Abstract
Ischemic stroke poses significant challenges in terms of mortality and disability rates globally. A key obstacle to the successful treatment of ischemic stroke lies in the limited efficacy of administering therapeutic agents. Leveraging the unique properties of nanoparticles for brain targeting and crossing the blood–brain barrier, researchers have engineered diverse nanoparticle-based drug delivery systems to improve the therapeutic outcomes of ischemic stroke. This review provides a concise overview of the pathophysiological mechanisms implicated in ischemic stroke, encompassing oxidative stress, glutamate excitotoxicity, neuroinflammation, and cell death, to elucidate potential targets for nanoparticle-based drug delivery systems. Furthermore, the review outlines the classification of nanoparticle-based drug delivery systems according to these distinct physiological processes. This categorization aids in identifying the attributes and commonalities of nanoparticles that target specific pathophysiological pathways in ischemic stroke, thereby facilitating the advancement of nanomedicine development. The review discusses the potential benefits and existing challenges associated with employing nanoparticles in the treatment of ischemic stroke, offering new perspectives on designing efficacious nanoparticles to enhance ischemic stroke treatment outcomes.
Graphical Abstract

Similar content being viewed by others
Introduction
Stroke represents a formidable challenge to global health, ranking as the world's second-leading cause of mortality and the primary cause of chronic disability. This condition poses a significant risk to patient safety and carries a substantial societal toll. With the global population aging, the incidence of stroke is on the rise, highlighting the pressing need for effective interventions and treatment strategies [1]. Among the various types of strokes, ischemic stroke (IS) is the most common, accounting for over 85% of cases. Characterized by acute and persistent cerebral ischemia and hypoxia, IS results in a detrimental disruption of brain blood circulation, leading to severe and often irreversible consequences [2].
Acute ischemic stroke (AIS) arises from the obstruction of blood flow in a cerebral artery due to a clot or embolus. This sudden blockage results in decreased perfusion to brain tissue, leading to reduced levels of oxygen, ATP, and glucose. Consequently, widespread neuronal death, brain tissue infarction, and subsequent neurological deficits ensue [3]. Although collateral circulation may offer partial compensation, it is often insufficient to salvage the entire ischemic injury area. The ischemic brain tissue can be categorized into two main regions: the ischemic core and the penumbra. The ischemic core, situated at the center of the injury, represents the most severely damaged area where nerve cells endure severe ischemia, leading to necrosis. Cells in the core zone have either perished or are nearing complete necrosis, rendering the damage irreversible. In contrast, the penumbra undergoes milder ischemia, where inadequate blood supply compromises normal neurophysiological function without prompting immediate cell death. Restoration of blood flow promptly allows cells in the penumbra to recover and regain normal function. Failure to restore blood flow promptly results in progressive degeneration and necrosis of neurons in the penumbra, ultimately contributing to the infarct core and worsening brain damage [4].
Recently, nanoparticle (NP)-based drug delivery systems have gained significant traction in biomedical applications as a result of their distinct physicochemical properties. These properties comprise their nano-scale dimensions, expansive surface area, and notable capacity for drug encapsulation, targeted delivery, and simultaneous administration of therapeutic agents. NPs also shield drugs from degradation, enable controlled release, extend circulation time, and diminish toxicity [5, 6]. Consequently, diverse NP-based drug delivery systems, such as liposomes, micelles, dendrimers, nanogels, inorganic NPs, and natural NPs, have been investigated for therapeutic and diagnostic purposes in cerebral stroke [7]. The versatility of nanomaterials allows for customized design to meet specific therapeutic goals or modifications with various ligands and responsive linkers for intelligent drug delivery. This aids in their traversal across the blood–brain barrier (BBB), enhances drug accumulation in ischemic regions, and modulates drug release based on the pathological features of IS [8]. Research indicates that NPs, either independently or in conjunction with neuroprotective drugs, can effectively address challenges like short half-life, low water solubility, poor bioavailability, limited BBB permeability, and potential kidney and liver toxicity, thereby significantly improving the management of AIS [9, 10]. This improvement encompasses promoting neuronal recovery, extending treatment windows, mitigating neuronal death in the ischemic penumbra, and enhancing patient prognosis. Moreover, NPs have substantially propelled the understanding of the pathogenesis of AIS and the creation of targeted pathophysiological treatments, ushering in new dimensions and innovative strategies for AIS management [11, 12]. Overall, NPs show great promise in the treatment of IS, offering enhanced therapeutic approaches and better patient outcomes.
In this review, firstly, to intuitively grasp the current research focal points and trends of NPs in IS, we searched the Web of Science Core Collection for articles and reviews on the application of NPs in IS published from January 1, 2003 to February 29, 2024. Qualitative and quantitative analysis was conducted using CiteSpace and GraphPad prism 8 software. The results showed that 610 articles were included, and in the past 20 years, the number of publications on the study of NPs in IS has significantly increased (Fig. 1A). The co-occurrence analysis of keywords showed the top ten most frequently occurring keywords. It is worth noting that, except for “ischemic stroke” and “nanoparticles”, “drug delivery”, “blood–brain barrier”, “cerebral ischemia” and “oxidative stress” are the most common keywords, indicating that they are currently the research hotspots of NPs in IS.
A Annual number of publications worldwide from January 1, 2003 to February 29, 2024. B The keyword co-occurrence analysis provides the top 10 keywords with co-occurrence frequency rankings. C Keyword co-occurrence analysis provides a centrality ranking of keywords. D Visualization of keyword co-occurrence analysis (The size of each node indicates the frequency of the word's occurrence in outputs, with larger circles representing higher frequencies. The thickness of the pink outer ring around each node signifies its centrality. The thickness of the connecting lines reflects the proximity of the relationship between two words)
(Fig. 1B). In addition, keyword visualization shows the highest centrality in the BBB and drug delivery (Fig. 1C). In short, considerable advancements have occurred in the past two decades within this research domain. The prominent focus in NP therapy for IS primarily revolves around enhancing drug delivery across the BBB. Secondly, the traditional treatment strategies for IS were reviewed, with a focus on its pathophysiological changes, as well as targeted therapy of NP-mediated drug delivery systems targeting specific pathological and physiological mechanisms of IS. Finally, we discussed the perspectives and prospects of NP-mediated delivery systems for the treatment of IS, providing insights into the future development of nanomaterial therapy for IS. In conclusion, we aspire for this comprehensive review to inspire researchers to purposefully design NP-based therapeutics based on the pathophysiological characteristics of IS, ultimately achieving effective treatment for this condition.
Traditional treatments for IS
Traditional treatments for IS encompass recanalization/reperfusion and neuroprotection, aiming to improve neurological outcomes [13,14,15]. Intravenous thrombolysis with rt-PA and mechanical thrombectomy are critical interventions for AIS, often used in combination to enhance efficacy and safety for selected patients (Fig. 2) [16, 17]. Despite these advances, most patients are unable to benefit due to limitations such as narrow treatment time windows and adverse reactions. Research continues to focus on neuroprotective agents to extend treatment windows and promote recovery. Neuroprotective agents developed to enhance neuron survival post-IS, show promise in preclinical studies (Table 1) but have not demonstrated significant clinical benefits, primarily due to poor BBB penetration and inadequate animal model representation [18, 19]. Pharmacotherapy for IS, including reperfusion and neuroprotective strategies, is vital for neuroprotection and brain function restoration but faces drawbacks such as poor solubility, short half-lives, toxicity, and low bioavailability. Monotherapies are inadequate for IS's complex pathophysiology, necessitating higher doses and combination therapies. Moreover, the BBB hinders effective drug delivery to ischemic regions, limiting treatment efficacy. Although transiently compromised during IS, the BBB's permeability is insufficient for optimal drug concentrations. NP-based drug delivery systems offer a promising solution to enhance drug delivery and therapeutic outcomes in IS treatment [20].
NP-based targeted pathophysiological therapy for IS
In recent times, there have been notable advancements within the biomaterials sector concerning the creation of therapeutic drug delivery systems aimed at treating IS [33]. Among the various biomaterials explored, those based on NPs have emerged as a particularly promising avenue. Nanoparticles (NPs), defined as ultrafine particles with dimensions ranging from 1 to 100 nm (nm), exhibit distinct physical and chemical properties due to their high surface area to volume ratio and come in various types including metallic NPs like gold and silver, metal oxide NPs such as iron oxide and titanium dioxide, carbon-based NPs like fullerenes and carbon nanotubes, polymeric NPs made from organic polymers, and ceramic NPs including silica and alumina. In medicine, these versatile particles are employed for targeted drug delivery systems that enhance therapeutic efficacy and minimize side effects by delivering drugs directly to diseased cells, improved imaging techniques such as enhanced MRI and CT scans that offer higher resolution and better contrast, and innovative therapeutic approaches like photothermal therapy for cancer where NPs generate heat to selectively destroy cancer cells, while also being used in biosensing for detecting biomarkers and in the creation of scaffolds for tissue engineering, all of which highlight their significant potential in advancing medical diagnostics and treatments [34,35,36]. The unique localization of NPs is that they are smaller than cells but larger than most small-molecule substances. This allows them to act as efficient drug carriers, capable of controlling the release of therapeutics, which can lead to enhanced treatment outcomes across a spectrum of diseases [37,38,39]. Moreover, NPs are capable of being taken up by various cell types through a process known as clathrin-mediated endocytosis [40]. Following internalization, NPs navigate the intracellular environment via the endolysosomal pathway. This journey culminates in the fusion of NP-loaded vesicles with the abluminal membrane of the BBB, facilitating the transport of NPs into the CNS [41]. Under the conditions of AIS, the paracellular permeability of the BBB is significantly increased due to the disruption of tight junctions (TJs) [42]. This disruption can further augment the delivery of NPs to the brain. Consequently, NP-based drug delivery systems hold the potential to address the current treatment challenges associated with IS.
Superlative NP-based drug delivery systems must harness and regulate the aberrant pathophysiological features of IS. Consequently, a thorough comprehension of the pathophysiological traits associated with IS is fundamental for the development of such systems. In IS, the blockage of a cerebral artery results in the disruption of blood flow, leading to insufficient delivery of oxygen and glucose to neurons and other brain cells. Consequently, this deprivation leads to disrupted ATP synthesis, energy deficiency, compromised ion balance, and acid–base disturbances [43, 44]. All of these functional impairments can lead to neuropathological changes in the brain, ultimately resulting in severe neurological damage and defects [45].
This section will comprehensively explore various pathophysiological changes related to IS, including glutamate excitotoxicity, neuroinflammation, BBB disruption, oxidative stress, mitochondrial dysfunction, and cell death (Fig. 3), as well as the application of NP-based drug delivery systems targeting the aforementioned pathophysiological treatments for IS.
Oxidative stress and mitochondrial dysfunction
The pathological mechanism of oxidative stress
Oxidative stress, stemming from disrupted redox reactions, generates harmful free radicals damaging cellular structures. In IS, ischemia/reperfusion exacerbates oxidative stress, compromising BBB integrity and triggering neuroinflammatory responses [46]. Uncontrolled ROS production leads to red blood cell membrane peroxidation, impairing gas exchange and red blood cell function [47]. This contributes to microthrombosis, exacerbating cerebral ischemic damage. Oxidative stress, primarily from heightened ROS production via mitochondrial oxidative phosphorylation, intertwines with various pathological mechanisms in IS [48]. Mitochondria, double-membraned organelles found in all eukaryotic cells except mammalian red blood cells, play a crucial role in ATP production via oxidative phosphorylation. The outer membrane, with its channel structures, allows molecules under 10 kDa to pass, while the inner membrane hosts the electron transport chain (ETC) and ATP synthase complexes, facilitating oxygen, carbon dioxide, and water movement. In IS, mitochondria are highly susceptible to damage from ROS bursts, calcium overload, and excitotoxicity, leading to structural and functional abnormalities. These include deformation of the inner mitochondrial membrane (IMM), increased ROS production, and calcium overload. Mitochondrial responses to these stressors include mitochondrial enlargement, increased cytoplasmic density, and disruption of the mitochondrial membrane potential (ΔΨm), which activates the mitochondrial permeability transition pore (MPTP) [49, 50]. This leads to a cycle of heightened ROS production, reduced ATP synthesis, PINK1 accumulation, and activation of the unfolded protein response (UPR), culminating in mitophagy [51]. Mitophagy is further stimulated by autophagosome formation from mitochondrial DNA damage due to excess ROS. The opening of the MPTP releases cytochrome C and ROS, activating caspases and leading to cellular apoptosis and necrosis (Fig. 4) [52].
In essence, the relationship between oxidative stress and mitochondria is intricately intertwined in the context of IS. Oxidative stress significantly impacts mitochondrial function, potentially causing mitochondrial dysfunction, which in turn can compromise cell viability and operation. Conversely, mitochondrial dysfunction can also contribute to oxidative stress, given that the oxidative phosphorylation process within mitochondria stands as a primary generator of free radicals. Consequently, a detrimental cycle forms between oxidative stress and mitochondria, amplifying cellular harm and mortality while worsening the condition of IS [53].
