Abstract
The epididymal function and gene expression in mammals are under the control of the testis. Sex steroids are secreted from the testis and act on the epididymis in an endocrine manner. There is another, non-sex steroidal secreted signaling, named lumicrine signaling, in which testis-derived secreted proteins go through the male reproductive tract and act on the epididymis. The effects of such multiple regulations on the epididymis by the testis have been investigated for many genes. The recent development of high-throughput next-generation sequencing now enables us a further comparative survey of endocrine and lumicrine action-dependent gene expression. In the present study, testis-derived endocrine and lumicrine actions on epididymal gene expression were comparatively investigated by RNA-seq transcriptomic analyses. This investigation utilized experimental animal models in which testis-derived endocrine and/or lumicrine actions were interfered with, such as unilateral or bilateral orchidectomy. By bilateral orchidectomy, which interferes with both endocrine and lumicrine actions, 431 genes were downregulated. By unilateral orchidectomy, which also interferes with endocrine and lumicrine actions by the unilateral testis, but the endocrine action was compensated by the contralateral testis, 283 genes were downregulated. The content of such genes downregulated by unilateral orchidectomy was like those of lumicrine action-interfered efferent duct-ligation, W/Wv, and Nell2−/− mice. When genes affected by unilateral and bilateral orchidectomy were compared, 154 genes were commonly downregulated, whereas 217 genes were specifically downregulated only by bilateral orchidectomy, indicating the distinction between endocrine and lumicrine actions on the proximal epididymal transcriptome. Comparative transcriptome analyses also showed that the expressions of genes emerging since Amniota were notably impacted by bilateral orchidectomy, unilateral orchidectomy, and lumicrine action-interfering treatments; the degree of influence from these treatments varied based on the evolutionary stage beyond Amniota. These findings unveil an evolutional transition of regulated gene expression in the proximal epididymis by two different testis-derived signaling mechanisms.
Similar content being viewed by others
Introduction
The epididymis is a highly coiled epithelial duct constituting a part of the sperm transport route. After production in the testis, the testicular spermatozoa are transported through the efferent duct toward the epididymis, where sperm undergo further maturation of sperm functions such as motility and binding to oocytes necessary for their full fertilizing ability [1,2,3,4]. If the spermatozoa are not properly matured by the epididymis, they will not be able to acquire the cellular functions necessary for fertilization, eventually resulting in a significant decrease in male reproductive ability.
Such epididymal functions are mediated by the specific expressions of various genes. The epididymal expressions of specific genes are often responsible for the physiological functions of the epididymis and eventually the downstream sperm maturation [5, 6]. Interestingly, the epididymal gene expressions are known to be regulated by extra-epididymal or testicular factors. There are the endocrine and the non-endocrine signaling mechanisms, as signaling systems between the testis and the epididymis. In endocrine regulation, sex steroids originating from testicular Leydig cells reach the epididymis through the bloodstream. They act on epididymal cells by binding with androgen or estrogen receptors [7,8,9,10,11,12]. In non-endocrine regulation, secreted proteins synthesized by the testicular germ cells located inside the seminiferous tubule are secreted into the seminiferous fluid and reach the epididymis via the reproductive tract by the luminal flow [13,14,15]. The secreted proteins act on epididymal cells by binding to their receptors expressed on the cell surface of the epididymal luminal epithelium [15,16,17]. Since this type of secretion signaling between the testes and epididymis acts through the lumen, it has been referred to as “lumicrine signaling,” a terminology introduced by Barry T. Hinton [18]. Eventually, these testis-derived signals regulate the physiological functions of the epididymis by modifying gene expressions.
