Abstract
Background
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for 90% of cases worldwide and a significant contributor to cancer-related deaths. This study comprehensively compares the safety and efficacy of laparoscopic liver resection (LLR) versus laparoscopic or percutaneous radiofrequency ablation (LRFA or PRFA) in patients with early and small HCC.
Methods
We systematically searched Cochrane Library, PubMed, Scopus, and Web of Science databases to include studies comparing LLR versus LRFA or PRFA in patients with early HCC meets the Milan criteria (defined as solitary nodule < 5 cm or three nodules ≤ 3 cm with no extrahepatic spread or vascular invasion). Pooled results were examined for overall survival, disease-free survival, recurrence-free survival, local, intrahepatic and extrahepatic recurrence rates, and complications. We conducted subgroup analyses based on the type of RFA. Meta-regression analyzed the association between overall survival, local recurrence, and various factors. The quality of the included studies was assessed using the Newcastle–Ottawa Scale. We analyzed the data using the R (v.4.3.0) programming language and the “meta” package of RStudio software.
Results
We included 19 observational studies, compromising 3756 patients. LLR showed higher 5-year overall survival compared to RFA (RR = 1.17, 95% CI [1.06, 1.3], P > 0.01). Our subgroup analysis showed that LLR had higher 5-year survival than PRFA (RR = 1.15, 95% CI [1.02, 1.31], P = 0.03); however, there was no significant difference between LLR and LRFA (RR = 1.26, 95% CI [0.98, 1.63], P = 0.07). LLR was associated with higher disease-free survival) RR = 1.19, 95% CI [1.05, 1.35], P < 0.01; RR = 1.61, 95% CI [1.31, 1.98], P < 0.01(and recurrence-free survival) RR = 1.21, 95% CI [1.09, 1.35], P < 0.01; RR = 1.45, 95% CI [1.15, 1.84], P < 0.01(at 1 and 3 years. LLR was associated with lower local (RR = 0.28, 95% CI [0.16, 0.47], P < 0.01) and intrahepatic recurrence (RR = 0.7, 95% CI [0.5, 0.97], P = 0.03) than RFA. However, complications were significantly higher with LLR (RR = 2.01, 95% CI [1.51, 2.68], P < 0.01). Our meta-regression analysis showed that younger patients had higher risk for local recurrence (P = 0.008), while age wasn’t significantly linked to overall survival (P = 0.25). Other covariates like total bilirubin, alpha-fetoprotein levels, and tumor size also showed no significant associations with either overall survival or local recurrence.
Conclusion
LLR offers improved long-term outcomes and lower recurrence rates than PRFA. However, no significant distinctions were observed between LRFA and LLR in overall survival, recurrence-free survival, and local recurrence. More robust well-designed RCTs are essential to validate our findings.
Similar content being viewed by others
Introduction
Liver cancer poses a global health challenge, with expanding incidence worldwide. It is expected that one million individuals annually will face liver cancer by 2025 [1]. Hepatocellular carcinoma (HCC) dominates, accounting for 90% of cases of liver cancers. It is the fifth most common cancer worldwide and the second major cause of cancer-related deaths due to its aggressiveness [2]. In East Asia and Africa, HCC exhibits notable prevalence and mortality rates, with China at the forefront, housing 466,000 HCC patients – accounting for 55% of global cases – among the yearly count of 854,000 new cases [3, 4]. Additionally, the emergence of increasing cases is evident in various regions of Europe and the USA [1].
Chronic liver disease is the predominant cause of HCC, contributing to 90% of cases. Cirrhosis is the most significant risk factor for HCC, regardless of its etiology. HCC is now the leading cause of death in cirrhotic patients, with an annual occurrence rate of 1–6%. HCC risk factors involve persistent alcohol use, diabetes, and non-alcoholic steatohepatitis related to obesity and HBV or HCV infection [1].
The Barcelona Clinic Liver Cancer (BCLC) algorithm outlines diverse treatment choices for HCC, spanning liver transplantation, surgical resection, and ablation [5]. Due to donor scarcity, liver transplantation is seldom the primary choice. In addition, the effectiveness of surgery and ablation remains a topic of ongoing discussion.
Open hepatic resection is a key curative approach for HCC; however, it presents certain risks and can negatively impact liver function. As a result, this method may not be ideal for patients with severe cirrhosis [6]. Radiofrequency ablation (RFA) emerges as an alternative for small HCC cases, noted for its minimally invasive nature and simplicity. In fact, only 30% of HCC patients are considered good candidates for hepatic resection, underscoring the importance of RFA. Studies indicate that RFA produces comparable outcomes to open resection but with shorter hospital stays and fewer complications. Therefore, both RFA and hepatectomy are recommended for treating early-stage HCC [7].
Recent developments in laparoscopic technology expand the treatment options for HCC, with laparoscopic liver resection (LLR) and laparoscopic radiofrequency ablation (LRFA) gaining traction, especially for cases with small HCC. LLR combines the strengths of RFA and open resection to reduce recurrence risks [8]. While percutaneous RFA is widely used for early-stage HCC, its limitations arise from tumor visibility and positioning. LRFA offers a solution for challenging cases, like subcapsular tumors, where percutaneous methods face difficulties. Previous research emphasizes LRFA's effectiveness and safety for subcapsular HCCs [9,10,11,12,13].
The debate over the most effective and safe treatment for hepatocellular carcinoma is ongoing [9, 14, 15]. Based on previous research, there is a recognized need for a comprehensive assessment of the effectiveness and safety of LLR, LRFA, and PRFA in patients with early HCC. While previous meta-analyses [16,17,18,19] have made valuable contributions, they have been limited in study numbers and scope, potentially missing essential insights. For example, Mou‐Bo Si et al. [16], Shan Jin et al. [17], and Xiaocheng Li et al. [20] included 6, 7, and 10 studies, respectively. In contrast, Zhijun Li et al. [19] adopted a more focused approach, scrutinizing Chinese literature and solely including studies from China, with a total of 19 articles (3 in English and 16 in Chinese). However, new studies have emerged in the English literature, providing an opportunity to bolster the impact of the meta-analysis. Surprisingly, previous meta-analyses have yet to concentrate on comparing LLR and LRFA.
Given the advancements in medical knowledge and techniques, an updated systematic review and meta-analysis is essential. This updated analysis aims to fill crucial gaps by directly comparing LLR and laparoscopic/percutaneous RFA and giving the medical community scientifically informed insights to facilitate enhanced clinical decision-making.
Methods
Our methodology and findings followed systematic review and meta-analysis guidelines, including PRISMA 2020 [21] and the Cochrane Handbook [22]. Transparency was ensured by registering our protocol on PROSPERO with reference “CRD42023436948.”
Literature search
We performed an extensive search across various databases, including the Cochrane Library, PubMed, Web of Science, and Scopus. Our search spanned from the databases' earliest records to July 31, 2023. We used the following key terms: laparoscopic liver resection, radiofrequency ablation, and hepatocellular carcinoma. We provide our detailed search strategy in the Supplementary file.
Eligibility criteria and study selection
Two authors (B.E. and N.Y.) screened the article to determine their eligibility for our study focusing on RCTs, non-randomized comparative studies, and observational studies (prospective and retrospective cohorts). Initial screening involved titles and abstracts, followed by a detailed review of chosen study texts.