NP-based therapeutics in antioxidant therapy for IS
The use of antioxidant therapy to neutralize excess ROS has been shown to mitigate oxidative stress damage and decrease subsequent apoptotic and inflammatory reactions. As a result, this form of therapy has emerged as a principal approach for addressing injuries sustained from ischemia and reperfusion [54]. In preclinical studies, antioxidants typically encompass small molecular compounds, antioxidant enzymes, and various organic/inorganic substances. NPs have the potential to enhance the pharmacokinetic properties of antioxidants, facilitating their passage across the BBB and enabling them to concentrate in the affected areas of the brain [55].
Small-molecule antioxidants are frequently utilized for the antioxidant therapy of IS. The utilization of the NP-based drug delivery system is chiefly intended to improve the bioavailability of the therapeutic agents. Ashafaq and colleagues developed a nanostructured lipid carrier (NLC) encapsulating resveratrol (NR) and tested its neuroprotective effects in a rat model of MCAO [56]. Polysorbate-80 coating on NPs enhanced brain targeting and BBB penetration. NR reduced oxidative stress by increasing glutathione (GSH) levels and protecting antioxidant enzyme activity. The efficacy of 250 μg of encapsulated resveratrol was comparable to 20 mg of free resveratrol, showing NR to be 40–160 times more effective than free resveratrol in previous studies, significantly improving its bioavailability and therapeutic potential. Further investigations into the therapeutic efficacy and post-treatment protocols are needed to confirm whether NR treatment could be a promising candidate for a stroke. EDV, the sole neuroprotective drug approved for clinical use, neutralizes ROS to protect ischemic brain tissue. Jin et al. developed a micelle NP loaded with EDV-AM to enhance BBB crossing and neural protection during IS [57]. The EDV-AM significantly increased the permeability of the endothelial monolayer in vitro, leading to EDV concentrations that were 2.0 and 7.7 times higher than those of EDV-PM and unencapsulated EDV, respectively. HPLC studies revealed that EDV-AM delivered more EDV to brain ischemia than free EDV following intravenous injection. Furthermore, magnetic resonance imaging showed that EDV-AM more rapidly salvaged ischemic tissue compared to free EDV. Diffusion tensor imaging demonstrated that EDV-AM was most effective in accelerating axonal remodeling in the ipsilesional white matter and improving functional behaviors in IS models. This agonistic micelle shows promise in enhancing the therapeutic efficacy for IS patients who miss the window for thrombolytic treatment.
Small-molecule antioxidants frequently necessitate targeting specific pathways or signaling targets, potentially resulting in drug resistance. Consequently, addressing how NP-based drug delivery systems can overcome the resistance that may develop from prolonged small-molecule antioxidant use stands as a crucial challenge to be tackled in the future. Furthermore, in addition to a single small-molecule antioxidant, multiple small-molecule antioxidants could be co-delivered by NPs to synergistically treat IS. The co-delivery of multiple drugs by NPs can not only improve the therapeutic effect, but also reduce side effects, and provide new possibilities for the treatment of IS through precision treatment and the integration of diagnosis and treatment.
Macromolecular antioxidants, such as melanin (Me) and superoxide dismutase (SOD), are known for their excellent biocompatibility and potent antioxidant properties. Despite these benefits, their clinical application in treating strokes is significantly hindered by their brief biological half-lives and the low permeability of the BBB. To overcome these challenges, a novel approach involving NP-based delivery systems that harness these macromolecular antioxidants has been devised for the antioxidant treatment of IS. Reddy and colleagues developed SOD-encapsulated biodegradable NPs (SOD-NPs) using PLGA and tested their efficacy in a rat model of transient focal cerebral I/R [58]. SOD-NPs preserved the integrity of the BBB, preventing edema, reducing the levels of ROS produced after reperfusion, and protecting neurons from apoptosis. Animals treated with SOD-NPs showed significantly higher survival rates compared to those treated with saline control (75% vs. 0% at 28 days) and eventually regained most critical neurological functions. SOD-NPs could be an effective treatment option when used alongside a thrombolytic agent for stroke patients. In addition, the biodegradability of PLGA may bring greater advantages to this system. Besides SOD, other antioxidant enzymes like catalase and glutathione peroxidase can be employed in the treatment of IS through NP-based delivery. However, these proteins are prone to degradation, thus requiring careful consideration when employing NPs for their encapsulation.
In contrast, melanin, a polyphenolic polymer, exhibits greater resistance to degradation and enhanced load operability. Liu et al. developed PEG-coupled melanin NPs (PEG-MeNPs) and evaluated their antioxidative potential in IS [59]. PEG-MeNPs effectively countered various reactive oxygen and nitrogen species (RONS) and demonstrated neuroprotective and anti-inflammatory effects in vitro. In vivo results further confirm that the distinctive multi-antioxidative, anti-inflammatory, and biocompatible properties of MeNPs effectively protect ischemic brains while causing negligible side effects. This innovative approach combines melanin's natural antioxidant properties with sustained NP release, enhancing IS treatment.
In addition to serving as carriers, NPs can synergize with antioxidants for enhanced efficacy. Varlamova et al. developed Se-TAX, a nanocomposite of selenium NPs (SeNPs) and taxifolin (TAX), to improve neuroprotection in ischemic/reoxygenated cortical cells [60]. Se-TAX, TAX, and SeNPs all reduced ROS in neurons and astrocytes under oxidative stress, with Se-TAX showing superior performance and minimal pro-oxidant activity. Se-TAX activated antioxidant enzymes and inhibited ROS-generating systems during OGD/reoxygenation, outperforming its components. Unlike SeNPs alone, which partially inhibited Ca2+ increase, Se-TAX mitigated both calcium surges and hyper-excitation. TAX was effective only against hyper-excitation. Se-TAX also significantly reduced necrosis and apoptosis post-OGD/reoxygenation, ranking in effectiveness as Se-TAX > SeNPs > TAX. Combining SeNPs with taxifolin creates a promising neuroprotective strategy for brain ischemia with low toxicity. However, further research is needed to explore the neuroprotective effects of this stroke model in vivo.
In biomedicine, inorganic NPs are popular due to their large surface area, customizable architecture, versatile surface chemistry, and unique optical and physical properties. Many have been engineered for antioxidant treatments targeting IS. Tian et al. created selenium-containing metal–organic framework-coated dual-iron-atom nanoenzymes (Fe2NC@Se NPs) with multi-enzymatic cascade activities to mimic natural antioxidants [61]. These NPs showed superior SOD, catalase, and oxidase activities compared to single-atom iron counterparts, with the Se-MOF layer enhancing stability and biocompatibility. Density functional theory suggests a synergistic effect boosts Fe2NC activity. In vitro and in vivo studies demonstrated that Fe2NC@Se NPs effectively reduce oxidative damage and neuronal apoptosis post-cerebral I/R injury by scavenging ROS and inhibiting the ASK1/JNK apoptotic pathway. These NPs significantly reduced cerebral infarction and edema, suggesting their potential for novel antioxidant therapies to improve IS treatment.
Inorganic NPs can catalyze the decomposition of ROS directly and can also release specific elements or ions that regulate molecules or pathways associated with ROS. Huang et al. developed a bioinspired manganese oxide nanoenzyme (HSA-Mn3O4) to mitigate reperfusion damage post-IS [62]. This nanosystem demonstrates reduced inflammation, extended circulation time, and robust ROS scavenging capabilities. HSA-Mn3O4 effectively prevents cell apoptosis and endoplasmic reticulum (ER) stress induced by oxygen and glucose deprivation, showcasing its neuroprotective effects against IS and reperfusion injury in brain tissue. Additionally, it facilitates the release of Mn ions, enhancing SOD2 activity. Thus, HSA-Mn3O4 mitigates brain tissue damage by mitigating cell apoptosis and ER stress in vivo. Overall, this research not only informs the design of biomimetic and translational nanomedicine but also elucidates the mechanisms underlying its neuroprotective effects against IS and reperfusion injury.
In addition to inorganic NPs, some organic NPs can also directly promote the decomposition of ROS. Hosoo et al. developed nitroxide radical-containing NPs (RNPs) embedded with TEMPOL to neutralize ROS and provide neuroprotection [63]. Their study showed RNPs significantly reduced superoxide anions in both the peri-infarct zone and ischemic core, confirmed by decreased oxidative DNA damage via 8-OHdG staining. Compared to PBS-treated controls, arterial RNP administration significantly reduced BBB permeability, improved neurological function, and decreased infarct volume. Additionally, RNPs reduced neuronal apoptosis near the infarct. This study demonstrates that RNPs, as high-performance antioxidants, can effectively exert their effects while prolonging the half-life and circulation time of TEMPOL in vivo, reducing its toxicity. These findings highlight the potential of RNPs as a therapeutic intervention for cerebral I/R injuries, with intra-arterial RNP injection emerging as a novel approach to protect the neurovascular unit by reducing infarct size, BBB damage, and scavenging multiple ROS.
It should be noted that organic/inorganic NP-based nanoenzymes have been widely studied, but a major obstacle for these nanoantioxidants is their high specificity in antioxidant activity against ROS (mainly H2O2). Considering the multiple ROS produced in diseases, it may not be able to fully prevent oxidative damage. By comparison, the advantages of endogenous antioxidant-based NPs are as follows: (i) Strong clearance of multiple primary and secondary ROS and reactive nitrogen species (RONS); (ii) Highly stable antioxidant activity against oxidative damage; (iii) Good biocompatibility. Therefore, it is necessary to consider the pathophysiological characteristics of IS and the physicochemical properties of NPs in selecting the system for IS treatment.
The above research indicates that NP-based treatment methods can help various antioxidant drugs cross the blood–brain barrier, improve the efficacy and bioavailability of antioxidant drugs in vitro and in vivo treatment of IS, and help explore some antioxidant mechanisms, contributing new content to the antioxidant treatment of IS. However, the cascade reaction of ischemic pathology is complex and the targeting efficiency of drugs on ischemic penumbra needs to be further improved. To further improve the brain penetration and therapeutic effect of ischemic injury, NP-based drugs with precise targeted pharmacological effects have been designed and developed recently.
Smart-responsive NPs are already being used to deliver antioxidants for IS treatment. Wu et al. developed PEG-terminated poly (2,2'-thiodiethylene 3,3'-thiodipropionate) (PEG-PTT) polymers with ROS-sensitive thioether motifs and thrombin-sensitive peptides, creating NPs that shrink in response to thrombin in ischemic environments [64]. To enhance brain penetration, AMD3100, which binds to the upregulated CXCR4 receptor in ischemic areas, was grafted onto these NPs (ASPTT NPs). In MCAO-induced stroke models in mice, ASPTT NPs selectively accumulated in the ischemic brain and exerted strong antioxidant effects. This work shows ASPTT NPs are capable of efficient encapsulation and delivery of glyburide to achieve anti-edema and antioxidant combination therapy, resulting in therapeutic benefits significantly greater than those by either the NPs or glyburide alone. ASPTT NPs can encapsulate and deliver glibenclamide, an antiedematous agent that has shown promise in human patients. Despite its limited brain penetration, encapsulating glibenclamide within the Np provides additional therapeutic benefits. ASPTT NPs demonstrated exceptional brain penetration and therapeutic benefits, making them a promising platform for targeted stroke therapies and potential clinical translation for effective stroke management.
The current trend in the development of NP-based drug delivery systems is transitioning gradually from single to multiple targeting, a shift with significant implications for precision targeted therapy. Dong et al. developed a biomimetic drug delivery platform, stroke-homing peptides (SHp)-NM@Edv/RCD (SNM-NPs), for advanced, multi-phased targeting [65]. Firstly, by NM encapsulation, SNM-NPs can successfully penetrate the BBB and target inflammatory sites. Secondly, SHp modification allows SNM-NPs to specifically target damaged neurons at CIRI sites. In addition, SNM-NPs significantly reduced the drug concentration in vivo by stepwise targeting and inhibited neuroinflammation by scavenging excessive ROS. Furthermore, the scavenging of ROS upregulated Bcl2 expression, inhibited Bax function, and further inhibited Caspase 3 activation, thereby suppressing neuronal apoptosis and neuronal microtubule repair. Preliminary experiments also showed that SNM-NPs exhibited a good safety profile both in intravenous therapy and in vitro cell experiments; therefore, they can be further developed as effective and safe agents for targeted therapy of CIRI. In addition, stepwise targeting and precision drug delivery strategy can be used for the precision treatment of other diseases related to local oxidative stress and inflammation in the brain.