The testicular regulations of the epididymal cell functions and gene expressions can be investigated experimentally; in the early studies, when the testes were experimentally removed by orchidectomy (OD) or testis-epididymis luminal communication was interfered with by the efferent duct ligation (EDL), then the epididymal protein synthesis, which was monitored by the metabolic labeling using radioisotopes, was critically affected [19,20,21]. In addition to these OD or EDL-treated animals, gene-modified animals, in which the expression and function of endocrine or lumicrine signaling components are genetically ablated, are also available [8, 15,16,17, 22]. By using such animal models, various genes have been identified to be expressed in the epididymis in an endocrine action-dependent and/or lumicrine action-dependent manner [15, 16, 23,24,25,26,27,28,29,30,31,32].
Recently, it has become possible to examine gene expression by next-generation sequencing and analyze them not only by the expression levels of individual genes but with information about the structure, function, and evolution of gene products. Such data-driven analyses may allow for grouping genes based on their respective regulatory mechanisms or extracting the unknown features of regulated gene expression for gene groups classified according to specific criteria. In the present study, the gene expressions of the proximal epididymis, subjected to OD or lumicrine action-interfering treatments, were investigated through RNA sequencing (RNA-seq) and the obtained transcriptomes were subsequently evaluated to characterize the endocrine and lumicrine regulations of epididymal gene expression.
Materials and methods
Animals
B6D1F1 male mice were purchased from Japan SLC. Unilateral and bilateral ODs were performed as follows. Eight-week-old wild-type (WT) B6D1F1 males were unilaterally orchidectomized (n = 3), in which the contralateral untreated side served as control. Eight-week-old WT B6D1F1 males were bilaterally orchidectomized (n = 3) or sham-operated (n = 3), which served as controls. The initial segment (IS)-caput epididymides were isolated from the animals four weeks after OD or sham operation.
Dissection of epididymis
The IS was dissected together with the caput and such a tissue dissection was indicated by the description “IS-caput” as described previously [16, 30]. This is because of the difficulty in dissecting IS separately from caput epididymides, especially in mice in which IS differentiation is ablated by the experimental treatments.
RNA-seq
Total RNAs were isolated from the isolated IS-caput epididymides using RNeasy mini (Qiagen). On-column DNase treatment was performed during RNA purification using an RNase-free DNase set (Qiagen). The amount of RNAs was determined by absorbance at 260 nm. The RNA-seq of epididymal transcripts was performed as follows: libraries for sequencing were prepared from isolated RNAs using a TruSeq stranded mRNA sample prep kit (Illumina, #20,020,594) and sequenced on a NovaSeq6000 (Illumina) using 101 bp single-ended mode. The mapping of the obtained sequence reads onto a mouse reference genome (mm10) was performed using TopHat ver. 2.1.1 [33]. To calculate fragments per kilobase of exon per million mapped reads (FPKM) values for each gene, Cufflinks ver. 2.2.1 was used [34]. The obtained RNA-seq data have been deposited in the Gene Expression Omnibus database under the accession code GSE247764.
Transcriptome analyses
The IS-caput epididymal transcriptomes of unilateral OD, bilateral OD, and their controls were comparatively analysed. The IS-caput epididymal transcriptomes of EDL (EDL performed at 10 weeks old and the ipsilateral epididymis was at 14 weeks old) [30], W/Wv, a Kit compound heterozygous mutant (14 weeks old) [30], and Nell2−/− (14 weeks old) [15] mice were also used for comparison (datasets are publicly available from the NCBI Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo/). The transcriptome data were incorporated into Microsoft Excel software for further analysis. Gene ontology (GO) information was obtained from the Mouse Genome Informatics website (https://www.informatics.jax.org/vocab/gene_ontology).
Mouse genes were classified into nine classes, i.e., Craniata, Gnathostomata, Teleostomi, Tetrapoda, Amniota, Mammalia, Theria, Euthelia, and others according to the emergence of genes during vertebrate evolution, using the information provided by NCBI Gene (https://www.ncbi.nlm.nih.gov/gene).
Drawings
Schematic drawings were generated using Microsoft PowerPoint (Microsoft Corporation). Plot representations, heatmap representations, and bar graphs were generated using Microsoft Excel 2019 (Microsoft Corporation).
Statistical analysis
Two-tailed t-tests under the assumption of unequal variances were performed using Microsoft Excel 2019.
