Abstract
Background
Anti-programmed death 1/anti-programmed death ligand 1 (PD-1/PD-L1) combined with radiotherapy (RT) has a synergistic effect on systemic tumor control. A dissociated response (DR), characterized by some lesions shrinking and others growing, has been recognized with immune checkpoint inhibitor (ICI) monotherapy or combination therapy. The objective of this study was to assess the frequency and clinical benefit of DR in patients with advanced metastatic solid tumors receiving PD-1 inhibitors in combination with RT.
Methods
We conducted a single-center retrospective analysis of patients with advanced metastatic solid tumors receiving PD-1 inhibitor combined with RT at the Department of Radiotherapy & Oncology, The Second People’s Hospital Affiliated with Soochow University. Treatment response was assessed for each measurable lesion according to the Response Evaluation Criteria in Solid Tumours ( RECIST) v 1.1 guidelines. Patterns of response are divided into four groups: (1) DR, (2) uniform response, (3) uniform progression, and (4) only stable lesions. The overall survival (OS) of different groups was compared using Kaplan–Meier methods and log-rank tests.
Results
Between March 2019 and July 2022, 93 patients were included. The median follow-up was 10.5 months (95% CI 8.8–12.1). The most common tumor types were lung cancer (19.8%), colorectal adenocarcinoma (17.2%), and esophageal cancer (10.8%). DR was observed in 22 (23.7%) patients. The uniform progression and DR are two different patterns of progression. After confirming progression, the overall survival of patients with DR was significantly longer than that of patients with uniform progression (9.9 months (95%CI 5.7-14.1) vs. 4.2 months (95%CI 1.9-6.5), P = 0.028). Compared with DR patients who did not continue PD-1 inhibitor combined with RT or PD-1 inhibitor monotherapy (n = 12), DR patients who continued treatment (n = 10) had significantly longer OS (15.7 (95%CI 3.5-27.9) vs 8.2 (95%CI 5.6-10.8) months, P = 0.035).
Conclusions
DR is not uncommon (23.7%) in patients with advanced metastatic solid tumors treated with PD-1 inhibitors combined with RT and shows a relatively favorable prognosis. Some patients with DR may benefit from continued PD-1 inhibitor therapy in combination with RT or PD-1 inhibitor monotherapy and may have longer OS.
Similar content being viewed by others
Introduction
In recent years, based on the breakthrough of tumor immunobiology, the development of tumor immunotherapy has opened a new chapter for the treatment of malignant tumors. Immunotherapy does not target the tumor cells themselves, but overcomes the immunosuppression caused by the tumor and its microenvironment, enhances the immunogenicity of tumor antigens, stimulates and improves anti-tumor immune response, and enables the immune system to target and kill cancer cells [1, 2]. Immune checkpoint inhibitors (ICIs), including anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4), PD-1, and PD-L1 antibodies, have dramatically changed the paradigm of oncology treatment and its assessment, achieving significant therapeutic outcomes in major types of advanced solid tumors [3]. However, many patients do not respond or respond only briefly to PD-1/PD-L1 monotherapies due to immunosuppressive factors in their bodies [4,5,6,7]. There is growing evidence that radiotherapy (RT) can both stimulate local and systemic immunostimulatory effects, which can be synergistic with immunotherapy in systemic tumor control. More and more clinical studies have demonstrated the potential synergistic effect of PD-1/PD-L1 blocking therapy and radiotherapy in patients with advanced cancer [8,9,10].
Due to ICIs’ unique mechanism of action [11], the tumor response pattern of ICI monotherapy or combination therapy may be different from that of conventional cytotoxic chemotherapy or targeted therapy (remission, stabilization, progression), such as pseudo-progressive disease (PsPD), DR, delayed response (DeR), hyperprogressive disease (HPD), and more recently fast progressive disease (FastPD) [12,13,14,15,16,17,18]. These atypical radiological response patterns do not fully conform to the RECIST v 1.1 guidelines and are relevant to clinical treatment decisions. DR is simply defined as an increase in the size of some lesions with shrinkage of others [19]. In recent years, the definition of DR is more detailed and specific, but there is still a lack of unity. This atypical response confused stopping or continuing ICI therapy.
To date, there is limited data on the incidence of DR in ICIs combined with RT, and its clinical significance is not fully understood [20]. Therefore, in this study, we aim to evaluate the occurrence of DR in patients with advanced metastatic solid tumors treated with PD-1 inhibitors combined with radiotherapy and its correlation with prognosis, to provide a reference for clinical treatment.