Hypoxia occurring in AIS before thrombolysis or the subsequent increase in oxygen levels post-thrombolysis can lead to elevated levels of free radicals, causing successive damage to neurocytes. Shi et al. introduced engineered nano-erythrocytes for AIS and I/R injury treatment by encapsulating Mn3O4 within natural erythrocytes and attaching T7 peptides for BBB penetration, forming Mn3O4@nanoerythrocyte-T7 (MNET) (Fig. 5A) [66]. MNET exhibited efficient localization within infarcted areas, leveraging the stealth effect of erythrocytes and the BBB-penetrating ability of T7 peptides (Fig. 5B). MNET significantly inhibited phagocytosis and enhanced BBB traversal, effectively eliminating free radicals and mitigating cellular hypoxia in vitro (Fig. 5C). In vivo, MNET provided therapeutic benefits by salvaging neurocytes before thrombolysis through rapid free radical clearance and oxygen delivery, and post-thrombolysis by curbing oxygen influx and neutralizing free radicals to prevent reperfusion injuries (Fig. 5D, E). MNET's dual ability to scavenge free radicals and absorb oxygen offers a promising stepwise approach for IS management. It is crucial to highlight that during the treatment of IS, the primary objective is to promptly restore flow in occluded blood vessels. Nevertheless, the oxidative stress induced by surplus oxygen post-reperfusion must not be overlooked. Consequently, strategically managing oxygen levels pre- and post-reperfusion of blood vessels could potentially be a pivotal approach in utilizing NPs for the treatment of IS. Currently, normobaric hyperoxia (NBO) is the primary treatment for reducing penumbral tissue hypoxia. However, as previously reported, NBO can cause side effects due to the risk of systemic exposure to high-dose oxygen. The oxygen sponge effect of MNET has effectively regulated oxygen levels before and after thrombolysis in ischemic stroke, showing promising clinical application potential. The elegant design, straightforward preparation process, and encouraging results of the nanosystem proposed in this study offer an effective therapeutic strategy with broad potential for clinical application in IS.
Bioinspired nanosponge for salvaging IS via free radical scavenging and self-adapted oxygen regulating. A Schematic diagram of MNET salvaging in an acute IS via combining free radical scavenging and natural oxygen sponge effect. B Blood stability and BBB-crossing ability of MNET. C Protective effect of MNET via ROS scavenging and hypoxia relief in vitro. D The therapeutic effect of MNET in vivo for rescuing IS before thrombolysis. E The therapeutic effect of MNET in vivo for rescuing IS after thrombolysis.
In addition to various peptide-mediated and receptor-mediated NPs targeting the ischemic site of the brain, NPs with organelle targeting (especially mitochondrial targeting) function are designed to more accurately regulate and treat the pathological and physiological processes of IS. Liao et al. designed and synthesized a nanoplatform based on ceria nanoenzymes TPP@(CeO2 + ROF) that exerts mitochondria-targeting and antioxidant activities [67]. This system consists of triphenylphosphine-modified ceria and is loaded with the PDE4 inhibitor roflumilast. This innovative design offers multiple advantages, including enhanced biocompatibility, mitochondrial targeting, excess ROS scavenging, mitochondrial function restoration, anti-inflammatory activity, antioxidant synergism, and microglial cell phenotype and cytokine secretion modulation. Their results suggest that TPP@(CeO2 + ROF) effectively mediates mitochondrial damage, attenuates oxidative damage and apoptosis, reduces cerebral infarct volume and BBB injury, and has a favorable biosafety profile. Transcriptome analysis further elucidated the neuroprotective mechanism of TPP@(CeO2 + ROF). We believe that this nanoplatform is potentially a promising strategy for the treatment of IS. Zhang et al. developed multifunctional nanocarriers (SPNPs) for I/R injury treatment, targeting mitochondria, responding to ROS, and providing antioxidative effects (Fig. 6A) [68]. The thioketal cross-linked SPNPs reacted to ROS, neutralizing them and releasing the therapeutic compound PU. The SS-31 peptide enabled mitochondrial targeting. Integrated into a thermosensitive hydrogel for intranasal administration, SPNPs protected SH-SY5Y cells from oxidative damage and effectively transported them to ischemic regions in MCAO rats (Fig. 6B, C). The ROS-triggered release of PU restored mitochondrial function, sustained ATP production, preserved membrane integrity, and inhibited apoptosis. In vivo, SPNPs improved neurological scores, reduced infarct volumes, and mitigated brain swelling (Fig. 6D). By utilizing intranasal administration, SPNPs encapsulated in the thermosensitive gel can bypass the BBB and directly target the mitochondria of ischemic penumbra neurons, thereby enhancing delivery efficiency. This groundbreaking method provides substantial therapeutic advantages for treating ischemic damage and other CNS disorders.

© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V
Mitochondrial-targeted and ROS-responsive nanocarrier via nose-to-brain pathway for IS treatment. A Schematic illustration of targeted treatment of IS by ROS-responsive NPs loaded with PU and decorated with SS31. B Neuroprotection on SH-SY5Y cells simulated oxidative stress environment after acute IS. C Characterization of thermo-sensitive gels containing different therapeutic agents and ex vivo biodistribution of therapeutic NPs. D In vivo anti-IS efficacy.
In recent years, advancements in materials science and biology have facilitated the transition of NP-mediated oxidative stress regulation in IS from a macroscopic to a microscopic scale. The targeting of NPs in IS has progressed from ischemic site targeting to precise cell and organelle targeting. Future NP designs must exhibit precise molecular targeting capabilities related to oxidative stress. Moreover, the mechanisms through which NPs regulate oxidative stress demand further elucidation through genomics, proteomics, metabolomics, and other analytical approaches.
The current approach to treating IS with NPs primarily revolves around enhancing the permeability of the BBB and increasing the bioavailability of established antioxidant medications. Additionally, there is a focus on developing novel NP-based drug delivery systems with superior antioxidant capabilities. Particularly noteworthy are the single and multi-targeted NPs that specifically target the ischemic region and mitochondria, thereby augmenting both the permeability of the BBB and the precise therapeutic impact of the medication. Moreover, the combined benefits of multifunctional NPs, acting as antioxidants while also releasing oxygen and reducing edema, have resulted in more favorable treatment outcomes for IS. Future NP designs must demonstrate precise molecular targeting capabilities associated with oxidative stress, rather than only targeting free radicals.
Glutamate excitotoxicity
The pathological mechanism of glutamate excitotoxicity
Glutamate, a key excitatory neurotransmitter in the CNS, is crucial for synaptic transmission, learning, memory, movement, cognition, and development [69]. Approximately 50% of glutamate is involved in synaptic transmission regulation. Since it cannot cross the BBB, glutamate is synthesized in the CNS from glucose, which undergoes glycolysis and the TCA cycle to form α-ketoglutarate (α-KG), the precursor for glutamate [70]. This synthesis is catalyzed by glutamate dehydrogenase (GDH). Another synthesis route involves the glutamate-glutamine cycle: glutamate is absorbed into astrocytes and converted to glutamine-by-glutamine synthetase (GS). Glutamine is then transported to neurons via transporters like the SLC38 family (SNAT) [71]. In neurons, glutaminase (GLS) converts glutamine back to glutamate and ammonium ions [72]. Additionally, glutamate can be converted to α-KG through GDH or aspartate aminotransferase (AAT) [73]. This intricate exchange network, known as the "glutamate-glutamine cycle," is essential for maintaining glutamate balance and provides neuroprotection for post-cerebral ischemia. During cerebral ischemia and hypoxia resulting from IS, diminished ATP synthesis in neurons results in energy deficiency, causing the malfunction of key proteins such as Na+/K+-ATPase (NKA), Na+/Ca2+ exchanger (NCX), and Ca2+-ATPase across both plasma and intracellular organelle membranes. The disrupted ionic balance leads to heightened glutamate release and impaired glutamate reuptake, leading to aberrant glutamate processing. Additionally, it induces intracellular calcium overload, triggering excessive glutamate secretion and activation of calcium-dependent enzymes. Elevated levels of glutamate in the synaptic cleft overstimulate the N-methyl-D-aspartate receptor (NMDAR) on the postsynaptic membrane, initiating cellular demise. Moreover, NMDAR hyperactivation exacerbates intracellular Ca2+ accumulation, exacerbating the progression of cell death. The sequence of excitotoxic events in IS predominantly entails the death of postsynaptic cells mediated through the glutamate-NMDAR pathway (Fig. 7).
In summary, prolonged exposure to elevated extracellular glutamate levels during IS causes significant Ca2+ influx into neurons and glial cells, triggering neurotoxic events. This excess Ca2+ activates proteases, phosphatases, endonucleases, and lipases, leading to the overproduction of free radicals, mitochondrial damage, membrane disruption, and DNA fragmentation, ultimately resulting in apoptotic or necrotic cell death.
NP-based therapeutics in excitotoxicity therapy for IS
Excitotoxicity mediated by glutamate is a key mechanism in IS, triggering early stroke damage. Regulating this process can improve intervention outcomes. Glutamate receptor antagonists and calcium channel blockers reduce excitotoxicity, offering neuroprotection for IS treatment [74]. However, current therapies face challenges like limited duration, poor targeting, and inadequate drug release control. To address these issues, NPs based on excitotoxicity inhibitors have been developed to enhance IS treatment efficacy.
Enhancing the solubility and BBB permeability of glutamate receptor antagonists using NP-based delivery systems can improve IS treatment efficacy. PSD-95 activates neuronal nitric oxide synthase (nNOS) via the NMDA receptor, forming the NMDAR/PSD-95/nNOS complex. Disrupting this interaction can reduce excitotoxic damage. Drugs like ZL006 and NR2B9C selectively block the nNOS-PSD-95 interaction but face challenges in penetrating the ischemic brain effectively. Zhao et al. developed T7 and SHp dual-conjugated PEGylated liposomes (T7&SHp-P-LPs) for targeted delivery of ZL006 to treat IS [75]. In vivo imaging showed efficient BBB crossing and accumulation in the ischemic area of MCAO rats. The treatment reduced infarct size, improved neurological function, and alleviated histopathological damage. Cellular studies confirmed enhanced BBB crossing and cellular uptake of T7&SHp-P-LPs/ZL006, reducing glutamate-induced cell apoptosis. The research findings indicate that dual-targeted liposomes, modified with T7 and shp, can transport more drugs to the brain and selectively deliver them to ischemic tissue. This approach increases the local drug concentration while minimizing side effects. To further improve the therapeutic effect of drugs, Lv et al. developed SHp-modified RBC membrane-coated ROS-responsive NPs (SHp-RBC-NP/NR2B9C) for delivering the neuroprotective drug NR2B9C to treat ischemic brain injury (Fig. 8A) [76]. SHp-RBC-NPs enhanced NR2B9C transport across the BBB, targeting injured neurons in the ischemic region. SHp-RBC-NP/NR2B9C improved PC-12 cell performance under glutamate-induced stress, suggesting a protective role (Fig. 8B). Pharmacokinetic studies showed extended circulation time (beyond 48 h) for both RBC-NP/NR2B9C and SHp-RBC-NP/NR2B9C groups. The SHp-RBC-NP group demonstrated higher fluorescence intensity in ischemic brain regions, indicating precise targeting (Fig. 8C). SHp-RBC-NP/NR2B9C significantly improved neurological impairments and reduced cerebral infarct size in I/R models (Fig. 8D). This system prolongs the circulation time of NR2B9C in the body and enhances its targeting ability and greater neuroprotective effect. Therefore, it suggested that SHp-RBC-NPs may be utilized as a potential formulation strategy to enhance the treatment of IS in the clinic.
Bioengineered boronic ester-modified dextran polymer NPs as ROS responsive nanocarrier for IS treatment. A Schematic design of the SHp-RBC-NP/NR2B9C. B In vitro cell-based studies. C In vivo pharmacokinetics and fluorescent image of rhodamine-labeled free NR2B9C, NP, RBC-NP, and SHp-RBC-NP in the ischemic brain sections. D The neuroscore and infarct size were evaluated 24 h after the I/R and in vivo safety evaluation.
Ca2+ channel blockers reduce excitatory amino acid release, prevent excessive intracellular Ca2+ accumulation, and promote cerebral blood flow through vasodilation [77]. However, their use is limited by low bioavailability and gastrointestinal side effects. Advances in neuroprotective treatments for IS have been made with NP-based Ca2+ antagonists. Nimodipine, an L-type voltage-gated calcium channel inhibitor, reduces excessive Ca2+ in nerve cells but causes gastrointestinal side effects when taken orally. Orsolya et al. addressed this by using pH-responsive chitosan NPs to deliver nimodipine, mitigating nerve damage from cerebral ischemia [78]. Their study showed that nimodipine remained encapsulated at neutral pH and was released during ischemia-induced acidosis, increasing cerebral blood flow and blocking Ca2+ influx. The chitosan NPs prevented depolarization without causing neuro-immune reactions, effectively reducing excitotoxicity. In summary, the data from this study avoided gastrointestinal side effects and accurately delivered nimodipine to the ischemic site. This method can pave the way for the development of an intelligent drug delivery system that can selectively target the ischemic penumbra in IS.