Results
Comparative transcriptome analyses of orchidectomized mouse proximal epididymis
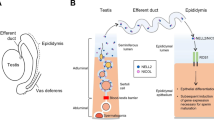
A schematic representation of endocrine and lumicrine actions from the testis to the epididymis is represented in Fig. 1. In the bilateral OD, both testes are removed and both endocrine and lumicrine actions are ablated. In the unilateral OD, the unilateral testis is removed, then the ipsilateral lumicrine and endocrine actions are ablated but the contralateral testis-derived endocrine action is expected to be still active. In the EDL, only lumicrine is ablated because the testis-epididymis luminal connection is interfered with. Since W/Wv and Nell2−/− mice lack germ cells that secrete lumicrine ligands and lumicrine ligand NELL2, respectively, only lumicrine but not endocrine signaling is dysfunctional in these animals [15].
Testis-derived endocrine and lumicrine actions on the epididymis. (A) A schematic representation of testis-derived endocrine and lumicrine actions on the epididymis. IS, initial segment; Cap, caput; ED, efferent duct; VD, vas deferens. Blue arrows indicate the lumicrine action through the male reproductive tract. Red arrows indicate the endocrine action through the bloodstream. (B) A summary of experimental conditions and their effects on endocrine and lumicrine actions
The unilateral or bilateral OD was performed at 8 weeks old for 4 weeks and the IS-caput epididymides were isolated for RNA isolation and the subsequent transcriptome analysis. For unilateral OD, contralateral epididymides were used as controls. For bilateral OD, the epididymides of sham-operated animals were used as controls. The obtained RNA-seq results were summarized in Supplementary Data File 1. The IS-caput epididymal gene expression in the orchidectomized animals and those in their control animals were plot-represented (Fig. 2A-B). The unilateral OD did not significantly affect the gene expression of the contralateral side epididymis, as evidenced by the comparison between the sham-operated control and the contralateral side control of unilateral OD (Fig. 2C). For the comparative study, the IS-caput transcriptomes of EDL, W/Wv, and Nell2−/− animals, all of which were previously done by the author based on the experimental procedure identical to that employed in the present study [15, 30], were also shown (Fig. 2D–F).
RNA-seq analyses of orchidectomized and lumicrine signaling-deficient IS-caput epididymis. A–C, RNA-seq of sham-operated control vs. bilateral OD (A), contralateral side control vs. ipsilateral side in unilateral OD (B), sham-operated control vs. contralateral side control in unilateral OD (C). D-F, RNA-seq of WT vs. EDL (D), WT vs. W/Wv (E), and WT vs. Nell2−/− (F). FPKM values are plotted. Statistically significantly downregulated (fold change < 0.1, and t-test P < 0.05) and upregulated (fold change > 10, and t-test P < 0.05) genes are represented in green and yellow, respectively
In the bilateral OD treatment, 431 genes were significantly downregulated (Table 1). Among such genes downregulated in bilateral OD, 27.8 ∼ 41.5% are common with those with unilateral OD (154 genes), EDL (179 genes), W/Wv, (165 genes), and Nell2−/− (120 genes). The proportion of unique genes whose expression was significantly downregulated in the bilateral OD animals was 50.3% (217 genes), which was very high compared with those in unilateral OD (14.8%), EDL (22.1%), W/Wv (9.0%), and Nell2−/− (10.4%) (Table 1). In the unilateral OD, 283 genes were significantly downregulated (Table 1). Among them, 49.1 ∼ 77.0% are common with those with bilateral OD (154 genes), EDL (218 genes), W/Wv, (187 genes), and Nell2−/− (139 genes). The proportion of unique genes whose expression was significantly downregulated in the unilateral OD animals was 14.8% (42 genes). Thus, while many genes were commonly downregulated among experimentally treated animals including bilateral OD and unilateral OD, there were a considerable number of genes specifically downregulated by bilateral OD.