We included studies comparing LLR versus RFA (percutaneous or laparoscopic) in patients with early-stage HCC meets the Milan criteria (defined as solitary nodule < 5 cm or three nodules ≤ 3 cm with no extrahepatic spread or vascular invasion) [23] or meets University of California San Francisco criteria (defined as a solitary tumor smaller than 6.5 cm or up to three nodules, each less than 4.5 cm in diameter) [24]. Furthermore, eligible patients should exhibit liver function classified as Child–Pugh class A or B (less than 10% fall into the Child–Pugh class C).
Our primary investigation centered on direct comparisons of clinical effectiveness, evaluating parameters such as overall survival, recurrence-free survival rate, disease-free survival rate, local recurrence, intrahepatic recurrence, and extrahepatic recurrence. In terms of safety assessments, we examined the overall incidence of all complications, major complications rated as grade 3 or above, 90-day mortality, 30-day mortality, as well as hospital stay duration. Discrepancies were resolved by a third author.
Exclusion criteria
We excluded case series, case reports, editorials, cross-sectional and non-human studies, and. Moreover, studies exploring alternative treatments like trans-arterial chemoembolization and percutaneous ethanol injection were excluded. Finally, non-English studies and those with unreliable data were also excluded.
Quality assessment
Two independents’ authors (B.E and M.E) assessed the quality of included studies using the Newcastle–Ottawa Scale (NOS) [25], which covers the following domains selection, comparability, and outcomes. A top score of 9 is possible, with 7 or higher indicating high quality. Discrepancies were resolved through discussion or involving a third reviewer if necessary.
Data extraction and study outcomes
Two authors (N.Y AND B. E) used standardized method for data extraction in a predefined Excel sheet, covering study characteristics, patient descriptions, and outcomes of interest. Disagreements were resolved through discussion or consultation with the senior author. Pertinent data were gathered in a predefined Excel sheet, covering study characteristics, patient descriptions, and LLR and RFA outcomes for safety and efficacy. If any study reported their outcomes at different time points, we extracted the data at each timepoint separately, aiming to perform subgroup analysis to explore the change of this outcome overtime.
Outcome definition
This study rigorously assessed the effectiveness and safety of treatments, employing a comprehensive range of metrics. These measures encompassed overall survival (from treatment onset to death or latest follow-up), recurrence-free survival rate (proportion of patients without HCC recurrence), disease-free survival rate (proportion without disease), hospital stay (duration of patient admission for treatment and recovery), major (grade 3 or above) complications (complications significantly impacting postoperative progress, necessitating interventions), local recurrence (tumor reappearance within liver or nearby original site), intrahepatic recurrence (new tumor nodules or growth within liver separate from primary tumor or previously treated lesions), and extrahepatic recurrence (spread to distant organs).
Data synthesis and heterogeneity assessment
We conducted our analysis using the R (v.4.3.0) programming language and the “meta” package of RStudio software [26]. We computed the risk ratio (RR) for dichotomous outcomes using the “metabin” function; however, the “metacont” function was used to pool the standardized mean difference (SMD) for continuous outcomes. Given the substantial heterogeneity among the included studies, we preferred to use the random-effects model. We used the 95% confidence intervals (CI) for all outcomes. A p-value < 0.05 indicated significance; however, a chi-square P value < 0.10 indicated significant heterogeneity among the included studies. We performed subgroup analysis based on the time point of outcome assessment (i.e., at 1, 3, or 5 years). Also, we performed another subgroup analysis based on type of RFA (i.e., LRFA versus PRFA). In addition, we performed sensitivity analyses using the leave-one-out model to explore the effect of each individual study on our results. To assess publication bias, we employed funnel plots, Egger’s test, and trim-and-fill analysis [27]. Finally, we conducted meta-regression analyses to explore whether there was any significant association between the local recurrence and overall survival at 1 year with continuous covariates, such as the age, tumor size, total bilirubin, and alpha-fetoprotein [28].
Results
Literature search results
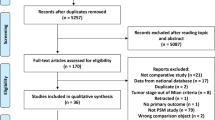
Our comprehensive search yielded 527 records. After removal of duplicates, only 334 records remained for the title and abstract screening. After which, 22 articles seemed eligible for the full-text screening. Finally, we included 19 observational studies in our systematic review and meta-analysis. Reviewing the reference list of all included studies did not retrieve any additional eligible studies. The PRISMA flow diagram is shown in Fig. 1.
Characteristics of individual studies
Our meta-analysis included 19 observational studies [15, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], compromising 3756 patients. Of which, only one study was prospective [44], while all remaining studies were retrospective [15, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43, 45, 46]. The included studies were conducted in five different countries: China (n = 7), Japan (n = 4), Korea (n = 3), Italy (n = 3), and Taiwan (n = 2). The follow-up duration ranged from one year in Wu 2020 [44] to about 17 years in Cheng 2022 [43]. All included studies used percutaneous RFA, except for Casaccia 2017 [15], Santambrogio 2017 [33], Tsukamoto 2019 [31], and Ko 2022 [39], which used laparoscopic RFA. According to the NOS, the quality of included studies ranged from six to nine points, indicating good to fair quality and low risk of bias in the included studies. Only one study scored nine [32]; however, 12 studies scored eight [15, 29, 31, 33,34,35,36,37, 39, 40, 42, 44, 45], four studies scored seven [30, 38, 41, 43], and three studies scored six [36, 46]. We summarized the included studies and their patients’ baseline characteristics in Table 1 and Supplementary Table 1 respectively.
Efficacy outcomes
Overall survival
Our pooled analysis showed that the overall survival rate at 1, 3, and 5 years was significantly higher with LLR compared to RFA (RR = 1.01, 95% CI [1, 1.02], P = 0.05; RR = 1.09, 95% CI [1.02, 1.16], P < 0.01; RR = 1.17, 95% CI [1.06, 1.3], P < 0.01, respectively). The pooled studies at 1 year were homogenous (I2 = 0%, P = 0.55). However, the pooled studies at 3 and 5 years were heterogenous (I2 = 83%, P < 0.01; I2 = 75%, P < 0.01, respectively) (Fig. 2). Heterogeneity at 3 and 5 years was not resolved by sensitivity analysis (Supplementary file Figs. S1 and S2, respectively).
Additionally, our subgroup analysis for 1-year overall survival based on RFA type found no significant difference between PRFA or LRFA and LLR (RR = 1.01, 95% CI [1, 1.02], P = 0.09; RR = 1.01, 95% CI [0.96, 1.07], P = 0.64, respectively), with homogeneity in both subgroups (I2 = 0%, P = 0.57; I2 = 46%, P = 0.14) (Supplementary file Fig. S3).
For 3-year survival, LLR significantly improved rates compared to PRFA (RR = 1.08, 95% CI [1, 1.16], P = 0.05), while no difference was seen between LRFA and LLR (RR = 1.13, 95% CI [0.96, 1.34], P = 0.14). Studies were heterogeneous in both subgroups (I2 = 84%, P < 0.01; I2 = 74%, P < 0.01) (Supplementary file Fig. S4). Heterogeneity in the laparoscopic subgroup resolved by excluding Santambrogio 2017 [33], but not resolved in the percutaneous subgroup (Supplementary file Figs. S5 and S6).
For 5-year survival, LLR significantly outperformed PRFA (RR = 1.15, 95% CI [1.02, 1.31], P = 0.03), but no difference was noted between LRFA and LLR (RR = 1.26, 95% CI [0.98, 1.63], P = 0.07). Heterogeneity was present in both subgroups (I2 = 77%, P < 0.01; I2 = 81%, P < 0.01) (Supplementary file Fig. S 7). Heterogeneity in the percutaneous subgroup resolved by excluding Liu 2022 [36] (Supplementary file Fig. S8), and in the laparoscopic subgroup by excluding Ko 2022 [39] (Supplementary file Fig. S9).
