cardiovascular Risk. Curr Atheroscler Rep. 2019;21:40.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2015;385:331–40.
Blom DJ, Harada-Shiba M, Rubba P, et al. Efficacy and Safety of Alirocumab in Adults With Homozygous Familial Hypercholesterolemia: The ODYSSEY HoFH Trial. J Am Coll Cardiol. 2020;76:131–42.
Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–40.
Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:618–28.
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8.
Kastelein JJP, Kereiakes DJ, Cannon CP, et al. Effect of alirocumab dose increase on LDL lowering and lipid goal attainment in patients with dyslipidemia. Coron Artery Dis. 2017;28:190–7.
Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N Engl J Med. 2017;376:1527–39.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–22.
Schwartz GG, Szarek M, Bhatt DL, et al. Transiently achieved very low LDL-cholesterol levels by statin and alirocumab after acute coronary syndrome are associated with cardiovascular risk reduction: the ODYSSEY OUTCOMES trial. Eur Heart J. 2023;44:1408–17.
Ray KK, Haq I, Bilitou A, et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023;29: 100624.
Kotseva K, De Backer G, De Bacquer D, et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: A report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur J Prev Cardiol. 2021;28:370–9.
Kazi DS, Penko J, Coxson PG, et al. Updated Cost-effectiveness Analysis of PCSK9 Inhibitors Based on the Results of the FOURIER Trial. JAMA. 2017;318:748–50.
Annemans L, Packard CJ, Briggs A, Ray KK. “Highest risk-highest benefit” strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur Heart J. 2018;39:2546–50.
Marco-Benedí V, Bea AM, Sánchez Hernández RM, Plana N, Valdivielso P, Civeira F. Dyslipidemia treatment strategies in primary and secondary prevention. Dyslipemia Registry of the Spanish Arteriosclerosis Society. Clin E Investig En Arterioscle. 2022;34:303–10.
Civeira F, Arca M, Cenarro A, Hegele RA. A mechanism-based operational definition and classification of hypercholesterolemia. J Clin Lipidol. 2022;16:813–21.
Anon. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) - PubMed. https://pubmed.ncbi.nlm.nih.gov/21882396/. Accessed 21 Aug 2024.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143.
de Rojas FD, De Frutos T, Ponte A, Chacón JM, Vitale GC, PRINCEPS Investigators. Coronary heart disease and dyslipidemia: a cross-sectional evaluation of prevalence, current treatment, and clinical control in a large cohort of Spanish high-risk patients: the PRINCEPS study. Prev Cardiol. 2009;12:65-71.
Bytyçi I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43:3213–23.
Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med. 2023;388:1353–64.
Seijas-Amigo J, Cordero A, Olmo RF, et al. Patients With High Cardiovascular Risk as Candidates to Bempedoic Acid, After Treatment With Statins, Ezetimibe and PCSK9 Inhibitors: An Estimation and Cost-Effectiveness Analysis. J Cardiovasc Pharmacol. 2023;81:70–5.
Sabatine MS, Giugliano RP. Low-Density Lipoprotein Cholesterol Treatment in the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Era: Getting Back on Target. JAMA Cardiol. 2017;2:935–6.
Jiménez A, Viñals C, Marco-Benedí V, et al. Sex Disparities in Familial Hypercholesterolemia. J Am Coll Cardiol. 2023;81:203–5.
Okunrintemi V, Valero-Elizondo J, Patrick B, et al. Gender Differences in Patient-Reported Outcomes Among Adults With Atherosclerotic Cardiovascular Disease. J Am Heart Assoc. 2018;7: e010498.
Gargiulo P, Basile C, Cesaro A, et al. Efficacy, safety, adherence and persistence of PCSK9 inhibitors in clinical practice: A single country, multicenter, observational study (AT-TARGET-IT). Atherosclerosis. 2023;366:32–9.
Gargiulo P, Basile C, Galasso G, et al. Strike early-strike strong lipid-lowering strategy with PCSK9i in ACS patients. Real-world evidence from AT-TARGET-IT registry. Eur J Prev Cardiol. 2024:zwae170. Online ahead of print.
Michaeli DT, Michaeli JC, Boch T, Michaeli T. Cost-Effectiveness of Lipid-Lowering Therapies for Cardiovascular Prevention in Germany. Cardiovasc Drugs Ther. 2023;37:683–94.
Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nasir K. Updated Cost-effectiveness Assessments of PCSK9 Inhibitors From the Perspectives of the Health System and Private Payers: Insights Derived From the FOURIER Trial. JAMA Cardiol. 2017;2:1369–74.
Stam-Slob MC, van der Graaf Y, de Boer A, Greving JP, Visseren FLJ. Cost-effectiveness of PCSK9 inhibition in addition to standard lipid-lowering therapy in patients at high risk for vascular disease. Int J Cardiol. 2018;253:148–54.
Mostaza JM, Pintó X, Armario P, et al. SEA 2024 Standards for Global Control of Vascular Risk. Clin E Investig En Arterioscler Publicacion Of Soc Espanola Arterioscler. 2024;36:133–94.
Acknowledgements
Authors want to particularly acknowledge the patients for their collaboration
Funding
This study was supported by grants PI22/01595 and PI19/00694 from the Spanish Ministry of Economy and Competitiveness, CIBERCV, and Gobierno de Aragón B-14. These projects are co-funded by Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) of the European Union, “A Way to Make Europe.”
Author information
Authors and Affiliations
Contributions
Conceptualization, VMB and FC; Data Curation MVB, RSH, JLD, EJ, MST, XP, CM, NP, JPB and FC. Formal Analysis VMB and FC; Funding Acquisition, FC; Investigation, VMB, RSH and FC; Methodology, VMB and FC; Project Administration, FC; Resources, VMB and FC; Software VMB; Writing—Original Draft Preparation: VMB, RSH and FC; Review: all authors. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All subjects provided written informed consent for a protocol approved by the Ethical Committee of Clinical Research of Aragón, Spain. The study was conducted in accordance with the Declaration of Helsinki for the protection of the rights and welfare of people participating in biomedical research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marco-Benedí, V., Sánchez-Hernández, R.M., Díaz, J.L. et al. PCSK9 inhibitors on the management of primary and secondary cardiovascular prevention. Lipids Health Dis 23, 290 (2024). https://doi.org/10.1186/s12944-024-02283-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02283-x