The fold changes of gene expression in the bilateral and unilateral OD-treated IS-caput epididymides were shown by heatmap representation (Fig. 3A). For genes significantly downregulated in bilateral OD-treated IS-caput epididymis (fold change < 0.1, and t-test P value < 0.05), their expression fold changes were compared with those of unilateral OD, EDL, W/Wv, and Nell2−/− animals (Fig. 3B and Supplementary Data File 2). Among genes significantly downregulated in the bilateral OD animals, there are many genes not affected or only moderately downregulated in the unilateral OD, EDL, W/Wv, and Nell2−/− animals, endorsing the above observation that a larger number of genes were downregulated in the bilateral OD mice compared with unilateral OD, EDL, W/Wv, and Nell2−/− ones.
Comparative representation of genes downregulated in lumicrine signaling-deficient and endocrine signaling-deficient mouse IS-caput epididymides (A) Fold change of gene expressions in bilateral OD (n = 3) and unilateral OD (n = 3). Green and magenta represent downregulation and upregulation, respectively. (B) Genes downregulated in bilateral OD IS-caput epididymis (fold change < 0.1, and t-test P < 0.05) compared with fold changes in unilateral OD, EDL, W/Wv, and Nell2−/− IS-caput epididymis. Average values are shown. Color indications are the same as in panel A
For upregulated genes, there are 196 and 69 genes upregulated by bilateral OD and unilateral OD, respectively (Table 2). However, among such upregulated genes, only 1 ∼ 3 genes were common between genes upregulated in the IS-caput epididymis of EDL, W/Wv, or Nell2−/− mice, implying that the observed upregulations are resulting from experimental variation than from a common mechanism.
Collectively, these results indicate that unilateral OD, EDL, W/Wv, and Nell2−/− animals are similar whereas the bilateral OD animals are rather unique in their gene downregulation. Hereafter in the present study, unilateral OD is therefore treated as a variation of the lumicrine action-interfering treatments (see also Discussion).
The feature of gene products downregulated in endocrine and/or lumicrine actions-interfered mouse epididymis
The expression of many genes was influenced differently by the bilateral OD and lumicrine action-interfering treatments. To investigate whether there are common characteristics shared among such genes whose expressions were affected, genes were classified based on their function or their evolution. Subsequently, the total expressions of genes classified in such a manner were compared between the experimental groups.
Using GO information, genes were selected based on the localization of the encoded proteins (extracellular, plasma membrane, cytosol, mitochondrion, endoplasmic reticulum, Golgi apparatus, and nucleoplasm). Gene expression was then accumulated for each classification group and compared between experimental groups (Fig. 4A and Supplementary Data File 3). An apparent reduction by bilateral OD was observed in GO “extracellular” genes. In other GO classifications, prominent downregulation or upregulation of the accumulated gene expression was not recognized in the specific experimental group.
Expression of genes classified based on the properties of resulting proteins. Gene expressions in sham-operated control, contralateral control, WT, bilateral OD, unilateral OD, EDL, W/Wv, and Nell2−/− IS-caput epididymis. (A) Genes are classified by subcellular localization using GO information. The expression levels of genes were accumulated for each group. (B) Genes are classified according to their evolution. The expression levels of genes were accumulated for each group. (C) Magnified representations of Mammals and Theria in panel B. All values are shown as mean ± S.E.M (n = 3)
Transcriptomes can be analyzed not only based on gene functions but also based on gene evolution. Genes were therefore classified into nine evolutional classes, i.e., Craniata, Gnathostomata, Teleostomi, Tetrapoda, Amniota, Mammalia, Theria, Euthelia, and others (evolutionally newer genes), and the accumulated gene expressions for each class were compared between experimental groups (Fig. 4B and C and Supplementary Data File 4). There was no critical difference in the accumulated expression of genes acquired before Tetrapoda between the experimental groups, whereas apparent reductions in the accumulated expression by the experimental treatments were observed in genes acquired since Amniota. The accumulated expressions of Amniota-specific genes were reduced in mice with bilateral OD and mice with lumicrine action-interfering treatments such as unilateral OD, EDL, W/Wv, and Nell2−/− mice. For Mammalia- and Theria-specific genes, their accumulated expressions were also reduced similarly in the unilateral OD, EDL, W/Wv, and Nell2−/− animals, whereas the reduction in the bilateral OD was even greater. Decreased expression was also observed in the genes of For Euthelia-specific and evolutionally newer genes, the reduction of accumulated gene expression was apparent only in the bilateral OD animals but not prominent in those of unilateral OD, EDL, W/Wv, and Nell2−/− animals. Thus, the expressions of genes acquired since Amniota were affected by bilateral OD and other lumicrine action-interfering treatments but to a different extent.