Glyburide acts by obstructing the influx of calcium ions, a mechanism that involves the inhibition of the Trpm4 channel, which is a non-selective cation channel regulated by the sulfonylurea receptor 1 (Sur1-Trpm4 or Sur1-NCCa-ATP). To enhance delivery precision, Ma et al. encapsulated glyburide in PLGA NPs coated with neuronal stem cell membranes, creating Gly-CMNPs [79]. These engineered NSC membranes, overexpressing CXCR4, improved the homing ability of PLGA NPs to ischemic regions via CXCR4 and SDF-1 chemotaxis. In vivo trials with Gly-CMNPs showed improved survival rates, reduced infarct volumes, and better neurological scores in MCAO mice compared to free glyburide. This novel formulation could significantly enhance IS treatment options. The study suggests a new approach to improving drug delivery to the ischemic brain and establishes a novel formulation of glyburide that can be potentially translated into clinical applications to improve the management of human patients with stroke.
In summary, numerous studies have convincingly demonstrated the significant promise of NPs carrying excitotoxicity inhibitors for the treatment of IS. Despite this potential, the development of such targeted NPs remains relatively uncommon. One critical aspect to consider is the timing of administration, as the efficacy of neurotoxicity-blocking agents is closely tied to their delivery within a specific window following the onset of a stroke event. Delaying this administration may reduce the protective effects, thereby restricting both the duration and method of action of NPs. Moreover, the pathophysiological mechanisms underlying excitotoxicity are multifaceted and intricate. As such, NPs must possess a versatile design to effectively inhibit this complex process. Hence, from our perspective, the advancement of NPs engineered with excitotoxicity inhibition capabilities signifies a highly promising avenue for the future treatment of IS.
The BBB breakdown and neuroinflammation
The pathological mechanism of neuroinflammation
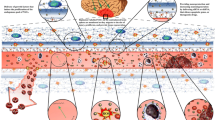
The CNS features protective barriers, notably the BBB, which consists of endothelial cells (ECs) with TJs, pericytes, astrocytic endfeet, and ECM components [80]. ECs form vessel linings, pericytes are within the basement membrane, and astrocytic processes envelop capillaries. While ECs and TJs primarily block permeability, pericytes and astrocytes regulate it. The BBB is crucial for maintaining CNS homeostasis by controlling fluid, solute, and cell movement at the blood–brain interface. In IS, the BBB undergoes significant damage due to glucose and oxygen deprivation followed by reperfusion injury, leading to local energy disturbances and oxidative stress. This causes deterioration of vascular endothelial cells and disruption of TJs, increasing BBB permeability [81]. The BBB breakdown facilitates continuous inflammatory pathway activation by inflammatory factors from infiltrating peripheral cells and CNS-activated cells [82]. Early IS-induced excitotoxicity and oxidative stress trigger the release of cytokines, MMPs, and GFAP from microglia and astrocytes, prompting endothelial cells to elevate cell adhesion molecules like selectins, VCAM, and ICAM. This promotes white blood cell attachment and immune cell infiltration [81]. Dying neurons release DAMPs, further activating microglia and peripheral immune cells, which produce additional pro-inflammatory factors, exacerbating BBB damage [83]. This creates a vicious cycle where initial inflammation disrupts the BBB, enabling peripheral white blood cells to migrate to the injured brain, releasing pro-inflammatory cytokines, ROS, and MMPs, which further damage the BBB and perpetuate inflammation (Fig. 9) [84].
In brief, dysfunction of the BBB, characterized by the structural breakdown of TJs and heightened permeability, stands out as a key pathological feature in IS. The compromise of the BBB is not solely a consequence of injury, rather, it actively contributes to the damage and often correlates with a bleak prognosis [85]. In the context of IS, various blood-borne elements such as cells, chemicals, and fluids breach the BBB and enter the brain tissue due to increased paracellular and transcellular permeability, as well as substantial damage to the endothelium [86]. This breach disrupts the balance of water and ions in the brain, leading to cerebral edema. Furthermore, the migration of infiltrating leukocytes intensifies inflammatory responses, worsening brain injury [87]. Although numerous consequences of BBB dysfunction are negative, one potential benefit is that it could enhance the targeted transportation of therapeutic substances to specific locations within the brain.
NP-based therapeutics in anti-inflammatory therapy for IS
Neuroinflammation significantly contributes to neuronal cell death in IS, with initial neuron death triggering an immune and inflammatory response involving microglia/macrophages and pro-inflammatory cytokines [88]. Anti-inflammatory drugs like fingolimod, glycyrrhizic acid, and ligustrazine show promise in preclinical IS studies by modulating microglial polarization and reducing inflammatory mediators and cell infiltration. However, their clinical use is limited by poor bioavailability, high lipophilicity, low solubility, and rapid clearance. To address these issues, various nano-formulations have been developed to enhance the therapeutic properties and bioavailability of these agents.
Polymers such as PEG are often used to construct nanomedicine carriers. Wang et al. developed mPEG-b-PLA copolymer NPs (NPcurcumin) to enhance curcumin delivery for IS treatment, showing improved therapeutic effects, BBB protection, and reduced neuroinflammation by modulating microglial activity, aiding neurological recovery in mice post-I/R injury [89]. NPcurcumin effectively addresses the challenges posed by curcumin's poor water solubility and unstable chemical properties. It enhances curcumin's stability, prolongs its circulation time in vivo, and amplifies its therapeutic efficacy. Similarly, Thomas et al. demonstrated that atorvastatin in PEG-modified liposomes (LipoStatin) reduced brain damage and improved neurological recovery in rats with cerebral I/R injury [90]. It efficiently accumulated at the ischemic injury site, significantly reducing infarct volume and improving neurological function recovery compared to the control group. Additionally, LipoStatin markedly improved brain metabolism and demonstrated significant anti-inflammatory effects. Protein analysis indicated considerable recovery of blood–brain barrier integrity and endothelial function. PEGylated LipoStatin can be more effectively delivered to the ischemic brain and have significant neuroprotective effects. Thus, PEGylated LipoStatin can be further developed as a promising targeted therapy for IS and other major vascular diseases.
Furthermore, polymer NPs can facilitate the co-delivery of multiple drugs. Zhu et al. developed an amphiphilic polymer with a Ce4+ ligand, forming NTA/Ce4+/C-176 NPs to co-deliver drugs for IS treatment (Fig. 10A) [91]. These NPs counteract neuroinflammation by inhibiting dsDNA accumulation and STING protein overexpression (Fig. 10B). In vivo studies showed that intraventricular injection of these NPs at the stroke site effectively reduced neuroinflammation, promoted neurogenesis, and improved motor function in a MCAO model (Fig. 10C). This approach resulted in a smaller ischemic penumbra and better recovery than standard NP treatments. This strategy offers a promising new avenue for modulating CNS inflammatory pathways and improving stroke prognosis, despite potential tissue damage risks from the intraventricular injection.
C-176-loaded Ce4+ DNase NPs synergistically inhibit the cGAS-STING pathway for IS treatment. A The therapeutic mechanism of NTA/Ce4+/C-176 NPs. B NTA/Ce4+/C-176 decreased neuroinflammation and increased neurogenesis in vivo. C Effect of NTA/Ce4+/C-176 on brain infarct volume and functional motor recovery after stroke.
Besides small-molecule drugs, macromolecular drugs including protein drugs and nucleic acid drugs can be delivered by polymer NPs. Jin et al. enhanced IVIg delivery to ischemic regions by creating MPC-n (IVIg) nanocapsules via in situ polymerization with MPC monomer and EGDMA [92]. These nanocapsules crossed the BBB through high-affinity choline transporter 1 (ChT1) in ischemic endothelial cells, targeting IVIg accumulation. Early MPC-n (IVIg) administration reduced stroke-induced inflammation by suppressing neural C3 complement activation, monocyte/macrophage infiltration, and glial cell activation, while promoting a protective microglial phenotype. This treatment decreased neurological deficits, infarct volume, and mortality rates, effectively reducing inflammation-induced brain damage and improving outcomes. In summary, MPC-n (IVIg) can effectively and selectively deliver therapeutic IVIg to the ischemic region following a stroke. Even at low doses, it can penetrate the BBB and accumulate in the ischemic area, enhancing its clinical feasibility. Phosphatidylcholine (PC), a key component of cell membranes, is commonly utilized to modify NPs to evade the reticuloendothelial system and prolong systemic circulation time. Our study has further revealed that PC can actively cross the BBB via receptor-mediated transport, indicating its promise as a feasible candidate for the development of nanomedicines for IS treatment [93].
Nucleic acid-based drugs are gaining prominence in drug development due to their precise targeting, broad therapeutic applications, sustained effects, potential for personalized medicine, low drug resistance, minimal off-target effects, and the ability to modify diseases. Choi et al. developed PLGA NPs encapsulating PINK1 siRNA (PINK1 NPs) to target PINK1 in mitochondrial autophagy within microglia, offering neuroprotection in a photothrombotic-induced IS mouse model [94]. PINK1 NPs promoted anti-inflammatory microglia states, reduced microglial migration, and increased phagocytic activity, preventing delayed neuronal loss. Pre-stroke administration of PINK1 NPs decreased mitochondrial autophagy factors, reduced infarct volume, and improved motor deficits. In summary, to effectively apply gene therapy in clinical practice, a technology minimizing non-specific gene expression and dysfunction in normal cells has been developed. NPs enhance drug therapeutic efficacy and offer benefits in cost, economy, and productivity. PLGA polymers, approved by the FDA and EMA for various therapeutic applications, are a promising gene delivery vector. PLGA NPs protect delivery materials from external stress in vitro and in vivo and maintain circulation until reaching the target site. This study leverages these advantages to deliver siRNA from PLGA NPs specifically to microglia, demonstrating that PINK1 is a key signaling molecule in mitochondrial autophagy dysregulation induced by IS, potentially identifying a neurotoxic mechanism of the condition.
Wang et al. demonstrated the potential of C3-siRNA-encapsulated NPs (NPsiC3) to suppress IS-induced inflammation [95]. Using BHEM-Chol and PEG-PLA, NPsiC3 efficiently inhibited C3 expression increase in microglia during hypoxia/re-oxygenation, reduced C3b accumulation on neurons, and alleviated microglia-induced neuronal injury. NPsiC3 crossed the BBB, delivering C3-siRNA to the ischemic penumbra, reducing C3 expression in microglia and brain tissues. This decreased inflammatory cell infiltration, pro-inflammatory factors, neuronal apoptosis, and ischemic area size, while improving functional recovery post-ischemia/reperfusion injury, suggesting NPsiC3 as an effective IS therapy. The BBB poses a significant obstacle to using C3 inhibitors for stroke treatment, resulting in a lack of effective methods to inhibit microglial neurotoxicity following brain I/R injury in clinical practice. This study explores the use of PEG NPs as a brain delivery system to transport siRNA across the BBB. This approach can modify NPs distribution, extend their circulation half-life, and enhance drug delivery within the BBB, offering an innovative alternative strategy for drug delivery. It is worth considering that as an acute-onset disorder, the timely efficacy of nucleic acid drugs in the treatment of IS is a matter of concern. The potential of nucleic acid drugs may lie more prominently in the realm of preventive interventions for IS.
Natural polymer-based NPs, such as those from chitosan, hyaluronic acid, and sodium alginate, are increasingly favored for drug delivery. These renewable biopolymers offer high hydrophilicity, viscoelasticity, biodegradability, low allergenic potential, excellent biocompatibility, and unique targeting abilities, making them ideal for pharmaceutical applications. Zhao et al. developed carboxymethyl chitosan NPs (GA-NPs) to deliver Gallic acid (GA) for neuroprotection in IS [96]. GA-NPs demonstrated high encapsulation efficiency, sustained release, and extended GA half-life. The pharmacological findings, which include assessments of neurological deficits, cerebral infarction, inflammation levels, and oxidative stress, demonstrate that GA-NP exhibits superior neuroprotective effects compared to GA alone. This encapsulation method enhanced GA bioavailability and neuroprotective effects. However, the brain penetration mechanism of GA-NPs remains unclear, and the capabilities of natural polymers in this regard need to be further explored.
Jin and colleagues developed ROS-sensitive 18 β-glycyrrhetinic acid-conjugated DEAE-dextran NPs (DGA) for neuroprotection in IS by suppressing HMGB1 and promoting anti-inflammatory M2 microglial phenotypes [97]. In vitro, GA effectively targeted and restrained intracellular HMGB1. In vivo testing demonstrated the therapeutic effect on stroke mice, with smaller infarct volume, better motor function, and more neurogenesis. This study avoided the limitations of non-specific drug release and rapid plasma elimination to reduce side effects while achieving effective concentrations. The dextran-based nanomaterials show promise for mitigating CNS inflammation and advancing nerve repair. Similarly, the BBB traversal mechanism is unclear, it is hypothesized that NPs passively target stroke sites via abnormal blood vessel permeation, with active targeting enhancing efficiency.