Finally, Meta-regression indicated no significant associations between 1-year overall survival and age (P = 0.25), total bilirubin level (P = 0.49), alpha-fetoprotein level (P = 0.2), tumor size within the range of 1.6 to 3.5 cm (P = 0.86) (Supplementary file Fig. S10).
Overall survival PSM
LLR significantly improved overall survival PSM at 3 years. However, no significant differences were observed between LLR and RFA in overall survival PSM at 1 and 5 years (RR = 1.1, 95% CI [1.03, 1.18], P < 0.01; RR = 1, 95% CI [0.98, 1.02], P = 0.99; RR = 1.06, 95% CI [0.86, 1.31], P = 0.6, respectively). While studies at 1 and 3 years were homogeneous, those at 5 years exhibited heterogeneity (I2 = 13%, P = 0.33; I2 = 28%, P = 0.22; I2 = 82%, P < 0.01, respectively) (Supplementary file Fig. S11). Heterogeneity at 5 years was not resolved by sensitivity analysis (Supplementary file Fig. S12).
Disease-free survival
Our analysis found higher disease-free survival rates with LLR at 1 and 3 years, but no significant difference between LLR and RFA at 5 years (RR = 1.19, 95% CI [1.05, 1.35], P < 0.01; RR = 1.61, 95% CI [1.31, 1.98], P < 0.01; RR = 1.61, 95% CI [0.98, 2.64], P = 0.06, respectively). Studies at 1, 3, and 5 years were heterogeneous (I2 = 69%, P < 0.01; I2 = 56%, P = 0.03; I2 = 81%, P < 0.01, respectively) (Fig. 3). Heterogeneity at 3 years improved by excluding Kim 2021 [45] (Supplementary file Fig. S13); however, sensitivity analysis did not resolve heterogeneity at 1 and 5 years (Supplementary file Figs. S14 and S15, respectively).
Disease-free survival PSM
LLR significantly improved disease-free survival PSM at 1 and 3 years. However, there was no significant difference between LLR and RFA in terms of disease-free survival rate at 5 years (RR = 1.37, 95% CI [1.09, 1.71], P < 0.01; RR = 1.99, 95% CI [1.24, 3.2], P < 0.01; RR = 2.27, 95% CI [0.78, 6.64], P = 0.13, respectively). Studies in all subgroups were heterogeneous (I2 = 74%, P = 0.02; I2 = 79%, P < 0.01; I2 = 92%, P < 0.01, respectively) (Supplementary file Fig. S16). Heterogeneity at 1 year improved by excluding Chong 2019 [41] (Supplementary file Fig. S17); however, sensitivity analysis did not resolve heterogeneity at 3 years (Supplementary file Fig. S18).
Recurrence-free survival
Our pooled analysis showed that compared to RFA, LLR was associated with higher recurrence-free survival rate at 1, 3, and 5 years (RR = 1.21, 95% CI [1.09, 1.35], P < 0.01; RR = 1.45, 95% CI [1.15, 1.84], P < 0.01; RR = 2, 95% CI [1.21, 3.33], P < 0.01, respectively). The pooled studies at 1, 3, and 5 were heterogenous (I2 = 77%, P < 0.01; I2 = 88%, P < 0.01; I2 = 91%, P < 0.01, respectively) (Fig. 4). Heterogeneity was not resolved by sensitivity analysis (Supplementary file Figs. S19, S20 and S21, respectively).
Our subgroup analysis based on RFA type revealed that LLR was linked to higher recurrence-free survival rates at 1 and 3 years compared to PRFA (RR = 1.24, 95% CI [1.09, 1.41], P < 0.01; RR = 1.63, 95% CI [1.29, 2.07], P < 0.01, respectively), but no significant difference was observed between LLR and LRFA (RR = 0.99, 95% CI [0.65, 1.51], P = 0.97; RR = 1.11, 95% CI [0.52, 2.38], P = 0.78, respectively). Heterogeneity was present in both PRFA and LRFA subgroups (I2 = 82%, P < 0.01; I2 = 86%, P < 0.01; I2 = 85%, P < 0.01; I2 = 93%, P < 0.01, respectively) (Supplementary file Figs. S22, S23). Heterogeneity in the percutaneous subgroup was partially resolved by omitting Lee 2020 [46] at 1 and 3 years (Supplementary file Figs. S24 and S25 respectively), but not resolved in the laparoscopic subgroup at 1 and 3 years (Supplementary file Figs. S26 and S27 respectively).
Regarding recurrence-free survival at 5 years, LLR was associated with significantly higher rates compared to PRFA (RR = 2.24, 95% CI [1.5, 3.34], P < 0.01), while no significant difference was found between LRFA and LLR (RR = 1.57, 95% CI [0.57, 4.33], P = 0.39). Both percutaneous and laparoscopic subgroups exhibited heterogeneity (I2 = 64%, P = 0.04; I2 = 94%, P < 0.01, respectively) (Supplementary file Fig. S28). Heterogeneity in the percutaneous subgroup was partly resolved by omitting Harada 2016 [40], but not resolved in the laparoscopic subgroup (Supplementary file Figs. S29 and S30 respectively).
Recurrence-free survival PSM
We found that LLR was associated with higher recurrence-free survival PSM at 1, 3, and 5 years (RR = 1.2, 95% CI [1.04, 1.38], P = 0.01; RR = 1, 49% CI [1.1, 2.02], P < 0.01; RR = 2.33, 95% CI [1.13, 4.79], P = 0.02, respectively). The pooled studies in all subgroups were heterogenous (I2 = 71%, P < 0.01; I2 = 80%, P < 0.01; I2 = 74%, P = 0.02, respectively) (Supplementary file Fig. S31). Heterogeneity at 1 and 5 years was best resolved by omitting Lee 2020 [46] and Harada 2016 [40], respectively (Supplementary file Figs. S32 and S33 respectively) However, heterogeneity at 3 years was not resolved by sensitivity analysis (Supplementary Fig. S34).
Local recurrence
The risk for local recurrence was significantly lower with LLR (RR = 0.28, 95% CI [0.16, 0.47], P < 0.01). The pooled studies were heterogenous (I2 = 65%, P < 0.01) (Fig. 5). Heterogeneity was best resolved by omitting Song 2015 [32] (Supplementary file Fig. S35). In addition, our subgroup analysis based on the type of RFA showed that the risk for local recurrence was significantly lower with LLR than with percutaneous RFA; however, there was no significant difference between LLR and laparoscopic RFA (RR = 0.28, 95% CI [0.16, 0.5], P < 0.01; RR = 0.16, 95% CI [0.01, 1.84], P = 0.65, respectively). The pooled studies were heterogenous in both subgroups (I2 = 70%, P < 0.01; I2 = 74%, P = 0.02, respectively) (Supplementary file Fig. S36). Heterogeneity in both subgroups was not resolved by sensitivity analysis (Supplementary file Figs. S37 and S38, respectively). Finally, the results of meta-regression indicated significant association between the risk for local recurrence and the age (P = 0.008) (Fig. 6). In contrast, there was no significant association between the risk for local recurrence and the tumor size (P = 0.07), alpha-fetoprotein level (P = 0.53) and total bilirubin level (P = 0.29) (Fig. 6).