There are several protein families known to be specifically expressed in the epididymis such as β-defensins [35, 36], cystatins [37, 38], cysteine-rich secretory proteins (CRISPs) [39,40,41], and lipocalins [42, 43]. The regulated expressions of genes encoding such protein families were also investigated from the evolutional aspect (Fig. 5). All genes encoding these protein families have emerged since Amniota. The expressions of Amniota-specific β-defensin, cystatin, and lipocalin genes are downregulated by either or both bilateral OD and lumicrine action-interfering treatments, whereas Amniota-specific CRISP genes were not expressed in both control and experimentally treated IS-caput epididymis. Also, the expressions of genes β-defensin, lipocalin, cystatin, and CRISP genes emerged since Theria were affected by both bilateral OD and lumicrine action-interfering treatments. Collectively, bilateral OD and lumicrine action-interfering treatments differently but critically affected the IS-caput epididymal expressions of genes acquired since Amniota.
Expression of gene families abundantly expressed in the epididymis. The accumulated expression levels of genes encoding β-defensins, lipocalins, cystatins, and CRISPs in sham-operated control, contralateral control, WT, bilateral OD, unilateral OD, EDL, W/Wv, and Nell2−/− IS-caput epididymis are shown according to their evolution. All values are shown as mean ± S.E.M (n = 3)
Discussion
There are non-autonomous mechanisms that regulate epididymal cell differentiation and gene expression. By observing changes in the epididymis following OD and EDL, endocrine and lumicrine actions have been identified as secretory signaling mechanisms originating from this testis [13, 14, 44]. The actions of these two regulatory mechanisms on the epididymal gene expression have been investigated for many genes but have not been investigated comprehensively at the genomic level. However, recent advances in the analytical capabilities of next-generation sequencing have made it possible to carry out comparative genome-wide expression analyses with higher precision and comprehensiveness than has been possible in the past. In the present study, the features of endocrine and lumicrine actions to regulate epididymal gene expression were examined by transcriptome analyzes. In the bilateral OD, the testicular endocrine and lumicrine actions are completely ablated. In the unilateral OD, the endocrine and lumicrine actions originating from the ipsilateral testis on the epididymis are ablated, but the endocrine action is still compensated by that from the contralateral testis. The comparative transcriptome analyses confirmed that the gene expression profile of unilateral OD is different from that of bilateral OD but rather like those of the lumicrine action-interfered animals such as EDL, W/Wv, and Nell2−/− mice (Figs. 2 and 3; Table 1). Thus, as briefly described in the Result section, unilateral OD is concluded to be a variation of the lumicrine action-interfering treatments.