Zhao et al. developed a triple-targeted drug delivery system based on hyaluronic acid and rutin (SHR) for treating cerebral ischemia [98]. Utilizing short peptides, SS31, and CD44-mediated endocytosis, this delivery vector can effectively penetrate the blood–brain barrier, target brain injury sites, and accumulate in damaged mitochondria. Hyaluronidase 1-mediated degradation and the acidic environment synergistically promote the sustained release of rutin in the ischemic brain area. This study revealed for the first time that rutin effectively binds to ACE2. Experimental results indicate that SHR micelles have significant anti-inflammatory, antioxidant, angiogenic, and vascular normalization effects, synergistically restoring damaged penumbra tissue by activating ACE2 and TFEB signals (Fig. 11A, B). It also confirms that ACE2 mediates the crosstalk between BMEC and microglia during SHR treatment. This study also addressed the issues of rutin's low water solubility and difficulty penetrating the BBB. Therefore, SHR's impact on vascular normalization offers potential treatment options for comorbidities such as cerebral ischemia and tumors.
Besides polymer NPs, many inorganic NPs are also used for the anti-inflammatory treatment of I/R injury. First, many NPs exhibit anti-inflammatory properties by releasing specific elements, such as selenium (Se) and zinc (Zn), that facilitate the direct treatment of IR. Amani et al. synthesized OX26-PEG-Se NPs, monoclonal antibodies targeting transferrin receptors conjugated with PEG selenide NPs, for IS treatment [99]. The surface modification of the OX26 antibody significantly enhances the targeted transport of Se NPs to the brain via transferrin receptor-mediated endocytosis. In vitro analysis showed that the NPs primarily localized in the nucleus. More importantly, Se NPs regulate the cellular metabolic state (TSC1/TSC2, p-mTOR, mTORC1), oxidative defense system (FoxO1, β-catenin/Wnt, Yap1), inflammatory response (jak2/stat3, ADAMS-1), autophagy, and apoptotic cell death (Mst1, ULK1, MTORC1) by targeting Bax, Caspase-3, and Bcl-2. They also influence various cellular signaling pathways in hippocampal neurons (rictor/mTORC2) to promote neuronal survival. The design of these NPs ensures efficient targeting with minimal side effects, presenting a promising new approach for stroke treatment.
Moreover, inorganic NPs are commonly utilized as passive carriers. Xiao and team developed SiO2@PAA-MT/ACh-aC5a nanospheres, which load melatonin (MT) and acetylcholine (ACh), to treat cerebral ischemia/reperfusion injury [100]. ACh stimulates the α7 nicotinic acetylcholine receptor (α7nAChR) on microglia, shifting them from pro-inflammatory (M1) to anti-inflammatory (M2) phenotypes, reducing pro-inflammatory cytokines. MT provides antioxidant properties, neutralizing ROS. The inclusion of aC5a aptamers ensures targeted delivery by binding to C5a, blocking its inflammatory effects. These nanospheres effectively mitigate the side effects of rhythm disorders caused by intravenous injection of MT, a circadian rhythm-dependent hormone. They address the critical issue of safely concentrating MT at an effective dose for treating I/R at the site of cerebral ischemia. However, the gradual degradation and potential long-term toxicity of inorganic NPs need careful consideration.
In recent years, several new types of NPs have emerged, especially those based on cells or cell membranes. These NPs with biomimetic properties offer significant advantages in the treatment of IS. As an example, the targeting capability of these NPs to ischemic sites, endowed by membrane proteins, exceeds that of other NPs. Dong et al. developed Resolvin D2-loaded nanovesicles (RvD2-HVs) to mitigate brain damage in IS [101]. These neutrophil-derived nanovesicles target inflamed endothelium by modulating adhesion molecules and integrins. Both in vitro and in vivo studies showed that RvD2-HVs enhance RvD2 delivery to the brain, reducing neutrophil infiltration at ischemic lesions, decreasing cerebral infarction size, and improving neurological recovery. The design of RvD2-HVs addresses the issue of Resolvin binding directly to plasma proteins, which reduces its bioavailability after intravenous administration. This research highlights the potential of neutrophil membrane-based nanocapsules for developing personalized nanomedicines for inflammatory diseases.
Besides neutrophil membranes (NMs), neutrophils can serve as direct vehicles for mediating NPs to ischemic sites. Mu et al. developed a cinnamyl-functionalized D-phenylalanine (CFLFLF) modified, ROS-responsive NP encapsulating ligustrazine (T-TMP) for treating cerebral I/R injuries by targeting neutrophils [102]. Given the presence of formyl peptide receptors (FPRs) on the surface of neutrophils, this study modified nanoplatforms by incorporating the peptide cinnamyl-F-(D)L-F-(D)L-F (CFLFLF), which specifically binds to FPRs. After intravenous injection, these NPs effectively adhere to neutrophils in peripheral blood via FPR, allowing them to "ride" with neutrophils and accumulate more efficiently at the inflammatory site of cerebral ischemia. The NP shell is composed of a polymer with reactive oxygen species (ROS)-responsive bonds, encapsulating ligustrazine, a natural neuroprotective product. This strategy overcomes the limitations of TMP, such as short half-life, poor water solubility, and low bioavailability, enhancing its bioavailability and targeting ability for cerebral ischemia. This innovative drug delivery system offers a universal platform for treating IS and other inflammation-related diseases using modern drug preparation technology.
Neutrophils possess robust phagocytic capabilities that can be harnessed to achieve the ‘Trojan Horse’ effect. Pan et al. developed OMV@PGZ, a brain-targeted nanoplatform using bacterial outer membrane vesicles (OMVs) loaded with pioglitazone (PGZ) for IS treatment [103]. Neutrophils take up OMV@PGZ via LPS-TLR4 interaction and transport it across the BBB to ischemic sites. PGZ activates PPARγ, reducing inflammation and oxidative stress, and promoting anti-inflammatory agents. It also restores mitochondrial activity and prevents ferroptosis, protecting the nervous system. Notably, the transcription factors Pou2f1 and Nrf1 of oligodendrocytes are identified for the first time to be involved in this process and promoted neural repair by single-nucleus RNA sequencing (snRNA-seq). OMV@PGZ shows promise for IS treatment with a good biosafety profile and potential for broader neurological disorder therapies.
Summarizing the aforementioned research findings, it can be observed that there are two primary strategies to mitigate inflammation. First, to limit the influx of inflammatory cells from the bloodstream into the site of injury, and second, to suppress the activation and multiplication of inflammatory cells at the site of focus. The vast majority of studies have adopted either one strategy or the other. However, employing both strategies concurrently may yield superior outcomes. Wang et al. engineered McM/RNPs, combining monocyte membrane-coated PLGA NPs with rapamycin to create targeted anti-inflammatory ‘shield and password’ nano-soldiers [104]. These NPs adhere to inflamed endothelial cells, preventing further inflammatory cell infiltration into the brain. Upon reaching the injury site, they release rapamycin, curbing inflammation by limiting microglial cell proliferation. McM/RNPs have shown promise in reducing inflammation, enhancing neurological scores, and decreasing infarct volume post-surgery for MCAO. This dual-functional monocyte membrane-functionalized drug delivery system (MCM/RNPs) achieves synergistic immunochemotherapy for IS. Thus, MCM/RNPs present a promising formulation strategy for enhancing the clinical treatment of IS.
In conclusion, various NPs, such as polymer-based, biomimetic, and inorganic types, are used for the anti-inflammatory treatment of IS. They improve drug delivery through the BBB, increase bioavailability, and target ischemic areas and inflammatory cells effectively. NPs modulate microglia activation to combat neuroinflammation, overcoming the limitations of traditional therapies. These innovative strategies promise improved outcomes for IS management through enhanced specificity and bioavailability.
Inflammation plays a dual role, acting as either a component of the body's reparative mechanisms or a potential cause of local tissue damage. Therefore, it is imperative to strike a balance between suppressing the inflammatory process and upholding normal immune function in the management of IS. NP-based drug delivery systems must be precisely targeted to the inflammatory sites to prevent harm to healthy tissues. Consequently, nanotechnology is leveraged to engineer more accurate and effective NPs to mitigate adverse effects on normal tissues. Moreover, the incorporation of molecular imaging technology into NP-based drug delivery systems is envisaged for real-time monitoring and assessment of inflammatory responses, thus guiding the therapeutic regimen for IS.
Cell death
The pathological mechanism of cell death
The damage resulting from mitochondrial dysfunctions, oxidative stress, inflammation, and excitotoxicity in IS can trigger a range of cellular signaling cascades. These events can drive neural cells to experience programmed cell death, such as apoptosis and autophagy, which are intrinsic cellular processes, or unprogrammed cell death, like necrosis, often instigated by external influences [105]. Necrosis is a form of pathological cell death triggered by severe physical, chemical, or other pathological stressors [106]. Necrotic cells experience an increase in membrane permeability, resulting in cell swelling, organelle distortion or enlargement, early absence of nuclear morphological changes, and eventual cell membrane rupture (Fig. 12A). The release of cellular contents through lysis by necrotic cells incites an inflammatory response [107]. Subsequent tissue and organ healing often involves fibrosis, leading to scar formation. Autophagy represents a crucial evolutionary mechanism for the turnover of intracellular components in eukaryotic organisms [108]. Within this process, impaired proteins or organelles are encapsulated by autophagic vesicles characterized by a double-layered membrane structure. Subsequently, these vesicles are directed to lysosomes for degradation and recycling [109]. The essence of autophagy is the membrane rearrangement in the cell, and its occurrence process can be roughly divided into the following four stages (Fig. 12B): (1) Initiation of autophagy. (2) Formation of isolation membranes and autophagosomes. (3) The autophagosome fuses with the lysosome. (4) Cleavage of autophagosomes [110]. Apoptosis, regulated by environmental cues, progresses through stages (Fig. 12C): activation, apoptotic body formation, and phagocytosis. Cells undergo volume reduction, cytoplasmic changes, mitochondrial dysfunction, and DNA fragmentation. Phosphatidylserine shifts outward, maintaining membrane integrity [111]. Apoptotic bodies form without inflammation, apoptosomes can be quickly engulfed by surrounding full-time or part-time phagocytes [112]. In circumstances where the ischemic core lacks sufficient oxygen and glucose, irreversible necrosis typically ensues. Conversely, minor injury in the penumbra area can trigger reversible cellular death mechanisms such as apoptosis and autophagy [113].
NP-based therapeutics in neuron regeneration therapy for IS
IS-induced CNS damage contributes to mortality and disability due to limited neuronal regeneration and glial scarring. Exogenous stem cell transplantation and gene therapy show promise in promoting neuronal regrowth and tissue restoration post-IS. NPs incorporating stem cells and gene therapy offer a cutting-edge approach to enhance neural recovery after cerebral ischemia, addressing the limitations of conventional treatments.
Stem cells, including neural stem cells (NSCs), mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and endothelial progenitor cells (EPCs), hold promise for treating IS by promoting neural recovery [114]. However, challenges such as the hostile post-IS environment, limited endogenous NSCs, lack of neural differentiation factors, and preferential astrocytic differentiation of transplanted NSCs hinder their therapeutic efficacy [115]. Complications like fibrosis, functional loss, and immune rejection further impede treatment success [116]. Innovative strategies, such as embedding functional NPs within stem cells, have shown promise in overcoming these barriers. This approach can boost the robustness and cytokine-secreting capacity of the stem cells, thereby enhancing their overall reparative potential for IS.
Jiang et al. developed a novel strategy using a ROS-responsive polymer, B-PDEA, to deliver BDNF plasmid DNA into NSCs, enhancing BDNF secretion [117]. The polyplex NPs disassembled within cells due to ROS, releasing the DNA for effective gene delivery. Transplanting BDNF-modified NSCs into mice increased brain BDNF levels significantly. BDNF-NSC administration improved outcomes in cerebral ischemia mice compared to non-transfected NSCs. The B-PDEA/pBDNF polyplex-transfected NSCs showed increased BDNF secretion in vitro and in vivo, leading to better recovery of neurological and motor functions and reduced mortality in MCAO mice. In conclusion, the present study investigates the ROS-responsive charge-reversal polymer B-PDEA as the first successful nonviral vector for effective genetic transfection of NSCs. Consequently, the B-PDEA/pBDNF polyplex-transfected NSCs secreted significantly more BDNF in vitro and in vivo in ischemic brain regions, leading to notably decreased mortality of MCAO mice and the improved reconstruction of neurological and motor functions.