Intrahepatic recurrence
The risk for intrahepatic recurrence was significantly lower with LLR (RR = 0.7, 95% CI [0.5, 0.97], P = 0.03). The pooled studies were heterogenous (I2 = 72%, P < 0.01) (Supplementary file Fig. S39). Heterogeneity was best resolved by omitting Chong 2019 [41] (Supplementary file Fig. S40).
Extrahepatic recurrence
There was no significant difference between LLR and RFA in terms of extrahepatic recurrence (RR = 1.41, 95% CI [0.62, 3.2], P = 0.41). The pooled studies were heterogenous (I2 = 0%, P = 0.83) (Supplementary file Fig. S41).
Duration of surgery
The duration of surgery was significantly higher with LLR (SMD = 2.78, 95% CI [1.38, 4. 18], P < 0.01). The pooled studies were heterogenous (I2 = 98%, P < 0.01) (Supplementary file Fig. S42). Heterogeneity was not resolved by sensitivity analysis (Supplementary file Fig. S43).
Incidence of blood transfusion during surgery
LLR was associated with higher incidence of blood transfusion compared to RFA (RR = 4.14, 95% CI [1.33, 12.88], P = 0.01). The pooled studies were homogenous (I2 = 42%, P = 0.14) (Supplementary file Fig. S44).
Safety outcomes
All complications
The risk for all complications was significantly higher with LLR (RR = 2.01, 95% CI [1.51, 2.68], P < 0.01). The pooled studies were homogenous (I2 = 36%, P = 0.1) (Supplementary file Fig. S45). Comprehensive details on complications have been incorporated into Table 1.
90-days mortality
The risk for 90-days mortality was significantly lower with LLR (RR = 0.54, 95% CI [0.36, 0. 81], P < 0.01). The pooled studies were homogenous (I2 = 0%, P = 0.9) (Supplementary file Fig. S46).
30-days mortality
The risk for 30-days mortality was significantly higher with LLR (RR = 3.42, 95% CI [1.5, 7. 79], P < 0.01). The pooled studies were homogenous (I2 = 0%, P = 0.39) (Supplementary file Fig. S47).
Major complications
The risk for major complications was significantly higher with LLR (RR = 2.02, 95% CI [1.26, 3. 24], P < 0.01). The pooled studies were homogenous (I2 = 0%, P = 0.83) (Supplementary file Fig. S48).
Duration of hospital stay
The duration of hospital stay was significantly higher with LLR (SMD = 1.14, 95% CI [0.66, 1. 62], P < 0.01). The pooled studies were heterogenous (I2 = 92%, P < 0.01) (Supplementary file Fig. S49). Heterogeneity was not resolved by sensitivity analysis (Supplementary file Fig. S50).
Publication bias
The funnel plots for the overall survival at 1, 3, and 5 years were symmetrical. This was confirmed by the insignificant results of Egger’s test (P = 0.7; P = 0.1; P = 0.98, respectively), indicating that there was no publication bias in terms of overall survival at 1, 3, and 5 years. In contrast, visual inspection of the funnel plot for the local recurrence showed asymmetry, which was confirmed by the significant results of Egger’s test (P = 0.03) (Supplementary file Fig. S51). Finally, the trim and fill analysis revealed that adding five studies showed that LLR was associated with lower risk for local recurrence (RR = 0.41, 95% CI [0.26; 0.64], P < 0.01), which was consistent with our findings (Supplementary Fig. S52).
Discussion
Summary of the findings
In our meta-analysis, LLR demonstrated higher overall survival (OS) at 1, 3, and 5 years compared to RFA. Subgroup analysis found no significant OS differences at 1 and 5 years among PRFA, LRFA, and LLR, while LLR exhibited improved 3-year survival over PRFA. Notably, LRFA showed no significant difference from LLR. Meta-regression analysis found no significant associations between 1-year OS and factors such as age, bilirubin, AFP, or tumor size. OS Propensity-score matching indicated a significant improvement at 3 years with LLR, while no differences were observed at 1 and 5 years.
LLR demonstrated enhanced disease-free survival at 1 and 3 years, and recurrence-free survival analysis favored LLR at 1, 3, and 5 years, particularly over PRFA, but no significant difference was found with LRFA.
LLR exhibited significantly lower local recurrence rates compared to RFA, with PRFA showing a notable reduction; however, no significant difference was seen with LRFA. Meta-regression linked this reduction to age. LLR showcased benefits in decreasing intrahepatic recurrence and 90-day mortality; however, it was associated with longer surgery, higher transfusion rates, more complications, and extended hospital stays. We summarized the results of our analysis in Table 2.
Explanation of the findings
Open hepatectomy (OH) is a well-established method for treating HCCs, but its drawbacks include large incisions, extensive resection, and significant blood loss causing trauma. OH, suits patients with normal liver function; however, it is not suitable for patients with severe cirrhosis. A recent analysis showed that laparoscopic liver resection (LLR) was associated with lower postoperative complications, such as ascites and liver failure than OH. Therefore, LLR emerged as a minimally invasive alternative for OH, particularly in patients with severe cirrhosis [47, 48].
However, not all cases are suitable for LLR because LLR is primarily indicated for easily reachable lesions and tumors in the outer part of anterolateral liver segments (segments 2, 3, 5, and 6). Lesions in the posterior or upper liver regions (segments 1, 7, and 8, and the upper part of segment 4) represent technical challenges due to bleeding control and limited visibility difficulties [49, 50]. LLR is particularly considered the preferred option for small HCC cases, even in cirrhotic patients, when feasible, as its effectiveness matches that of open surgery in achieving a cure [51].
Radiofrequency ablation (RFA) is a widely used minimally invasive approach for treating HCCs. Various randomized controlled trials (RCTs) and meta-analyses have compared RFA with OH [52, 53]. These studies have consistently demonstrated that RFA is effective for early-stage HCCs, offering comparable prognostic outcomes and a lower complication rate than OH. In recent years, this has led to an increasing focus among surgeons on comparing these minimally invasive methods for the curative treatment of HCCs.
Advancements in artificial hydrothorax, imaging-guided localization, and probes have considerably expanded the indications of RFA. RFA procedures are performed under conscious sedation. Furthermore, most patients undergoing RFA treatment experience brief hospital stays of 2 to 3 days; in some cases, they can even be discharged on the same day, eliminating the need for prolonged hospitalization [54]. As a result, it is evident that RFA treatment is associated with reduced postoperative complications, shorter surgical durations, and minimized hospitalization periods. It’s a viable supplemental therapy for cirrhotic livers without significant damage.
However, local recurrence at the RFA treatment site is a common limitation. Rhim et al. noted this due to limited ablation volume, technical difficulties for certain tumors based on location, and the heat sink effect caused by nearby large vessels. [55]. Therefore, our observations of the higher local recurrence rates may be attributed to the incomplete ablation of the primary HCC tumor, the heat sink impact, or venous invasion in the adjacent liver. On the other hand, LLR provides a broader safety margin during treatment and often involves completely removing segments containing tumors. This thorough approach may contribute to lower recurrence rates in HCC patients with LLR [56].
In our subgroup analyses, we found that LLR had better outcomes for OS, RFS, and local recurrence rates compared to PRFA. However, regarding 1 to 5 years of OS, RFS, and local recurrence rates, LRFA and LLR had similar effects. This may be attributed to the ability of laparoscopic techniques to detect microscopic tumor foci. In addition, laparoscopic approaches allow precise electrode placement, especially in difficult tumor locations, through comprehensive exploration and intraoperative ultrasound. [57] Laparoscopic RFA's superiority over the percutaneous approach, especially in complex cases or severe liver disease, broadens the scope of RFA treatments, effectively expanding their applications [58].