Materials and methods
Patients
We retrospectively analyzed patients with advanced metastatic solid tumors who received PD-1 inhibitors combined with RT in the Department of Radiotherapy & Oncology, The Second People’s Hospital Affiliated with Soochow University, from March 2019 to July 2022. The cutoff date for data collection was December 31, 2022. All patients were given stereotactic body radiotherapy (SBRT) or hypofractionated radiotherapy (HFRT; 5 Gy or 8 Gy *3), and anti-PD-1 antibody (200 mg per body) was injected intravenously within 1 week after radiotherapy. Clinically assessed patients with the stable disease continue to be treated with PD-1 inhibitors as maintenance monotherapy until clinical or radiological disease progression or unacceptable toxicity. Inclusion criteria are (1) patients with advanced metastatic solid tumor and age ≥ 18 years and (2) the score of the Eastern Cooperative Oncology Group performance status (ECOG ps) ≤ 3. Exclusion criteria are (1) combined with other therapies, including chemotherapy, targeted therapy, antiangiogenic therapy, etc.; (2) CT/MRI was not available at baseline (within 28 days before treatment) and follow-up to evaluate efficacy; (3) without measurable lesions at baseline; and (4) receiving PD-1 inhibitor combined with RT < 2 cycles. This study was approved by the Medical Ethics Committee of the hospital. Due to the retrospective study design, no written informed consent was required.
Clinical variables
Patients’ age, gender, primary tumor, ECOG status, number of previous systemic treatments, and number of organs involved in metastasis were collected from the electronic medical record (EMR). OS was defined as the time from the start of the first cycle of the PD-1 inhibitors combined with RT to death or the last follow-up. A durable clinical benefit was defined as treatment continuation over 6 months [21].
Tumor assessment
All patients were assessed every 6–8 weeks, and the relative diameter change of each non-irradiated measurable lesion between baseline and follow-up was evaluated. According to RECIST 1.1 criteria, measurable lesions were defined as non-lymph node lesions ≥ 10 mm in the long axis and lymph node lesions ≥ 15 mm in the short axis. A responding lesion was defined by a decrease in lesion size of > 30%. A progressive lesion was defined by an increase in lesion size of > 20% and the diameter increased by at least 5 mm. Patterns of tumor response are divided into four groups: (1) DR: ① the presence of both progressive and responding lesions and ② only responding lesions, but new lesions or unmeasurable lesions significantly worsened (irrespective of stable lesions); (2) uniform response: only responding lesions with no new lesions or unmeasurable lesions with significant deterioration (irrespective of stable lesions); (3) uniform progression: only progressive lesions (irrespective of stable lesions); and (4) only stable lesions. All radiological images were evaluated independently by two radiologists (8 and 14 years of experience in oncologic imaging), and in cases of disagreement, images were re-examined until a consensus was reached.
Statistical analysis
IBM SPSS (Armonk, New York) statistics version 26.0 was used for statistical analyses. Continuous variables with normal distribution were represented by mean ± standard deviation and analyzed by the Student’s t-test. Categorical variables were described by n (%) and compared using chi-square tests or Fisher exact tests. OS were generated using the Kaplan–Meier method and compared using a log-rank test. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
Patient characteristics
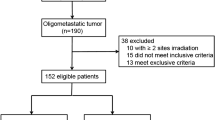
A total of 93 patients were enrolled (Fig. 1). The median follow-up was 10.5 months (95% CI 8.8–12.1). The mean age is 63 years (range: 31–87), 53.8% (50/93) were male, 78.5% (73/93) had ECOG PS of 2 or 3, 22.6% (21/93) had received 3 or more systemic therapies before treatment, and 26.9% (25/93) had 3 or more metastatic organs. Primary tumor types are lung cancer (19.8%), colorectal adenocarcinoma (17.2%), esophageal cancer (10.8%), stomach (7.5%), and cervical cancer (7.5%). 81.8% of patients had one or two irradiated sites (range: 1–5). 29.0% (27/93) showed a uniform response, 23.7% (22/93) showed DR, 25.8% (24/93) showed uniform progression, and 21.5 (20/93) presented only stable lesions. Patients’ characteristics are summarized in Table 1.