Extracellular vehicles (EVs) represent an innovative cell-free therapeutic alternative for the treatment of IS, offering benefits such as comparable physiological functions to parent cells, minimal volume, reduced immunogenicity, and the capacity to cross the BBB, a distinct advantage over traditional stem cell therapies. Ruan et al. utilized copper-free click chemistry to modify extracellular vesicles (EVs) from M2 microglia with the high-affinity ligand DA7R targeting damaged blood vessels and a variant of SDF-1 to attract NSCs, creating Dual-EVs for IS treatment (Fig. 13A) [118]. These engineered EVs enhanced NSC neuronal differentiation without compromising their functionality (Fig. 13B). The DA7R-modified EVs effectively targeted HUVECs, with the additional SDF-1 modification increasing uptake and promoting NSC migration (Fig. 13C). In a mouse tMCAO model, Dual-EVs carrying both ligands improved neurological recovery by enhancing NSC attraction to the ischemic site, stimulating neurogenesis, and improving overall neurological function (Fig. 13D). These Dual-EV nanocarriers offer new insights into treating neuronal regeneration following CNS injuries and targeting endogenous stem cells. The use of click chemistry with EV/peptide/chemokine and related nanocarriers represents a promising approach for enhancing human health.

© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V
Click chemistry extracellular vesicle/peptide/chemokine nanocarriers for treating central nervous system injuries. A Schematic illustration of DA7R-SDF-1-EV nano-missile (Dual-EV) treatment on IS. B Effect of M2-EV on NSC differentiation. C Internalization of different EVs by HUVECs and its recruitment effect on NSCs. D Ischemic brain-targeting ability of Dual-EV in vivo.
Cerebral ischemia can alter gene expression, prompting the use of gene therapy to introduce exogenous genes or modulate existing ones in the ischemic penumbra. This aims to reduce neuronal damage by enhancing blood flow or modifying cellular processes. Genes related to angiogenesis, antioxidant defense, anti-apoptosis, and hypoxia response, like VEGF, show promise in post-stroke treatment. However, gene therapy faces challenges due to poor targeting and transfection efficiency of DNA or RNA alone. NPs have emerged as crucial carriers for gene delivery, enhancing the success of gene therapy for IS.
Hypoxia-inducible factor 1-alpha (HIF-1α) plays a crucial role in angiogenesis post-ischemia. Deng et al. developed RGD-DMAPA-Amyp NPs by attaching RGD peptides to a cationic polymer, enhancing targeted HIF-1α delivery [119]. The results demonstrated that RGD-DMAPA-Amyp has good biocompatibility and a high cell uptake rate, indicating it is a safe nonviral gene vector for human cell endocytosis. In rat models of IS, RGD-DMAPA-Amyp NPs accumulated more in vascular endothelial cells of the peri-infarct region compared to the non-targeted nanocarrier group, significantly improving neurological function recovery. The RGD-modified nanomedicine promotes nerve function recovery more efficiently and shows the potential to significantly promote new blood vessel formation in vivo. These findings suggest that the RGD-modified nonviral gene vector containing HIF-1α-AA is a safe and promising therapeutic strategy for IS gene therapy.
Heme oxygenase-1 (HO1) is an important antioxidant enzyme that breaks down heme into carbon monoxide, biliverdin, and iron ions, leading to anti-inflammatory effects. Increasing HO1 expression in penumbral regions could help reduce reperfusion injury post-IS. Oh et al. developed self-assembling NPs (HSAP-NP/pHO1) containing hypoxia-targeted anti-RAGE peptides (HSAP), deoxycholate-conjugated polyethylenimine-2 k (DP2k), and an HO1 plasmid (pHO1) for IS treatment [120]. These NPs showed superior gene delivery and expression compared to HSAP or DP2k/pHO1 alone in ischemic areas. In rat models of MCAO, stereotaxic injection of HSAP-NP/pHO1 NPs had a significant therapeutic effect. Therefore, HSAP-NP may be a useful gene and peptide therapy system for stroke therapy with dual functions of hypoxia-specific gene delivery and cytoprotective effects. This system holds promise as an advanced platform for combined gene and peptide delivery in stroke therapy, offering precise gene transfer to hypoxic regions and enhanced cellular protection.
HMGB1 is a DAMP released by dying cells and immune cells, exacerbating inflammation in IS. Kim et al. used e-PAM-R dendrimers for intranasal delivery of HMGB1 siRNA in MCAO rats, achieving knockdown and reducing brain damage [121]. FITC-labeled control siRNA was intranasally delivered in normal adult rats using e-PAM-R, a biodegradable PAMAM dendrimer, as a gene carrier. Within 1 h fluorescence-tagged siRNA was observed in neurons and glial cells across various brain regions. The fluorescence persisted for at least 12 h. Intranasal delivery of siRNA targeting HMGB1 significantly reduced its expression in brain regions like the prefrontal cortex and striatum. Importantly, this approach markedly reduced infarct volume in postischemic rat brains (up to 42.8 ± 5.6% reduction at 48 h post-MCAO), leading to improvements in neurological and behavioral deficits. These results indicate that the intranasal delivery of HMGB1 siRNA offers an efficient means of gene knockdown-mediated therapy in the ischemic brain.
tFNAs, a type of DNA nanomaterial, have shown promise in enhancing NSCs proliferation, migration, and neuronal differentiation. Zhou et al. studied the neuroprotective effects of tFNAs on IS using a rat tMCAO model (Fig. 14A) [122]. tFNAs were able to penetrate the brain tissue, cross the BBB, and protect neuronal cells from apoptosis in an OGD/R model by disrupting the ischemic cascade (Fig. 14B). They improved the ischemic microenvironment by upregulating erythropoietin and reducing inflammation, leading to decreased neuronal loss, cell apoptosis, infarct volume reduction, and neurological deficit improvement in the tMCAO rat model (Fig. 14C). Inhibition of the TLR2-MyD88-NF-κB pathway may be a key mechanism of tFNAs' neuroprotective effects (Fig. 14D). tFNAs are a safe and versatile nano-neuroprotective agent for IS therapy. However, the absence or presence of normal function in these regenerated neurons that were induced by tFNAs requires further exploration.
A DNA nanostructure-based neuroprotectant against neuronal apoptosis via inhibiting toll-like receptor 2 signaling pathway in acute IS. A Schematic diagram of tFNAs as neuroprotective agents for the treatment of IS. B Protection of tFNAs for apoptosis of SH-SY5Y cells via interfering with the ischemia cascades in OGD/R models in vitro. C tFNAs reduce the infarct volume, alleviate mortality, and ameliorate functional outcomes of tMCAO models. D Potential mechanism of the protective effects of tFNAs on IS.
In addition, gene delivery based on NPs combined with NSC is used for neuronal regeneration therapy in IS. The regenerative processes following IS can be hindered by myelin-associated inhibitors (MAIs) such as oligodendrocyte myelin glycoprotein and Nogo-A, which are known to impede axonal regeneration and neuronal differentiation. Targeted therapies that aim to knock down the expression of genes related to these inhibitory proteins could potentially enhance neural regeneration and recovery. Lu et al. developed a novel strategy using a block copolymer PEI-PLA combined with SPIONs to create an MRI-visible nanocarrier (SPION-CP) for siRNA delivery targeting the NgR gene [123]. MRI demonstrates that nanomedicine provides non-invasive imaging of NSC migration and homing. Additionally, epigenetic downregulation of NgR genes in NSCs counteracts the inhibitory microenvironment of stroke, significantly enhancing neuronal differentiation of exogenous NSCs and promoting functional recovery in acute IS. The therapeutic potential of our siRNA nanomedicine allows for controlled stem cell differentiation, easily tracked by MRI. This has significant implications for developing new stem cell therapy paradigms for stroke and other neurological disorders.
Lin et al. developed an MRI-detectable nanocarrier for the simultaneous delivery of siRNA/ASO and SPIO NPs into NSCs targeting Pnky lncRNA in a mouse model of AIS (Fig. 15A) [124]. Pnky-SPIO-PALC nanocarriers increased neuronal differentiation and showed migration from the infarct core to the periinfarct cortex (Fig. 15B). Confocal microscopy revealed lysosomal localization of siRNA/ASO. Inhibition of Pnky lncRNA by SPIO-PALC complex reduced Pnky levels. Mice treated with Pnky-SPIO-PALC NSCs exhibited improved recovery compared to control (SCR)-SPIO-PALC (Fig. 15C). This multifunctional nanomedicine can be used to direct stem cell differentiation and in vivo track the stem cells after transplantation, which highlights its great potential as a promising platform to regulate the stem cell fate and thus lays down a foundation for developing stem cell-based therapies for strokes and other neurologic diseases.
Nanomedicine directs neuronal differentiation of neural stem cells via silencing long noncoding RNA for stroke therapy. A Schematic illustration of the preparation for MRI-Visible siRNA/ASO complexed nanomedicine, directed neuronal differentiation of transplanted NSCs by silencing pnky lncRNA and MRI tracking of NSCs in a mouse stroke model. B In vitro and in vivo neuronal differentiation mediated by Pnky downregulation. C Safety and efficiency of Pnky knockdown with MRI-visible nanomedicine.
Yang et al. used Ca-MOF to develop an effective miR-124 nano-carrier system (Ca-MOF@miR-124) for treating IS [125]. At safe concentrations, Ca-MOF@miR-124 NPs promoted NSC proliferation by releasing miR-124, which was internalized via endocytosis. The acidic lysosomal environment facilitated miR-124 release into the cytoplasm, accelerating neuronal differentiation. Ca-MOF@miR-124 NPs enhanced NSC maturation into functional neurons within 5 days, surpassing control group differentiation rates. Treatment with Ca-MOF@miR-124 NPs reduced ischemic brain area, leading to significant recovery by day 7. Ca-MOF@miR-124 NPs effectively overcome the instability of naked miR-124 degraded by nucleases and the difficulty in internalizing it through the cell membrane due to its multiple anionic charges, making it widely applicable in clinical treatment based on the characteristics of miR-124. Combining Ca-MOF@miR-124 NPs with NSC therapy shows promise for enhancing traumatic neural injury and neurodegenerative condition treatments, improving patient outcomes.
Regenerative medicine, focusing on stem cells and gene therapy, shows promise for nerve regeneration and treating IS and other nerve injuries. Stem cell and gene therapy NPs offer innovative treatments, aiming for nerve recovery. IS results from brain blood flow obstruction, causing neuronal damage and functional loss. Combining stem cell therapy, gene therapy, and nanotechnology offers a multi-modal approach to enhance recovery and regeneration post-IS. Furthermore, EVs isolated from various sources, including animal cells, plant cells, and bacteria, exhibit promising potential as NP-based drug delivery systems. These vesicles harbor bioactive constituents inherited from parent cells, enabling them to act as natural therapeutic agents. And they can take advantage of their natural structure to facilitate the transport of exogenous drugs. Consequently, we believe that EVs have significant utility in attenuating cell death and managing IS.
NPs-mediated combination therapy for IS
Integrating neuroprotective agents with distinct modes of action into a single formulation capable of influencing multiple pathological states can markedly amplify the therapeutic benefits in the management of IS. Yuan's team has developed TPCD NPs, which are nano-therapies with multi-targeted pharmacological actions for AIS. TPCD NPs are synthesized using an active oligosaccharide base, TPCD, covalently bonded with Tempol and phenylboronic acid pinacol ester (PBAP) on β-cyclodextrin [126]. These NPs reduce oxidative stress, enhance antioxidant enzyme expression, and reduce inflammation at the cellular level, preventing neuronal cell death (Fig. 16A, B). Intravenously delivered TPCD NPs target ischemic brain damage in MCAO mouse models, reducing tissue death and restoring neurological functions. They act as ROS-sensitive nanocarriers for the controlled release of the anti-inflammatory peptide Ac2-26, forming the ATPCD NP. In vivo tests show that ATPCD NP outperforms TPCD NP due to its superior anti-inflammatory efficacy. In summary, ATPCD NP integrates anti-inflammatory, antioxidant, and anti-apoptotic "three carriages" for precise targeted pathological and physiological treatment of IS, significantly improving in vivo efficacy. Therefore, TPCD NP-derived nanomedicines can be further developed as promising targeted therapies for stroke and other inflammation-related cerebrovascular diseases.
Liu et al. developed a neutrophil-mimicking nanobuffer (LA-NM-NP/CBD) to counteract the negative effects of elevated pro-inflammatory agents in stroke (Fig. 17A) [127]. The nanobuffer cleared inflammatory factors and ROS in cell experiments and preferentially accumulated in the infarct core in rat models (Fig. 17B). It neutralized harmful agents in the penumbra and modulated neuronal vitality with controlled CBD release (Fig. 17C). In vivo studies showed a reduction in harmful factors in the core and penumbra, decreased infarct size, and improved neurological outcomes. The nanobuffer strategy was first proposed in this study to remodel the hostile environment of the core and protect the salvageable penumbra for the treatment of IS. LA-NM-NP/CBD indeed played a comprehensive therapeutic role via remodeling the microenvironment of the ischemic core and simultaneously protecting the penumbra by neutralizing detrimental factors from the core and slowly releasing CBD. The nanobuffer provided a promising strategy for treating IS by modulating the core as well as the penumbra.