The findings from the meta-regression analysis demonstrate that certain factors significantly impact the local recurrence in early-stage HCC. Specifically, the analysis reveals a noteworthy correlation between the age of the patient and the incidence of recurrence.
It is interesting to note that there is an inverse correlation between age and recurrence risk, which may seem counterintuitive since one might expect older patients to have a higher risk due to compromised immune function and overall health. However, this observation is consistent with earlier studies on older breast cancer patients conducted by Anna Z. de Boer et al. in 2020, which found that individuals aged 75–79 were more likely to experience distant recurrence but not locoregional recurrence risk [59]. Similarly, research by R. A. M. Damhuis et al. in 1997 demonstrated that older age was associated with reduced local recurrence rates in rectal cancer across three different age groups (15–64, 65–74, and 75 and over). [60] Thus, advancing age may decrease local recurrence rates but potentially increase the likelihood of distant recurrence in the context of HCC.
However, it is essential to note that the variability in study designs and patient populations across the included studies limits our findings. Further research is needed to explore the molecular mechanisms and interactions with other unexplored factors.
Also, our meta-regression analysis found no significant link between tumor size and overall survival or local recurrence in HCC patients, challenging the prior consensus associating larger tumor size with worse outcomes [61, 62].
Interestingly, Anli Yang et al.'s [63] research has also found that for patients without vascular invasion, tumor size matters notably for overall survival in the radiofrequency ablation group, but this association is not observed in either the liver resection or transplantation group. Conversely, for patients with vascular invasion, tumor size affects survival in the liver resection and transplantation group. These findings suggest two possibilities: tumor size may not be as crucial a prognostic factor in HCC as believed, with factors like tumor stage, vascular invasion, and liver function playing more significant roles. Additionally, the relationship between tumor size and HCC survival may be more complex, influenced by age, gender, or underlying liver disease. So, the clinicians should be cautious about relying solely on tumor size for treatment decisions and consider multiple factors for more informed choices.
Given these uncertainties, further research is needed to better understand the tumor size and survival relationship in HCC.
In comparison to the previous meta-analyses conducted by Mou‐Bo Si in 2019 [16], Xiaocheng Li in 2019 [20], Shan Jin in 2020 [17], and Zhijun Li in 2021 [19], our current study provides a thorough and up-to-date assessment of various liver resection techniques, with a particular emphasis on the benefits associated with LLR and RFA approaches.
Agreements and disagreements with previous studies
Our analysis incorporates 19 studies and a substantial pooled sample size of 3756 patients, as presented in Table 3. Prior studies had differing numbers of included studies, ranging from 6 to 19, and sample sizes ranging from 597 to 2038.
After conducting a thorough and detailed analysis, we have discovered significant differences and similarities in the results of various meta-analyses. In the context of overall survival, our findings closely align with those of Xiaocheng Li et al. [20]. However, when examining the research conducted by Mou‐Bo Si et al. [16] and Zhijun Li et al. [19], their results demonstrate that there were no statistically significant differences observed at the 1 year, whereas the outcomes favored the LLR group at 3 years.
On the other hand, all the meta-analyses indicate that the LLR group has a better disease-free survival rate at one and three years. However, at five years, our study and Xiaocheng Li's et al. [20] highlight a lack of statistical differences.
Across all meta-analyses [16, 17, 19, 20], the RFA group consistently shows higher local recurrence rates and shorter duration of both surgery and hospital stay compared to the LLR group. Additionally, complications are uniformly more prevalent in the LLR group according to all analyses.
Strength points and limitations
To date, our study is the most comprehensive meta-analysis comparing LLR versus RFA in patients with early-stage HCC. We included 19 observational studies, compromising 3756 patients. We covered a five-year follow-up period, analyzing OS, DFS, and RFS using Propensity Score Matching while examining Intrahepatic and Extrahepatic recurrence. In addition, we comprehensively evaluated safety measures in terms of all complications, 30-day and 90-day mortality, and major complications. Moreover, our study is the first meta-analysis in this topic to conduct subgroup analysis based on RFA type, including four laparoscopic RFA studies, which is a significant improvement compared to previous meta-analyses that only featured one study. Finally, our study is the first to perform meta-regression analysis to explore the association between overall survival and local recurrence with multiple covariates such as age, tumor size, total bilirubin, and alpha-fetoprotein.
In addressing the limitations of our analysis, it's crucial to emphasize that our study exclusively incorporated English-language studies. It's also essential to acknowledge that most of the studies we examined were retrospective, potentially introducing an increased risk of bias, particularly concerning the selection of patients. The varying availability of resources and diverse levels of expertise among medical practitioners might have significantly influenced treatment choices, constraining our findings' broader applicability. Moreover, we observed heterogeneity across different outcomes, and indications of publication bias emerged in multiple studies. Our analysis did not compare outcomes such as quality of life, liver functions after treatment, and overall response rate as these data were not reported in our included studies. Furthermore, our ability to perform a subgroup analysis based on portal hypertension, cirrhosis, etiology of the underlying disease, or tumor location was hindered by inherent constraints.
Implications of our findings in practice
Based on our study, LLR provides better long-term survival outcomes at 1, 3, and 5 years compared to RFA, making it the preferred option. However, subgroup analysis indicates that LRFA yields similar survival rates to LLR at these time intervals, providing a less invasive alternative. It is important to consider individual patient characteristics and preferences when making treatment decisions. LLR has advantages in terms of disease-free and recurrence-free survival, especially over PRFA. Age has been identified as a factor in reducing local recurrence rates.
Additionally, our research indicates that tumor size may not be as critical a prognostic factor in HCC as previously thought. This information can aid clinicians in making treatment decisions. For instance, clinicians may be less inclined to exclude patients from surgery solely based on tumor size.
Nevertheless, clinicians must balance these benefits against LLR's longer surgery times, higher transfusion rates, complications, and extended hospital stays. Additionally, the study highlights the potential of laparoscopic RFA techniques, as no significant differences were found between LLR and LRFA in several key outcomes, suggesting future research in this area.
Recommendations
To improve HCC management, t is recommended to conduct larger, long-term comparative studies and prioritize well-designed randomized controlled trials. These efforts would validate current findings, assess treatment long-term effects, and provide robust evidence. Additionally, considering both survival outcomes and patients' quality of life is crucial, along with evaluating cost-effectiveness for informed healthcare decision-making. It is crucial to explore the impact of evolving technologies on outcomes, especially within laparoscopic radiofrequency ablation techniques. Incorporating patient-reported outcomes and satisfaction assessments can provide valuable insights into treatment preferences.
Furthermore, additional research is needed to comprehensively understand the correlation between tumor size and HCC survival rates. Additionally, exploring the impact of age on local recurrence, as well as both intrahepatic and extrahepatic recurrence, and to identify other covariates influencing overall survival and local recurrence. By conducting more research, we can better understand HCC management and improve patient outcomes.