Dissociated response
DR with the combination of PD-1 inhibitors and radiotherapy occurred in 23.70% (22/93) of patients (Fig. 2). Female patients in the DR were significantly more than those in the non-DR group (P = 0.018), and there was no significant difference in other clinical characteristics (Table 1). DR appeared between 7 and 38 weeks after the start of treatment. At DR diagnosis, 9.1% (2/22) patients showed RECIST-defined PR, 54.5% (12/22) patients showed RECIST-defined SD, and 36.4% (8/22) patients showed RECIST-defined PD. 54.5% (12 /22) of DR patients chose to continue PD-1 inhibitor combined with radiotherapy or PD-1 inhibitor monotherapy, of which 11 patients achieved a durable clinical benefit. Characteristics of DR patients are represented in Table 2.
Representative radiological data of patients with dissociated responses. A 67-year-old man with advanced lung adenocarcinoma. He started PD-1 inhibitors combined with radiotherapy as a fifth-line therapy. The radiation site was a metastatic lesion of the right iliac crest. After 22 weeks of treatment, his pulmonary metastasis lesions (the largest: 25 mm) reduced in size (c, d), although two new metastatic lymph nodes (the larger: 43 mm) in the mediastinum were detected on the CT scan (a, b); therefore, DR was diagnosed
Survival analysis
The median OS for DR patients was 13.5 (95% CI 8.1–18.9) months. Regarding the other subgroups including uniform response, only stable lesions, and uniform progression, the median OS was 27.7 (95% CI 16.9–38.5), 17.7 (95% CI 14.4–21.0), and 5.9 (95% CI 4.0–7.8) months, respectively. Patients with DR had significantly longer OS than patients who showed a uniform progression (P = 0.012). There was no significant difference in OS between patients exhibiting a uniform response and those exhibiting only stable lesions (P = 0.285), or between patients exhibiting a uniform response and those exhibiting DR (P = 0.088). The uniform progression and DR are two different patterns of progression. After confirming progression, the overall survival of patients with DR was significantly longer than that of patients with uniform progression (9.9 months (95% CI 5.7–14.1) vs. 4.2 months (95% CI 1.9–6.5), P = 0.028).
Patients with DR who continued PD-1 inhibitors in combination with RT or PD-1 inhibitor monotherapy (n = 12) experienced significantly prolonged overall survival (15.7 (95% CI 3.5–27.9) vs. 8.2 (95% CI 5.6–10.8) months, P = 0.035) compared with patients who did not continue PD-1 inhibitors in combination with radiotherapy or PD-1 inhibitor monotherapy (n = 10) (Fig. 3a–c).
a Kaplan–Meier survival curves showing the overall survival stratified by tumor response. (A) Uniform response; (B) dissociated response; (C) only stable lesions; (D) uniform progression. b Kaplan–Meier curves of overall survival (OS) after confirming progression stratified by different patterns of progression. (A) Dissociated response; (B) uniform progression. c Kaplan–Meier curves of overall survival (OS) stratified by continued PD-1 inhibitors in combination with RT or PD-1 inhibitor monotherapy after DR diagnosis. (A) PD-1 + RT/PD-1 therapy after DR diagnosis; (B) non-PD-1 + RT/PD-1 therapy after DR diagnosis
Discussion
DR corresponds to mixed radiological or heterogeneous patterns of response at the same time point [22]. In this retrospective study, we analyzed the response of all measurable lesions to PD-1 inhibitors combined with RT in patients with advanced metastatic solid tumors at the initial CT/MRI. DR was not uncommon in our cohort, 23.7% (n = 22).
The incidence of this atypical reaction is unknown. Depending on the definition, the incidence of DR varied between 3.3 and 47.8% in different histological subtypes [23]. However, studies on DR in immunotherapy combined with radiotherapy are rare. Sun et al. [24] retrospectively analyzed the first follow-up CT images (median time: 2.8 months, IQR (2.0–3.4)) of six independent IORT clinical studies of patients with advanced solid tumors receiving immunotherapy combined with radiotherapy and found that 12.8% of patients presented with DR. In our study, the incidence of DR was significantly higher than the results of the study. We believe there are two reasons for this. First, the incidence of DR in our cohort was assessed at every follow-up image after treatment, rather than just at the first follow-up images. Second, the definition of DR is different. In addition to patients with both progressive lesions and responding lesions, patients with only responding lesions but with the appearance of new lesions or significant deterioration of unmeasurable lesions are also included in DR.