Neutrophil-bomimetic “nanobuffer” for remodeling the microenvironment in the infarct core and protecting neurons in the penumbra via neutralization of detrimental factors to treat IS. A Schematic illustration of LA-NM-NP/CBD preparation and its nanobuffer effect. B Nanobuffer effect of LA-NM-NP/CBDs against the core and neuroprotective effect for the penumbra in vitro. C Nanobuffer formed at the infarct core by the accumulation of LA-NM-NPs and buffering detrimental erosions in vivo.
In summary, IS involves the occurrence and development of multiple pathophysiologies, and single-functional NPs exhibit limited efficacy in addressing the intricate and interconnected cascade reactions. Multi-functional targeted neuroprotective NPs have great potential for the treatment of IS ischemia and reperfusion. This approach signifies the direction for future advancements in the field.
Conclusions and perspectives
IS pathogenesis is marked by a complex interplay of pathophysiological processes, including excitotoxicity, oxidative stress, inflammation, and cellular death. The distinctive properties of the BBB present a significant challenge, as they impede the delivery of many potent therapeutic agents to areas affected by cerebral ischemia. This barrier has been a primary factor contributing to suboptimal clinical outcomes due to the inability to target ischemic zones with specific treatments effectively. In response to this challenge, the remarkable brain-targeting capabilities and BBB permeability of NPs have sparked innovative research. Scientists have developed an array of NP-based strategies for targeted therapeutic delivery to ischemic brain regions. These strategies primarily utilize NPs designed for neuroprotection and those optimized for combination therapy. These advancements in NP-mediated treatment modalities have demonstrated promising results in enhancing the efficacy of IS treatment during preclinical studies.
In addition, the drug delivery pathway is a crucial factor that influences the ability of NPs to target the brain. In the context of IS therapy, intravenous injection stands out as the preferred method for delivering NPs, offering operational convenience, the ability to administer high dosages, and minimal invasiveness. Following intravenous administration, the fate of NPs within the body is determined by factors such as their particle size, surface targeting groups, and surface charges. Notably, NPs tend to interact with plasma proteins, giving rise to a “protein corona” on their surfaces, which hinders their ability to target the brain effectively and permeate the BBB [128]. While an alternative, non-invasive approach via the intranasal route has been documented to bypass the BBB and swiftly achieve therapeutic drug concentrations, the drawback lies in the limited delivery dosage associated with intranasal administration [129]. Additionally, the motion of cilia and the presence of mucus present further obstacles by obstructing the delivery of medications to the brain. Alternative methods, including retro-orbital and intrathecal injections, provide a direct pathway for delivering therapeutic agents to the brain, circumventing the BBB. Nonetheless, these approaches are limited by the requirement for advanced injection skills and the significant discomfort they cause to patients. In an attempt to preserve the vitality of stem cells and boost the efficiency of RNA or DNA transfection, certain NPs tailored for stem cell or gene therapy have been utilized for highly invasive intracranial administration. Considering the varied mechanisms of action and targeting strategies of NPs, the achievement of optimal therapeutic results can be facilitated by the choice of suitable administration routes.
In conclusion, the advancements in NPs-mediated approaches for treating IS are evident in several key areas: (i) improving the stability of therapeutic substances in the blood and extending their circulation time; (ii) enhancing the targeting of the brain and permeability of the BBB for therapeutic agents; (iii) reducing the toxicity of therapeutic agents; (iv) enhancing the viability of stem cells and improving the efficiency of delivering therapeutic genes; (v) combining therapeutic agents targeting different aspects into a single nano-formulation for synergistic therapy. This suggests a promising future for the clinical application of NPs in IS treatment.
Despite the substantial progress in NP development, certain challenges impede their clinical translation. Initially, NPs frequently focus on individual pathological processes associated with IS, thereby neglecting to offer a holistic shield for the central nervous system. Additionally, the intricacy of integrating various therapeutic agents within a single nanocarrier system may impede their translation into clinical practice. Secondly, animal models of cerebral ischemia may not fully replicate the pathogenesis seen in human patients. Thirdly, while efforts to enhance brain targeting through modifications and biomimetic strategies have shown promise, the BBB remains a significant obstacle for NPs to overcome, resulting in many NPs failing to reach the ischemic regions in the brain. Utilizing the body's endogenous components or physiological mechanisms may represent a viable approach to facilitate the transport of NPs across the BBB. Lastly, understanding the intracerebral fate of NPs is crucial for improving the treatment effect of IS and ensuring their long-term safety in clinical settings. Further research and development are needed to address these challenges and optimize the efficacy and safety of NP-based therapies for IS treatment.
A substantial disparity persists between the preclinical trials and the practical utilization of NPs in treating IS. Given the unique brain structural characteristics and intricate injury mechanisms associated with this condition, NPs necessitate a thoughtful design approach that integrates their ability to penetrate the BBB with the specific microenvironment of ischemic regions. This strategic design aims to enhance targeted delivery to the lesion site. Furthermore, there is a crucial need for an in-depth exploration of the pathological mechanisms of IS and effective therapeutic drugs to restore impaired neurological functions, ultimately mitigating disability and mortality among patients. As nanomedicine advances and our understanding of IS's pathophysiology deepens, it becomes imperative to enhance the biological functionalities of NPs to facilitate their clinical translation for more effective treatment of IS.
Data availability
Data will be made available on request. No datasets were generated or analysed during the current study.
References
Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70.
Hankey GJ. Stroke. Lancet. 2017;389:641–54.
Powers WJ. Acute ischemic stroke. N Engl J Med. 2020;383:252–60.
Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–7.
Tang L, Fu C, Zhang A, Li X, Cao Y, Feng J, et al. Harnessing nanobiotechnology for cerebral ischemic stroke management. Biomater Sci. 2022;11:791–812.
Liao J, Li Y, Luo Y, Meng S, Zhang C, Xiong L, et al. Recent advances in targeted nanotherapies for ischemic stroke. Mol Pharm. 2022;19:3026–41.
Lv W, Liu Y, Li S, Lv L, Lu H, Xin H. Advances of nano drug delivery system for the theranostics of ischemic stroke. J Nanobiotechnology. 2022;20:1–31.
Tian X, Fan T, Zhao W, Abbas G, Han B, Zhang K, et al. Recent advances in the development of nanomedicines for the treatment of ischemic stroke. Bioact Mater. 2021;6:2854–69.
Ndemazie NB, Inkoom A, Morfaw EF, Smith T, Aghimien M, Ebesoh D, et al. Multi-disciplinary approach for drug and gene delivery systems to the brain. AAPS PharmSciTech. 2022;23:1–21.
Song YH, De R, Lee KT. Emerging strategies to fabricate polymeric nanocarriers for enhanced drug delivery across blood-brain barrier: an overview. Adv Colloid Interface Sci. 2023;320: 103008.
Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:1–25.
Zhang J, Chen Z, Chen Q. Advanced nano-drug delivery systems in the treatment of ischemic stroke. Molecules. 2024;29:1848.
Khandelwal P, Yavagal DR, Sacco RL. Acute ischemic stroke intervention. J Am Coll Cardiol. 2016;67:2631–44.
Psychogios K, Tsivgoulis G. Intravenous thrombolysis for acute ischemic stroke: why not? Curr Opin Neurol. 2022;35:10–7.
Silva GS, Nogueira RG. Endovascular treatment of acute ischemic stroke. Continuum. 2020;26:310–31.
Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum. 2020;26:268–86.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49:e46-110.
O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77.
Savitz SI, Fisher M. Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402.
Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46:2–18.
Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of α4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205.
Xu J, Wang A, Meng X, Yalkun G, Xu A, Gao Z, et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double-blind. Comparat Trial Stroke. 2021;52:772–80.
Van Dongen MME, Aarnio K, Martinez-Majander N, Pirinen J, Sinisalo J, Lehto M, et al. Use of statins after ischemic stroke in young adults and its association with long-term outcome. Stroke. 2019;50:3385–92.
Sabroe I, Read RC, Whyte MKB, David H, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2013;171:1630–5.
Hong JM, Lee JS, Lee YB, Shin DH, Shin DI, Hwang YH, et al. Nelonemdaz for patients with acute ischemic stroke undergoing endovascular reperfusion therapy: a randomized phase II trial. Stroke. 2022;53:3250–9.
Feigin VL, Doronin BM, Popova TF, Gribatcheva EV, Tchervov DV. Vinpocetine treatment in acute ischaemic stroke: a pilot single-blind randomized clinical trial. Eur J Neurol. 2001;8:81–5.
Leonard MG, Briyal S, Gulati A. Endothelin B receptor agonist, IRL-1620, provides long-term neuroprotection in cerebral ischemia in rats. Brain Res. 2012;1464:14–23.
Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–53.
Chen ZB, Huang DQ, Niu FN, Zhang X, Li EG, Xu Y. Human urinary kallidinogenase suppresses cerebral inflammation in experimental stroke and downregulates nuclear factor-B. J Cereb Blood Flow Metab. 2010;30:1356–65.
Malhotra K, Chang JJ, Khunger A, Blacker D, Switzer JA, Goyal N, et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol. 2018;265:1871–9.
Bei Y. Randomized, controlled, dose escalation trial of a protease- activated receptor-1 agonist in acute ischemic stroke: final results of the RHAPSODY trial. Mol Neurobiol. 2017;176:139–48.
Li R, Huang C, Chen J, Guo Y, Tan S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol Sci. 2015;36:1097–103.
He W, Zhang Z, Sha X. Biomaterials Nanoparticles-mediated emerging approaches for effective treatment of ischemic stroke. Biomaterials. 2021;277: 121111.
Vashist A, Manickam P, Raymond AD, Arias AY, Kolishetti N, Vashist A, et al. Recent advances in nanotherapeutics for neurological disorders. ACS Appl Bio Mater. 2023;6:2614–21.
Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K, et al. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018;97:1521–37.
Zhang C, Yan L, Wang X, Zhu S, Chen C, Gu Z, et al. Progress, challenges, and future of nanomedicine. Nano Today. 2020;35: 101008.
Sarmah D, Saraf J, Kaur H, Pravalika K, Tekade RK, Borah A, et al. Stroke management: an emerging role of nanotechnology. Micromachines. 2017;8:1–13.
Panagiotou S, Saha S. Therapeutic benefits of nanoparticles in stroke. Front Neurosci. 2015;9:1–6.
Kyle S, Saha S. Nanotechnology for the detection and therapy of stroke. Adv Healthc Mater. 2014;3:1703–20.
Østby-Deglum I, Dahl AA. How is an information programme for depressed patients perceived? Tidsskr Nor Laegeforen. 2002;122:1870–2183.
Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20:362–83.
Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–19.
Li D, Wang C, Yao Y, Chen L, Liu G, Zhang R, et al. MTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. 2016;30:3388–99.
Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, et al. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2015;212:712–22.
Xiong XY, Liu L, Yang QW. Refocusing neuroprotection in cerebral reperfusion era: new challenges and strategies. Front Neurol. 2018;9:1–11.
Zhao M, Zhu P, Fujino M, Zhuang J, Guo H, Sheikh I, et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci. 2016;17:2078.
Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–6.
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, et al. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med. 2014;71:186–95.
Zhou Y, Liao J, Mei Z, Liu X, Ge J. Insight into crosstalk between ferroptosis and necroptosis: novel therapeutics in ischemic stroke. Oxid Med Cell Longev. 2021;2021:9991001.
Bonora M, Patergnani S, Ramaccini D, Morciano G, Pedriali G, Kahsay AE, et al. Physiopathology of the permeability transition pore: molecular mechanisms in human pathology. Biomolecules. 2020;10:1–25.
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8: e1000298.
Tajeddine N. How do reactive oxygen species and calcium trigger mitochondrial membrane permeabilisation? Biochim Biophys Acta. 2016;1860:1079–88.
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95.
Kong N, Tao W, Ling X, Wang J, Xiao Y, Shi S, Ji X, Shajii A, Gan ST, Kim NY, Duda DG, Tian X, Omid C, Farokhzad JS. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci Transl Med. 2016;176:139–48.
Lu Y, Guo Z, Zhang Y, Li C, Zhang Y, Guo Q, et al. Microenvironment remodeling micelles for Alzheimer’s disease therapy by early modulation of activated microglia. Adv Sci. 2019;6:1801586.
Ashafaq M, Intakhab Alam M, Khan A, Islam F, Khuwaja G, Hussain S, et al. Nanoparticles of resveratrol attenuates oxidative stress and inflammation after ischemic stroke in rats. Int Immunopharmacol. 2021;94: 107494.
Jin Q, Cai Y, Li S, Liu H, Zhou X, Lu C, et al. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics. 2017;7:884–98.
Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23:1384–95.