Overview of testicular regulations of epididymal gene expression
The preceding studies unveiled that among genes expressed in the epididymis, some are regulated by the testicular endocrine and/or lumicrine signaling [8, 15, 16, 23, 25, 27,28,29,30,31, 45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214]. In the present study, genes whose expressions were regulated by testicular endocrine and/or lumicrine actions were explored further by comparing RNA-seq data obtained from bilateral or unilateral OD, EDL, W/Wv, and Nell2−/− mouse epididymis. Genes whose expression is reduced by experimental treatment are especially important because their gene expression is positively regulated under physiological conditions. Such downregulated genes can be classified into several groups: i, genes downregulated by lumicrine action-interfering treatments, and therefore affected equally also by bilateral OD; ii, genes not affected by lumicrine action-interfering treatments, but downregulated only by bilateral OD; iii, genes moderately downregulated by lumicrine action-interfering treatments and further by bilateral OD. Not all genes downregulated by unilateral OD are solely regulated by lumicrine signaling. The differentiation and associated gene expression of the IS epididymis require androgen action even if the luminal communication between testis and epididymis is intact; in Rnase10-cre; ArloxP mice, in which androgen receptors are conditionally knocked out in the proximal epididymis, the IS differentiation and associated gene expression are inhibited [8]. Therefore, the expression of genes downregulated by lumicrine action-interfering treatment is also regulated in a concerted way by lumicrine and endocrine signaling mechanisms. Collectively, the epididymal expression of individual genes is regulated not solely by either endocrine or lumicrine mechanisms, but rather to varying degrees.
Evolution of endocrine and lumicrine regulation of the epididymis by the testis
A variety of information for biological processes can be extracted by analyzing transcriptomes. In the present study, the transcriptomes were analyzed further based on the subcellular localization of gene products and gene evolution. Since such gene characterizations were based on the resulting proteins, genes encoding small non-coding RNAs, which are also enriched in the epididymis [215], were not analyzed in the present study. A comparison of the expression of genes selected based on GO protein localization information showed downregulation of GO “extracellular” genes by bilateral OD, indicating that the induction of extracellular proteins is one of the major targets of testicular endocrine signaling. On the other hand, expression comparisons of genes classified based on vertebrate evolutionary information showed there are selective regulations of gene expression by lumicrine and endocrine signaling mechanisms according to the evolutionary stage. The lumicrine signaling regulated the expression of genes acquired in Amniota, which corresponds to the early post-land expansion in the vertebrate evolutionary classification criteria adopted in this study. Since Amniota, regulation by both lumicrine and endocrine actions was found for genes acquired in Mammalia and Theria. For genes acquired evolutionarily more recently after Euthelia, only endocrine regulation was apparent. The expressions of several epididymis-specific genes such as encoding β-defensins, cystatins, CRISPs, and lipocalins were also specifically regulated by the testicular endocrine and lumicrine actions since Amniota. These findings suggest a possibility that testicular endocrine and lumicrine regulation of epididymal gene expression was active since the establishment of the epididymis in the Amniota but the extent of their contribution has been varying in the later evolution (Fig. 6). Among the genes acquired before Tetrapoda, there are genes such as Adam28, Etv1, Etv4, Etv5, Mfge8, and Ovch2 whose epididymal expressions are regulated by endocrine and/or lumicrine signaling [15, 24, 216]. However, in contrast to the genes acquired after Amniota, the total expressions of genes acquired before Tetrapoda were not critically affected by the testicular endocrine and lumicrine regulations. These observations suggest a possibility that genes acquired before Tetrapoda function rather as housekeeping genes in the proximal epididymis whereas genes acquired after Amniota function as those responsible for epididymis-specific functions. The evolution of the epididymis has been investigated mainly by comparative anatomy [217]. Although the findings in the present study do not clarify the genetic mechanisms of epididymis formation, they will provide new insights into how epididymal gene expressions have been regulated by testicular endocrine and lumicrine actions through vertebrate evolution.
A hypothesis for the testis-epididymis secreted signaling during evolution. Before Amniota, the epididymis and therefore testis-epididymis secreted signaling did not exist. In Amniota, in which the epididymis had developed from the mesonephros, testis-derived endocrine and lumicrine regulation of epididymal gene expression (represented by pink and blue arrows, respectively) emerged. Such contributions by endocrine or lumicrine action to the epididymal gene expression (represented by the size of arrows) can alter along evolution
In conclusion, the present study has unveiled the different characteristics of the endocrine and lumicrine actions on the regulation of epididymal gene expression.