Conclusion
In this meta-analysis, LLR yielded better oncological outcomes than RFA for patients with early and small HCC. LLR exhibited superior 5-year overall survival and lower recurrence rates, although it was associated with higher complication rates than RFA. The study also highlighted the potential of enhancing outcomes via laparoscopic RFA techniques, as no significant differences were found between LLR and LRFA in terms of overall survival, recurrence-free survival, and local recurrence. However, it is essential to emphasize that further well-designed prospective studies of high quality are necessary to validate and substantiate the conclusions drawn from this meta-analysis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Abbreviations
- AFP:
-
Alpha-Fetoprotein
- BCLC:
-
Barcelona Clinic Liver Cancer
- CI:
-
Confidence Interval
- DFS:
-
Disease-Free Survival
- HCC:
-
Hepatocellular Carcinoma
- HBV:
-
Hepatitis B Virus
- HCV:
-
Hepatitis C Virus
- LLR:
-
Laparoscopic Liver Resection
- LRFA:
-
Laparoscopic Radiofrequency Ablation
- NOS:
-
Newcastle-Ottawa Scale
- OH:
-
Open Hepatectomy
- OS:
-
Overall Survival
- PRFA:
-
Percutaneous Radiofrequency Ablation
- PSM:
-
Propensity-Score Matching
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RFA:
-
Radiofrequency Ablation
- RR:
-
Risk Ratio
- RFS:
-
Recurrence-Free Survival
References
Hepatocellular carcinoma | Nature Reviews Disease Primers. Available from: https://www.nature.com/articles/s41572-020-00240-3. Cited 2023 Aug 19.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/ijc.29210. Cited 2023 Aug 19.
Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition) - PMC. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7024893/. Cited 2023 Aug 19.
The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines from 2001 to 2017 - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/28904327/. Cited 2023 Aug 19.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Yamashita YI, Tsuijita E, Takeishi K, Ishida T, Ikegami T, Ezaki T, et al. Trends in surgical results of hepatic resection for hepatocellular carcinoma: 1,000 consecutive cases over 20 years in a single institution. Am J Surg. 2014;207(6):890–6. Available from: https://pubmed.ncbi.nlm.nih.gov/24144344/. Cited 2023 Aug 19.
Forner A, Reig ME, Rodriguez De Lope C, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. Available from: http://www.thieme-connect.de/products/ejournals/html/https://doi.org/10.1055/s-0030-1247133. Cited 2023 Aug 19.
Meta‐analysis of laparoscopic versus open liver resection for hepatocellular carcinoma - Jiang - 2018 - Hepatology Research - Wiley Online Library. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/hepr.13061. Cited 2023 Aug 19.
De La Serna S, Vilana R, Sánchez-Cabús S, Calatayud D, Ferrer J, Molina V, et al. Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB (Oxford). 2015;17(5):387. Available from: /pmc/articles/PMC4402048/. Cited 2023 Aug 19.
Santambrogio R, Barabino M, De Nicola E, Galfrascoli E, Giovenzana M, Zappa MA. Laparoscopic ablation therapies for hepatocellular carcinoma: could specific indications for the laparoscopic approach influence the effectiveness? Updates Surg. 2020;72(2):435–43. Available from: https://pubmed.ncbi.nlm.nih.gov/32246409/. Cited 2023 Aug 19.
Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019;11(3):227. Available from: /pmc/articles/PMC6425331/. Cited 2023 Aug 19.
Worakitsitisatorn A, Lu DS, Lee MW, Asvadi NH, Moshksar A, Yuen AD, et al. Percutaneous thermal ablation of subcapsular hepatocellular carcinomas: influence of tumor-surface contact and protrusion on therapeutic efficacy and safety. Eur Radiol. 2020;30(3):1813–21. Available from: https://pubmed.ncbi.nlm.nih.gov/31822975/. Cited 2023 Aug 19.
Lee MW, Kim YJ, Park HS, Yu NC, Jung S Il, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194(5). Available from: https://pubmed.ncbi.nlm.nih.gov/20410384/. Cited 2023 Aug 19.
Lei JY, Wang WT, Yan LN, Wen TF, Li B. Radiofrequency ablation versus surgical resection for small unifocal hepatocellular carcinomas. Medicine. 2014;93(29):e271. Available from: https://pubmed.ncbi.nlm.nih.gov/25546668/. Cited 2023 Aug 19.
Casaccia M, Santori G, Bottino G, Diviacco P, Andorno E. Laparoscopic resection vs laparoscopic radiofrequency ablation for the treatment of small hepatocellular carcinomas: A single-center analysis. World J Gastroenterol. 2017;23(4):653. Available from: /pmc/articles/PMC5292339/. Cited 2023 Sep 14.
Si MB, Yan PJ, Hao XY, Du ZY, Tian HW, Yang J, et al. Efficacy and safety of radiofrequency ablation versus minimally invasive liver surgery for small hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33(8):2419–29. Available from: https://pubmed.ncbi.nlm.nih.gov/30989373/. Cited 2023 Aug 19.
Jin S, Tan S, Peng W, Jiang Y, Luo C. Radiofrequency ablation versus laparoscopic hepatectomy for treatment of hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol. 2020;18(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32787883/. Cited 2023 Aug 19.
Li X, Wu YS, Chen D, Lin H. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:5711. Available from: /pmc/articles/PMC6600087/. Cited 2023 Aug 28.
Li Z, Yu Q, Lu X, Liu Y, Ji B. Efficacy of radiofrequency ablation versus laparoscopic liver resection for hepatocellular carcinoma in China: a comprehensive meta-analysis. Videosurg Other Miniinvasive Techniques. 2021;16(3):455–71. https://doi.org/10.5114/wiitm.2021.105377. Cited 2023 Aug 20.
Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis - PMC. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6600087/. Cited 2023 Aug 28.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. Available from: https://systematicreviewsjournal.biomedcentral.com/articles/https://doi.org/10.1186/s13643-021-01626-4. Cited 2023 Aug 27.
Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Available from: https://training.cochrane.org/handbook/current. Cited 2023 Aug 27.
Incenzo Azzaferro VM, Nrico Egalia ER, Oberto Oci RD, Alvatore Ndreola SA, Ndrea Ulvirenti AP, Ederico Ozzetti FB, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–700. Available from: https://pubmed.ncbi.nlm.nih.gov/8594428/. Cited 2023 Sep 19.
Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–403. Available from: https://pubmed.ncbi.nlm.nih.gov/11391528/. Cited 2023 Sep 19.
Ottawa Hospital Research Institute. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Cited 2023 Aug 27.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. BMJ Ment Health. 2019;22(4):153–60. Available from: https://mentalhealth.bmj.com/content/22/4/153. Cited 2023 Sep 14.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Available from: https://www.bmj.com/content/315/7109/629. Cited 2023 Sep 14.
Chapter 15: Interpreting results and drawing conclusions | Cochrane Training. Available from: https://training.cochrane.org/handbook/current/chapter-15. Cited 2023 Aug 27.
Yamashita Y ichi, Imai K, Kaida T, Yamao T, Tsukamoto M, Nakagawa S, et al. Multimodal radiofrequency ablation versus laparoscopic hepatic resection for the treatment of primary hepatocellular carcinoma within Milan criteria in severely cirrhotic patients: long-term favorable outcomes over 10 years. Surg Endosc. 2019;33(1):46–51. Available from: https://pubmed.ncbi.nlm.nih.gov/29872945/. Cited 2023 Sep 14.
Xu H, Zhou L, Jin Q. The effects of ultrasound-guided radiofrequency ablation and laparoscopic hepatectomy in the treatment of small hepatocellular carcinoma: a retrospective analysis. Transl Cancer Res. 2021;10(11):4794–801. Available from: https://pubmed.ncbi.nlm.nih.gov/35116332/. Cited 2023 Sep 14.
Tsukamoto M, Imai K, Yamashita Y ichi, Kitano Y, Okabe H, Nakagawa S, et al. Endoscopic hepatic resection and endoscopic radiofrequency ablation as initial treatments for hepatocellular carcinoma within the Milan criteria. Surg Today. 2020;50(4):402–12. Available from: https://pubmed.ncbi.nlm.nih.gov/31680205/. Cited 2023 Sep 14.