Mohimani H, Gurevich A, Mikheenko A, Garg N, Nothias L-F, Ninomiya A, et al. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke yanlan. J Am Chem Soc. 2017;176:139–48.
Varlamova EG, Khabatova VV, Gudkov SV, Plotnikov EY, Turovsky EA, et al. Cytoprotective properties of a new nanocomplex of selenium with taxifolin in the cells of the cerebral cortex exposed to ischemia/reoxygenation. Pharmaceutics. 2022;14:2477.
Tian R, Ma H, Ye W, Li Y, Wang S, Zhang Z, et al. Se-containing MOF coated dual-Fe-atom nanozymes with multi-enzyme cascade activities protect against cerebral ischemic reperfusion injury. Adv Funct Mater. 2022;32:1–17.
Huang G, Zang J, He L, Zhu H, Huang J, Yuan Z, et al. Bioactive nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano. 2022;16:431–52.
Hosoo H, Marushima A, Nagasaki Y, Hirayama A, Ito H, Puentes S, et al. Neurovascular unit protection from cerebral ischemia-reperfusion injury by radical-containing nanoparticles in mice. Stroke. 2017;48:2238–47.
Wu H, Peng B, Mohammed FS, Gao X, Qin Z, Sheth KN, et al. Brain targeting, antioxidant polymeric nanoparticles for stroke drug delivery and therapy. Small. 2022;18: e2107126.
Dong Z, Tang L, Zhang Y, Ma X, Yin Y, Kuang L, et al. A homing peptide modified neutrophil membrane biomimetic nanoparticles in response to ROS/inflammatory microenvironment for precise targeting treatment of ischemic stroke. Adv Funct Mater. 2023;2309167:1–16.
Shi J, Yu W, Xu L, Yin N, Liu W, Zhang K, et al. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2020;20:780–9.
Liao J, Li Y, Fan L, Sun Y, Gu Z, Xu Q, et al. Bioactive ceria nanoenzymes target mitochondria in reperfusion injury to treat ischemic stroke. ACS Nano. 2024;24:3c10982.
Zhang Y, Zhang H, Zhao F, Jiang Z, Cui Y, Ou M, et al. Mitochondrial-targeted and ROS-responsive nanocarrier via nose-to-brain pathway for ischemic stroke treatment. Acta Pharm Sin B. 2023;13:5107–20.
Arteaga Cabeza O, Zhang Z, Smith Khoury E, Sheldon RA, Sharma A, Zhang F, et al. Neuroprotective effects of a dendrimer-based glutamate carboxypeptidase inhibitor on superoxide dismutase transgenic mice after neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 2021;148: 105201.
Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013;63:244–58.
Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: new views in health and disease. Trends Biochem Sci. 2018;43:752–89.
Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol. 2013;4:1–11.
Andersen JV, Markussen KH, Jakobsen E, Schousboe A, Waagepetersen HS, Rosenberg PA, et al. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology. 2021;196: 108719.
Jalini S, Ye H, Tonkikh AA, Charlton MP, Carlen PL. Raised intracellular calcium contributes to ischemia-induced depression of evoked synaptic transmission. PLoS ONE. 2016;11:1–23.
Zhao Y, Jiang Y, Lv W, Wang Z, Lv L, Wang B, et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71.
Lv W, Xu J, Wang X, Li X, Xu Q, Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12:5417–26.
Zhang J, Liu J, Li D, Zhang C, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev. 2019;13:CD001928.
Tóth OM, Menyhárt Á, Varga VÉ, Hantosi D, Ivánkovits-Kiss O, Varga DP, et al. Chitosan nanoparticles release nimodipine in response to tissue acidosis to attenuate spreading depolarization evoked during forebrain ischemia. Neuropharmacology. 2020;162: 107850.
Ma J, Zhang S, Liu J, Liu F, Du F, Li M, et al. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15:1–8.
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25.
Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77:53–63.
Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606.
Chang JJ, Stanfill A, Pourmotabbed T. The role of matrix metalloproteinase polymorphisms in ischemic stroke. Int J Mol Sci. 2016;17:1323.
Ma Y, Yang S, He Q, Zhang D, Chang J. The role of immune cells in post-stroke angiogenesis and neuronal remodeling: the known and the unknown. Front Immunol. 2021;12: 784098.
Ye Y, Zeng Z, Jin T, Zhang H, Xiong X, Gu L. The role of high mobility group box 1 in ischemic stroke. Front Cell Neurosci. 2019;13:127.
Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:73–7.
Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–16.
Crack P, Wong C. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14.
Wang Y, Luo J, Li SY. Nano-curcumin simultaneously protects the blood-brain barrier and reduces M1 microglial activation during cerebral ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11:3763–70.
Thomas RG, Kim JH, Kim JH, Yoon J, Choi KH, Jeong YY. Treatment of ischemic stroke by atorvastatin-loaded PEGylated liposome. Transl Stroke Res. 2023;15:388–98.
Zhu Z, Lu H, Jin L, Gao Y, Qian Z, Lu P, et al. C-176 loaded Ce DNase nanoparticles synergistically inhibit the cGAS-STING pathway for ischemic stroke treatment. Bioact Mater. 2023;29:230–40.
Jin W, Wu Y, Chen N, Wang Q, Wang Y, Li Y, et al. Early administration of MPC-n(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics. 2021;11:8197–217.
Han L, Liu C, Qi H, Zhou J, Wen J, Wu D, et al. Systemic delivery of monoclonal antibodies to the central nervous system for brain tumor therapy. Adv Mater. 2019;31:1–9.
Choi SG, Shin J, Lee KY, Park H, Kim SI, Yi YY, et al. PINK1 siRNA-loaded poly(lactic-co-glycolic acid) nanoparticles provide neuroprotection in a mouse model of photothrombosis-induced ischemic stroke. Glia. 2023;71:1294–310.
Wang Y, Li SY, Shen S, Wang J. Protecting neurons from cerebral ischemia/reperfusion injury via nanoparticle-mediated delivery of an siRNA to inhibit microglial neurotoxicity. Biomaterials. 2018;161:95–105.
Zhao Y, Li D, Zhu Z, Sun Y. Improved neuroprotective effects of gallic acid-loaded chitosan nanoparticles against ischemic stroke. Rejuvenation Res. 2020;23:284–92.
Jin L, Zhu Z, Hong L, Qian Z, Wang F, Mao Z. ROS-responsive 18β-glycyrrhetic acid-conjugated polymeric nanoparticles mediate neuroprotection in ischemic stroke through HMGB1 inhibition and microglia polarization regulation. Bioact Mater. 2023;19:38–49.
Zhao T, He F, Zhao K, Yuxia L, Li H, Liu X, et al. A triple-targeted rutin-based self-assembled delivery vector for treating ischemic stroke by vascular normalization and anti-inflammation via ACE2/Ang1-7 signaling. ACS Cent Sci. 2023;9:1180–99.
Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9:1–15.
Xiao X, Gao Y, Liu S, Wang M, Zhong M, Wang J, et al. A “Nano-Courier” for precise delivery of acetylcholine and melatonin by C5a-targeted aptamers effectively attenuates reperfusion injury of ischemic stroke. Adv Funct Mater. 2023;33:1–16.
Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13:1272–83.
Mu Q, Yao K, Syeda MZ, Zhang M, Cheng Q, Zhang Y, et al. Ligustrazine nanoparticle hitchhiking on neutrophils for enhanced therapy of cerebral ischemia-reperfusion injury. Adv Sci. 2023;10:1–10.
Pan J, Wang Z, Huang X, Xue J, Zhang S, Guo X, et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv Mater. 2023;35:1–12.
Wang Y, Wang Y, Li S, Cui Y, Liang X, Shan J, et al. Functionalized nanoparticles with monocyte membranes and rapamycin achieve synergistic chemoimmunotherapy for reperfusion-induced injury in ischemic stroke. J Nanobiotechnol. 2021;19:1–13.
Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42:259–305.
Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15.
Szabó C. Mechanisms of cell necrosis. Crit Care Med. 2005;33:S530–4.
Dou C, Zhang Y, Zhang L, Qin C. Autophagy and autophagy-related molecules in neurodegenerative diseases. Animal Model Exp Med. 2023;6:10–7.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12.
Kanamori H, Naruse G, Yoshida A, Minatoguchi S, Watanabe T, Kawaguchi T, et al. Morphological characteristics in diabetic cardiomyopathy associated with autophagy. J Cardiol. 2021;77:30–40.
Taatjes DJ, Sobel BE, Budd RC. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129:33–43.
Pereira WO, Amarante-Mendes GP. Apoptosis: a programme of cell death or cell disposal? Scand J Immunol. 2011;73:401–7.
Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24:687–702.
Goldenstein BL, Floriddia EM. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63:1469–82.
Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–42.
Luo ML, Pan L, Wang L, Wang HY, Li S, Long ZY, et al. Transplantation of NSCs promotes the recovery of cognitive functions by regulating neurotransmitters in rats with traumatic brain injury. Neurochem Res. 2019;44:2765–75.
Jiang XC, Xiang JJ, Wu HH, Zhang TY, Zhang DP, Xu QH, et al. neural stem cells transfected with reactive oxygen species-responsive polyplexes for effective treatment of ischemic stroke. Adv Mater. 2019;31:1–8.
Ruan H, Li Y, Wang C, Jiang Y, Han Y, Li Y, et al. Click chemistry extracellular vesicle/peptide/chemokine nanocarriers for treating central nervous system injuries. Acta Pharm Sin B. 2023;13:2202–18.
Deng L, Zhang F, Wu Y, Luo J, Mao X, Long L, et al. RGD-modified nanocarrier-mediated targeted delivery of HIF-1α-AA plasmid DNA to cerebrovascular endothelial cells for ischemic stroke treatment. ACS Biomater Sci Eng. 2019;5:6254–64.
Oh J, Lee J, Piao C, Jeong JH, Lee M. A self-assembled DNA-nanoparticle with a targeting peptide for hypoxia-inducible gene therapy of ischemic stroke. Biomater Sci. 2019;7:2174–90.
Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–39.
Zhou M, Zhang T, Zhang B, Zhang X, Gao S, Zhang T, et al. A DNA nanostructure-based neuroprotectant against neuronal apoptosis via inhibiting toll-like receptor 2 signaling pathway in acute ischemic stroke. ACS Nano. 2022;16:1456–70.
Lu L, Wang Y, Zhang F, Chen M, Lin B, Duan X, et al. MRI-visible siRNA nanomedicine directing neuronal differentiation of neural stem cells in stroke. Adv Funct Mater. 2018;28:1–12.
Lin B, Lu L, Wang Y, Zhang Q, Wang Z, Cheng G, et al. Nanomedicine directs neuronal differentiation of neural stem cells via silencing long noncoding RNA for stroke therapy. Nano Lett. 2021;21:806–15.
Yang H, Han M, Li J, Ke H, Kong Y, Wang W, et al. Delivery of miRNAs through metal-organic framework nanoparticles for assisting neural stem cell therapy for ischemic stroke. ACS Nano. 2022;16:14503–16.
Yuan J, Li L, Yang Q, Ran H, Wang J, Hu K, et al. Targeted treatment of ischemic stroke by bioactive nanoparticle-derived reactive oxygen species responsive and inflammation-resolving nanotherapies. ACS Nano. 2021;15:16076–94.
Liu S, Xu J, Liu Y, You Y, Xie L, Tong S, et al. Neutrophil-biomimetic “Nanobuffer” for remodeling the microenvironment in the infarct core and protecting neurons in the penumbra via neutralization of detrimental factors to treat ischemic stroke. ACS Appl Mater Interfaces. 2022;14:27743–61.
Cai R, Chen C. The crown and the scepter: roles of the protein corona in nanomedicine. Adv Mater. 2019;31:1–13.
Bonferoni MC, Eassu G, Gavini E, Sorrenti M, Catenacci L, Giunchedi P. Nose-to-brain delivery of antioxidants as a potential tool for the therapy of neurological diseases. Pharmaceutics. 2020;12:1–21.
Acknowledgements
W. Liu and L. Liu contributed equally to this work. This study was supported by the National Nature Science Foundation of China (No. 52103170), National Key Research and Development Project of China (No. 2018YFC1315200), the National Research and Development Program of China (No. 2017YFC1307701) and the traditional Chinese Medicine Science and Technology Project of Qingdao (No. 2022-zyyz07).
Funding
The National Nature Science Foundation of China, 52103170, National Key Research and Development Project of China, 2018YFC1315200, the National Research and Development Program of China, 2017YFC1307701, the traditional Chinese Medicine Science and Technology Project of Qingdao,2022-zyyz07
Author information
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, W., Liu, L., Li, H. et al. Targeted pathophysiological treatment of ischemic stroke using nanoparticle-based drug delivery system. J Nanobiotechnol 22, 499 (2024). https://doi.org/10.1186/s12951-024-02772-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02772-2