Data availability
All transcriptome data supporting the results of this study are available at the NCBI GEO under accession numbers GSE247764 (unilateral OD, bilateral OD, and Nell2-/-) and GSE232898 (W/Wv and EDL).
References
Breton S, Nair AV, Battistone MA. Epithelial dynamics in the epididymis: role in the maturation, protection, and storage of spermatozoa. Andrology. 2019;7:631–43.
Kiyozumi D. Lumicrine signaling: extracellular regulation of sperm maturation in the male reproductive tract lumen. Genes Cells. 2023;28:757–63.
Zhou W, De Iuliis GN, Dun MD, Nixon B. Characteristics of the Epididymal Luminal Environment responsible for sperm maturation and storage. Front Endocrinol (Lausanne). 2018;9:59.
Robaire B, Hinton BT, Orgebin-Crist M-C. The epididymis. Knobil Neill’s Physiol Reprod. 2006;1071–148.
Murashima A, Xu B, Hinton BT. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl. 2015;17:749–55.
Björkgren I, Sipilä P. The impact of epididymal proteins on sperm function. Reproduction. 2019;158:R155–67.
O’Hara L, Welsh M, Saunders PTK, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–29.
Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology. 2011;152:689–96.
Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152:1640–51.
Joseph A, Hess Ra, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, et al. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod. 2010;82:948–57.
Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod. 2010;82:958–67.
Cavalcanti FN, Lucas TFG, Lazari MFM, Porto CS. Estrogen receptor ESR1 mediates activation of ERK1/2, CREB, and ELK1 in the corpus of the epididymis. J Mol Endocrinol. 2015;54:339–49.
Moniem KA, Glover TD, Lubicz-Nawrocki CW. Effects of duct ligation and orchidectomy on histochemical reactions in the hamster epididymis. Reproduction. 1978;54:173–6.
Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod. 1979;20:162–81.
Kiyozumi D, Noda T, Yamaguchi R, Tobita T, Matsumura T, Shimada K, et al. NELL2-mediated lumicrine signaling through OVCH2 is required for male fertility. Science. 2020;368:1132–5.
Kiyozumi D, Shimada K, Chalick M, Emori C, Kodani M, Oura S, et al. A small secreted protein NICOL regulates lumicrine-mediated sperm maturation and male fertility. Nat Commun. 2023;14:2354.
Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Gödecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–93.
Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57.
Kanka J, Kopecný V. An autoradiographic study of macromolecular synthesis in the epithelium of the ductus epididymis in the mouse. I. DNA, RNA and protein. Biol Reprod. 1977;16:421–7.
Jones R, Brown CR, Von Glós KI, Parker MG. Hormonal regulation of protein synthesis in the rat epididymis. Characterization of androgen-dependent and testicular fluid-dependent proteins. Biochem J. 1980;188:667–76.
Brooks DE. Effect of androgens on protein synthesis and secretion in various regions of the rat epididymis, as analysed by two-dimensional gel electrophoresis. Mol Cell Endocrinol. 1983;29:255–70.
Jun HJ, Roy J, Smith TB, Wood LB, Lane K, Woolfenden S, et al. ROS1 signaling regulates epithelial differentiation in the epididymis. Endocrinology. 2014;155:3661–73.
Cooper TG. Gene and protein expression in the Epididymis of Infertile c-ros receptor tyrosine kinase-deficient mice. Biol Reprod. 2003;69:1750–62.
Sipila P, Sipilä P, Pujianto DA, Shariatmadari R, Nikkilä J, Lehtoranta M, et al. Differential Endocrine regulation of genes enriched in initial segment and distal caput of the mouse Epididymis as revealed by genome-wide expression profiling. Biol Reprod. 2006;75:240–51.
Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential Gene expression among the proximal segments of the rat epididymis is lost after Efferent Duct Ligation1. Biol Reprod. 2007;77:165–71.
Chauvin TR, Griswold MD. Androgen-regulated genes in the murine epididymis. Biol Reprod. 2004;71:560–9.