Song J, Wang Y, Ma K, Zheng S, Bie P, Xia F, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc. 2016;30(10):4249–57. Available from: https://pubmed.ncbi.nlm.nih.gov/26715020/. Cited 2023 Sep 14.
Santambrogio R, Barabino M, Bruno S, Mariani N, Maroni N, Bertolini E, et al. Surgical resection vs. ablative therapies through a laparoscopic approach for hepatocellular carcinoma: a comparative study. J Gastrointest Surg. 2018;22(4):650–60. Available from: https://pubmed.ncbi.nlm.nih.gov/29235004/. cited 2023 Sep 14.
Pan Y xun, Long Q, Yi M jiang, Chen J bin, Chen J cong, Zhang Y jun, et al. Radiofrequency ablation versus laparoscopic hepatectomy for hepatocellular carcinoma: a real world single center study. Eur J Surg Oncol. 2020;46(4 Pt A):548–59. Available from: https://pubmed.ncbi.nlm.nih.gov/31677940/. Cited 2023 Sep 14.
Ogiso S, Seo S, Eso Y, Yoh T, Kawai T, Okumura S, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma. HPB (Oxford). 2021;23(4):533–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32912835/. Cited 2023 Sep 14.
Liu YW, Yen YH, Li WF, Wang CC, Lu SN, Kee KM, et al. Minimally invasive surgery versus percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma: Results from a high-volume liver surgery center in East Asia. Surg Oncol. 2022;42. Available from: https://pubmed.ncbi.nlm.nih.gov/35468499/. Cited 2023 Sep 14.
Lin CH, Ho CM, Wu CH, Liang PC, Wu YM, Hu RH, et al. Minimally invasive surgery versus radiofrequency ablation for single subcapsular hepatocellular carcinoma ≤ 2 cm with compensated liver cirrhosis. Surg Endosc. 2020;34(12):5566–73. Available from: https://pubmed.ncbi.nlm.nih.gov/31993821/. Cited 2023 Sep 14.
Lai C, Jin R an, Liang X, Cai X jun. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B. 2016;17(3):236. Available from: /pmc/articles/PMC4794515/. Cited 2023 Sep 14.
Ko SE, Lee MW, Ahn S, Rhim H, Kang TW, Song KD, et al. Laparoscopic hepatic resection versus laparoscopic radiofrequency ablation for subcapsular hepatocellular carcinomas smaller than 3 cm: analysis of treatment outcomes using propensity score matching. Korean J Radiol. 2022;23(6):615–24. Available from: https://pubmed.ncbi.nlm.nih.gov/35289151/. Cited 2023 Sep 14.
Harada N, Shirabe K, Maeda T, Kayashima H, Takaki S, Maehara Y. Comparison of the outcomes of patients with hepatocellular carcinoma and portal hypertension after liver resection versus radiofrequency Ablation. World J Surg. 2016;40(7):1709–19. Available from: https://pubmed.ncbi.nlm.nih.gov/26911609/. Cited 2023 Sep 14.
Chong CCN, Lee KF, Chu CM, Chan AWH, Yu SCH, Lai PBS. Laparoscopic Hepatectomy (with or without Robotic Assistance) versus Radiofrequency Ablation as a Minimally Invasive Treatment for Very Early-Stage or Early-Stage Hepatocellular Carcinoma. Dig Surg. 2020;37(1):65–71. Available from: https://pubmed.ncbi.nlm.nih.gov/30917378/. Cited 2023 Sep 14.
Conticchio M, Delvecchio A, Ratti F, Gelli M, Anelli FM, Laurent A, et al. Laparoscopic surgery versus radiofrequency ablation for the treatment of single hepatocellular carcinoma ≤3 cm in the elderly: a propensity score matching analysis. HPB (Oxford). 2022;24(1):79–86. Available from: https://pubmed.ncbi.nlm.nih.gov/34167892/. Cited 2023 Sep 14.
Cheng KC, Ho KM. Pure laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma: a propensity score and multivariate analysis. Transl Cancer Res. 2022;11(1):43–51. Available from: /pmc/articles/PMC8841462/. Cited 2023 Sep 14.
Wu D, Yang Y, Chen J, Cai H, Duan Y, Sun D. Three Different Ways of Treating Primary Hepatocellular Carcinoma at an Early Stage: A Prospective Comparative Study. Gastroenterol Res Pract. 2020;2020. Available from: /pmc/articles/PMC7245688/. Cited 2023 Sep 14.
Kim S, Yoon CJ, Cho JY, Han HS, Yoon YS, Lee HW, et al. Comparative long-term outcomes of laparoscopic hepatectomy and radiofrequency ablation for hepatocellular carcinoma located in the anterolateral segments of the liver. J Hepatobiliary Pancreat Sci. 2022;29(3):349–58. Available from: https://pubmed.ncbi.nlm.nih.gov/34689415/. Cited 2023 Sep 14.
Lee DH, Kim JW, Lee JM, Kim JM, Lee MW, Rhim H, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer. 2021;10(1):25. Available from: /pmc/articles/PMC7923879/. Cited 2023 Sep 14.
Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–9. Available from: https://pubmed.ncbi.nlm.nih.gov/15082595/. Cited 2023 Aug 28.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. Available from: https://pubmed.ncbi.nlm.nih.gov/21374666/. Cited 2023 Aug 28.
Hikspoors JPJM, Peeters MMJP, Kruepunga N, Mekonen HK, Mommen GMC, Köhler SE, et al. Human liver segments: role of cryptic liver lobes and vascular physiology in the development of liver veins and left-right asymmetry. Sci Rep. 2017;7(1):1–12. Available from: https://www.nature.com/articles/s41598-017-16840-1. Cited 2023 Aug 28.
Meta-analysis of laparoscopic versus open liver resection for colorectal liver metastases - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/27811369/. Cited 2023 Aug 28.
Effective viral suppression is necessary to reduce hepatocellular carcinoma development in cirrhotic patients with chronic hepatitis B: Results of a 10-year follow up - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/29095292/. Cited 2023 Aug 28.
Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. Available from: https://pubmed.ncbi.nlm.nih.gov/22634125/. Cited 2023 Aug 28.
Xu XL, Liu X Di, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287(2):461–72. Available from: https://pubmed.ncbi.nlm.nih.gov/29135366/. Cited 2023 Aug 28.
Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27(1):51–8. Available from: https://pubmed.ncbi.nlm.nih.gov/22004366/. Cited 2023 Aug 28.
Rhim H, Lim HK. Radiofrequency Ablation of Hepatocellular Carcinoma: Pros and Cons. Gut Liver. 2010;4(Suppl 1):S113. Available from: /pmc/articles/PMC2989542/. Cited 2023 Aug 28.
Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4(1):1–9. Available from: https://www.nature.com/articles/srep07252. Cited 2023 Aug 28.
Lau WY, Lai ECH. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20–5. Available from: https://pubmed.ncbi.nlm.nih.gov/19106671/. Cited 2023 Aug 28.
Laparoscopic ultrasound with radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: technique and technical considerations - PubMed. . Available from: https://pubmed.ncbi.nlm.nih.gov/11768826/. Cited 2023 Aug 28.