At the DR diagnosis, 2 patients showed RECIST-defined PR, 11 patients showed RECIST-defined SD, and 9 patients showed RECIST-defined PD in our study. The median OS of patients with DR was 13.5 months, which was significantly longer than that of patients with uniform progression (13.5 months vs. 5.9 months; P = 0.012). After confirming uniform progression and DR, the overall survival of patients with DR was also significantly higher than that of patients with uniform progression (9.9 months vs. 4.2 months, P = 0.028). Our results are consistent with previous studies [25] showing that RECIST 1.1 also does not adequately capture the kinetics and heterogeneity of the combination of an immune checkpoint inhibitor and radiotherapy. New criteria such as immune-related response criteria (irRC), immune-related RECIST (irRECIST), and immune RECIST (iRECIST) have been proposed to evaluate ICIs’ response and survival benefit [26,27,28]. However, these specific radiological criteria only target the PsPD and do not capture other response patterns, such as HPD and DR. Therefore, the future immunotherapy-adapted guidelines and criteria in solid cancer should not only solve the problem of PsPD but also solve the problem of atypical reactions such as DR.
The basic mechanism of DR is not clear. The combination of multiple factors may explain the underlying biological mechanism of the separation reaction. Firstly, genomic instability occurs during the clonal evolution of solid tumors, and different tumor clones may produce multiple coexisting metastases [29]. Secondly, microenvironment differences among metastases also cause heterogeneous responses [22]. Thirdly, tissue penetration differences of anticancer drugs may also be a potential cause. This pattern of response presents particular challenges for patient management. At present, there is no consensus on the clinical management of DR. Sato et al. [30] believe that the occurrence of DR does not always mean ICI resistance, and it should not be changed to other treatments prematurely. Humbert et al. [22] proposed that continuation of immunotherapy after DR can achieve a durable response. Zhou et al. [31] showed that patients with advanced NSCLC who continued ICI treatment post-DR derived apparent OS benefit than discontinuing counterpart. In our cohort, there was no specificity between the response and progressive sites in DR patients. 54.5% (12/22) of DR patients chose to continue PD-1 inhibitor combined with radiotherapy or PD-1 inhibitor monotherapy and experienced significantly prolonged overall survival compared with patients who did not continue. Therefore, it is a possible treatment option to continue ICI monotherapy or combined radiotherapy for DR patients with stable clinical conditions after comprehensively considering the degree of disease progression, patient status, and risk of immune-related adverse events.
Different from previous studies, women in our cohort had a significantly higher probability of developing DR than men (P = 0.018), which required us to verify in a larger sample. Our study may contribute to the development of appropriate management protocols for patients who develop DR during ICIs combined with radiotherapy. However, the study has some limitations. First, this is a retrospective study. Second, the heterogeneity of population and tumor may also lead to bias. Third, a small number of patients from a single institution were included. Therefore, a larger sample study is needed to further confirm the reliability of our results.
Conclusion
DR is not uncommon in patients with advanced metastatic solid tumors treated with PD-1 inhibitors combined with radiotherapy and shows a relatively favorable prognosis. RECIST 1.1 does not adequately capture its dynamics and heterogeneity, which may underestimate the survival benefit in the combination treatment population. In patients who present with DR, continuing with a PD-1 inhibitor in combination with radiotherapy or PD-1 inhibitor monotherapy may be beneficial and may enable patients to achieve longer OS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- RT:
-
Radiotherapy
- DR:
-
Dissociated response
- OS:
-
Overall survival
- ICIs:
-
Immune checkpoint inhibitors
- CTLA-4:
-
Anti-cytotoxic T-lymphocyte antigen-4
- PsPD:
-
Pseudo-progressive disease
- DeR:
-
Delayed response
- HPD:
-
Hyperprogressive disease
- FastPD:
-
Fast progressive disease
- RECIST 1.1:
-
The Response Evaluation Criteria in Solid Tumours v 1.1 guidelines
- SBRT:
-
Stereotactic body radiotherapy
- HFRT:
-
Hypofractionated radiotherapy
- ECOG ps:
-
The Eastern Cooperative Oncology Group performance status
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- EMR:
-
The electronic medical record
- IQR:
-
Interquartile range
- IORT:
-
Radiotherapy with immunooncology
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- irRC:
-
Immune-related response criteria
- irRECIST:
-
Immune-related RECIST
- iRECIST:
-
Immune RECIST
- NSCLC:
-
Non-small cell lung cancer
References
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21.
Chmielewski M, Hombach AA, Abken H. Antigen-specific T-cell activation independently of the MHC: chimeric antigen receptor-redirected T cells. Front Immunol. 2013;4:371.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Nagasaki J, Ishino T, Togashi Y. Mechanisms of resistance to immune checkpoint inhibitors. Cancer Sci. 2022;113:3303–12.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94.
Sun JY, Zhang D, Wu S, Xu M, Zhou X, Lu XJ, Ji J. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. 2020;8:35.
Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, Bajwa R, Momtaz P, Callahan MK, Wolchok JD, Postow MA, Chapman PB. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. 2020;38:1655–63.
Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, Knepley C, Mutale F, Cohen RB, Langer CJ. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: a phase 2 trial. JAMA Oncol. 2019;5:1283–90.
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903.
Kong Y, Zhao X, Xu M, Pan J, Ma Y, Zou L, Peng Q, Zhang J, Su C, Xu Z, Zhou W, Peng Y, Yang J, Zhou C, Li Y, Guo Q, Chen G, Wu H, Xing P, Zhang L. PD-1 inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front Immunol. 2022;13: 952066.
Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, Goel S, Bedard P, Le Tourneau C. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385–96.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone , Wolchok JD. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016; 34:1510–1517.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Abramson RG, McGhee CR, Lakomkin N, Arteaga CL. Pitfalls in RECIST data extraction for clinical trials: beyond the basics. Acad Radiol. 2015;22:779–86.
Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16:655–70.
Chai LF, Prince E, Pillarisetty VG, Katz SC. Challenges in assessing solid tumor responses to immunotherapy. Cancer Gene Ther. 2020;27:528–38.
Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, Ferte C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–62.
Nishino M, Giobbie-Hurder A, Manos MP, Bailey N, Buchbinder EI, Ott PA, Ramaiya NH, Hodi FS. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res. 2017;23:4671–9.
Nishino M, Dahlberg SE, Adeni AE, Lydon CA, Hatabu H, Janne PA, Hodi FS, Awad MM. Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res. 2017;23:5737–44.
Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, Borchiellini D, Benisvy D, Ouvrier MJ, Zwarthoed C, Schiazza A, Ilie M, Ghalloussi H, Koulibaly PM, Darcourt J, Otto J. (18)FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. 2020;47:1158–67.
Humbert O, Chardin D. Dissociated response in metastatic cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol. 2020;10: 566297.
Guan Y, Feng D, Yin B, Li K, Wang J. Immune-related dissociated response as a specific atypical response pattern in solid tumors with immune checkpoint blockade. Ther Adv Med Oncol. 2022;14:17588359221096876.
Sun R, Sundahl N, Hecht M, Putz F, Lancia A, Rouyar A, et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J Immunother Cancer. 2020;8(2):e001429.
Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3.
Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, Beyer U, Rhee I, Fine G, Winslow N, Chen DS, Wolchok JD. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–8.
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–43.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz L.H, Mandrekar S, Lin NU, Litiere S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, Vries de EGE, R.w. group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152.
Tozuka T, Kitazono S, Sakamoto H, Yoshida H, Amino Y, Uematsu S, Yoshizawa T, Hasegawa T, Uchibori K, Yanagitani N, Horiike A, Horai T, Seike M, Gemma A, Nishio M. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer. 2020;20:207.
Sato Y, Morimoto T, Hara S, Nagata K, Hosoya K, Nakagawa A, Tachikawa R, Tomii K. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs. 2021;39:1170–8.
Zhou H, Sun Y, Xiu W, Han J, Zhong L, Suo J, Wei H, Wang Y, Zhu J. Overall survival benefit of continuing immune checkpoint inhibitors treatment post dissociated response in patients with advanced lung cancer. J Cancer Res Clin Oncol. 2020;146:2979–88.
Acknowledgements
The authors would like to thank all people involved in this work.
Funding
This study was supported by China Medical and Health Development Foundation (Grant no. C202212-006; C202212-0014). This study was supported by the Jiangsu Vocational College of Medicine Foundation (Grant no. 20229DT04; 20229DT11). This study was also supported by the Yancheng Science and Technology Bureau (Grant no. YCBK202222).
Author information
Authors and Affiliations
Contributions
Junkang Shen and Chen Chen: conception and design of the work; acquisition and interpretation of data; revision of the manuscript critically for important intellectual content and scientifc integrity. Qin Yu and Haiyan Zhang: drafting of the manuscript and analysis of the patient’s data. Yan Song and Jin Chen: clinical management of the patient and provision of patient’s data. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study received approval (JD—HG—2022—49) from the Ethics Committee of the Second Affiliated Hospital of Suzhou University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Q., Zhang, H., Song, Y. et al. Dissociated response to PD-1 inhibitors combined with radiotherapy in patients with advanced metastatic solid tumors: a single-center experience. World J Surg Onc 21, 228 (2023). https://doi.org/10.1186/s12957-023-03122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03122-6