Boer AZ de, Hulst HC van der, Glas NA de, Mheen PJM de, Siesling S, Munck L de, et al. Impact of older age and comorbidity on locoregional and distant breast cancer recurrence: a large population‐based study. Oncologist. 2020;25(1):e24. Available from: /pmc/articles/PMC6964133/. Cited 2023 Aug 28.
Damhuis RAM, Wiggers T, Wereldsma JCJ. Association between age and local recurrence of rectal cancer: Results from a retrospective study of 902 patients. Int J Colorectal Dis. 1997;12(4):235–9. Available from: https://springerlink.fh-diploma.de/article/https://doi.org/10.1007/s003840050096. Cited 2023 Aug 28.
Chen Z, Zheng H, Zeng W, Liu M, Chen Y. Prognostic Analysis on Different Tumor Sizes for 14634 Hepatocellular Carcinoma Patients. Eur J Cancer Care (Engl). 2023;2023.
Hong SK, Lee KW, Lee S, Hong S young, Suh S, Han ES, et al. Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis. Ann Surg Treat Res. 2022;102(4):193. Available from: /pmc/articles/PMC9010965/. Cited 2023 Sep 20.
Yang A, Xiao W, Chen D, Wei X, Huang S, Lin Y, et al. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. 2018;7(12):6040. Available from: /pmc/articles/PMC6308097/. Cited 2023 Sep 20.
Acknowledgements
I would like to extend my heartfelt gratitude to Dr. Mohamed Abd-ElGawad for his invaluable mentorship and unwavering support throughout my research journey.
Code availability
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). All author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Mahmoud Shaban Abdelgalil spearheaded the team, overseeing the development and execution of the search strategy, as well as conducting the data collection. He skillfully addressed conflicts during both the screening and quality evaluation phases and played a key role in crafting the discussion section. Basma Ehab Amer actively participated in title and abstract screening, full-text screening, and quality assessment. She conducted the analysis and authored the result section. Noha Yasen contributed to title and abstract screening, full-text screening, and data extraction. Mohamed El-Samahy engaged in full-text screening, quality assessment, and composed the methods section. Ahmed K. Awad played a significant role in data extraction and authored the introduction section. Bahaa Elfakharany participated in full-text screening and data extraction. Omar Saeed conducted the analysis and contributed to the introduction section. Mohamed Abd-ElGawad provided supervision throughout the process, performing a thorough peer-review. All authors actively participated in the manuscript review, and the author(s) collectively read and endorsed the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary figure S1. Sensitivity analysis of overall survival at 3 years. Supplementary figure S2. Sensitivity analysis of overall survival at 5 years. Supplementary file figure S3. Forrest plot illustrating subgroup analysis for 1-year overall survival based on RFA type. Supplementary file figure S4. Forrest plot illustrating Subgroup analysis for 3-years overall survival based on RFA type. Supplementary file figure S5. Sensitivity analysis of laparoscopic subgroup overall survival at 3 years. Supplementary file figure S6. Sensitivity analysis of percutaneous subgroup overall survival at 3 years. Supplementary file figure S7. Forrest plot illustrating Subgroup analysis for 5-years overall survival based on RFA type. Supplementary file figure S8. Sensitivity analysis of percutaneous subgroup overall survival at 5 years. Supplementary file figure S9. Sensitivity analysis of laparoscopic subgroup overall survival at 5 years. Supplementary file figure S10. Meta-Regression Analysis of Covariates and 1-Year overall Survival. Supplementary file figure S11. Forrest plot illustrating overall survival PSM. Supplementary file figure S12. Sensitivity analysis of overall survival PSM at 5 years. Supplementary file Figure S13. Sensitivity analysis of disease-free survival at 3 years. Supplementary file Figure S14. Sensitivity analysis of disease-free survival at 1 year. Supplementary file Figure S15. Sensitivity analysis of disease-free survival at 5 years. Supplementary file Figure S16. Forrest plot illustrating disease-free survival PSM. Supplementary file Figure S17. Sensitivity analysis of disease-free survival PSM at 1 year. Supplementary file Figure S18. Sensitivity analysis of disease-free survival PSM at 3 years. Supplementary file Figure S19. Sensitivity analysis of recurrence-free survival at 1 year. Supplementary file Figure S20. Sensitivity analysis of recurrence-free survival at 3 years. Supplementary file Figure S21. Sensitivity analysis of recurrence-free survival at 5 years. Supplementary file Figure S22. Forrest plot illustrating subgroup analysis for 1-year recurrence-free survival based on RFA type. Supplementary file Figure S23. Forrest plot illustrating subgroup analysis for 3-years recurrence-free survival based on RFA type. Supplementary file Figure S24. Sensitivity analysis of percutaneous subgroup recurrence free survival at 1 year. Supplementary file Figure S25. Sensitivity analysis of percutaneous subgroup recurrence free survival at 3 years. Supplementary file Figure S26. Sensitivity analysis of laparoscopic subgroup recurrence free survival at 1 year. Supplementary file Figure S27. Sensitivity analysis of laparoscopic subgroup recurrence free survival at 3 years. Supplementary file Figure S28. Forrest plot illustrating Subgroup analysis for 5-years Recurrence-free survival based on RFA type. Supplementary file Figure S29. Sensitivity analysis of percutaneous subgroup recurrence free survival at 5 years. Supplementary file Figure S30. Sensitivity analysis of laparoscopic subgroup recurrence free survival at 5 years. Supplementary file Figure S31. Forrest plot illustrating recurrence-free survival PSM. Supplementary file Figure S32. Sensitivity analysis of recurrence-free survival PSM at 1 year. Supplementary file Figure S33. Sensitivity analysis of recurrence-free survival PSM at 5 years. Supplementary file Figure S34. Sensitivity analysis of recurrence-free survival PSM at 3 years. Supplementary file Figure S35. Sensitivity analysis of local recurrence. Supplementary file Figure S36. Forrest plot illustrating Subgroup analysis for local recurrence based on RFA type. Supplementary file Figure S37. Sensitivity analysis of percutaneous subgroup local recurrence. Supplementary file Figure S38. Sensitivity analysis of laparoscopic subgroup local recurrence. Supplementary file Figure S39. Forrest plot illustrating intrahepatic recurrence. Supplementary file Figure S40. Sensitivity analysis of intrahepatic recurrence. Supplementary file Figure S41. Forrest plot illustrating extrahepatic recurrence. Supplementary file Figure S42. Forrest plot illustrating duration of surgery. Supplementary file Figure S43. Sensitivity analysis of duration of surgery. Supplementary file Figure S44. Forrest plot illustrating incidence of blood transfusion during surgery. Supplementary file Figure S45. Forrest plot illustrating all complications. Supplementary file Figure S46. Forrest plot illustrating 90-days mortality. Supplementary file Figure S47. Forrest plot illustrating 30-days mortality. Supplementary file Figure S48. Forrest plot illustrating major complications. Supplementary file Figure S49. Forrest plot illustrating duration of hospital stay. Supplementary file Figure S50. Sensitivity analysis of duration of hospital stay. Supplementary file Figure S51. Funnel plot for the local recurrence. Supplementary file Figure S52. Funnel plot (trim and fill method) for the local recurrence.
Additional file 2:
Supplementary Table 1. Baseline characteristics of enrolled patients in each included study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shaaban Abdelgalil, M., Amer, B.E., Yasen, N. et al. Efficacy and safety of laparoscopic liver resection versus radiofrequency ablation in patients with early and small hepatocellular carcinoma: an updated meta-analysis and meta-regression of observational studies. World J Surg Onc 22, 47 (2024). https://doi.org/10.1186/s12957-023-03292-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03292-3