Snyder EM, Small CL, Li Y, Griswold MD. Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod. 2009;81:707–16.
Xu B, Abdel-Fattah R, Yang L, Crenshaw Sa, Black MB, Hinton BT. Testicular lumicrine factors regulate ERK, STAT, and NFKB pathways in the initial segment of the rat epididymis to prevent apoptosis. Biol Reprod. 2011;84:1282–91.
Sipilä P, Krutskikh A, Pujianto DA, Poutanen M, Huhtaniemi I. Regional expression of androgen receptor coregulators and androgen action in the mouse epididymis. J Androl. 2011;32:711–7.
Kiyozumi D, Ikawa M. ADGRG2 is dispensable for lumicrine signalling regulating epididymal initial segment differentiation and gene expression. Biol Reprod. 2023;109:474–81.
Robaire B, Ewing LL, Zirkin BR, Irby DC. Steroid delta4-5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase in the rat epididymis. Endocrinology. 1977;101:1379–90.
Kiyozumi D. Busulfan administration replicated the characteristics of the epididymal initial segment observed in mice lacking testis-epididymis lumicrine signaling. J Reprod Dev. 2024.
Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5.
Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, Tyrrell C et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9.
Ribeiro CM, Silva EJR, Hinton BT, Avellar MCW. β-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian J Androl. 2016;18:323–8.
Fujihara Y, Noda T, Kobayashi K, Oji A, Kobayashi S, Matsumura T, et al. Identification of multiple male reproductive tractspecific proteins that regulate sperm migration through the oviduct in mice. Proc Natl Acad Sci U S A. 2019;116:18498–506.
Whelly S, Serobian G, Borchardt C, Powell J, Johnson S, Hakansson K, et al. Fertility defects in mice expressing the L68Q variant of human cystatin C: a role for amyloid in male infertility. J Biol Chem. 2014;289:7718–29.
Carvajal G, Brukman NG, Weigel Muñoz M, Battistone MA, Guazzone VA, Ikawa M, et al. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci Rep. 2018;8:17531.
Weigel Muñoz M, Carvajal G, Curci L, Gonzalez SN, Cuasnicu PS. Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility. Andrology. 2019;7:610–7.
Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch secretory protein 1 (CRISP1). Dev Biol. 2008;320:12–8.
Sakurai N, Fujihara Y, Kobayashi K, Ikawa M. CRISPR/Cas9-mediated disruption of lipocalins, Ly6g5b, and Ly6g5c causes male subfertility in mice. Andrology. 2022;10.1111/andr.13350.
Ong DE, Newcomer ME, Lareyre JJ, Orgebin-Crist MC. Epididymal retinoic acid-binding protein. Biochim Biophys Acta. 2000;1482:209–17.
Benoit J. Recherches anatomiques, cytologiques et histophysiologiques sur les voies excrétrices du testicule chez les mammifères. Arch Anat Histol Embryol (Strasb). 1926;5:173–412.
de Larminat MA, Monsalve A, Charreau EH, Calandra RS, Blaquier JA. Hormonal regulation of 5alpha-reductase activity in rat epididymis. J Endocrinol. 1978;79:157–65.
Brooks DE. Activity and androgenic control of enzymes associated with the tricarboxylic acid cycle, lipid oxidation and mitochondrial shuttles in the epididymis and epididymal spermatozoa of the rat. Biochem J. 1978;174:741–52.
Catayee G, Chalet M, Turchini J. Action of testosterone on 5’nucleotidase activity in the rat epididymal epithelium. Bull Assoc Anat (Nancy). 1978;62:217–23.
Pujol A, Bayard F. 5alpha-Reductase and 3alpha-hydroxysteroid oxidoreductase enzyme activities in epididymis and their control by androgen and the rete testis fluid. Steroids. 1978;31:485–93.
Purvis K, Hansson V. Androgens and androgen-binding protein in the rat epididymis. J Reprod Fertil. 1978;52:59–63.
Brooks DE. Control of glycolytic enzymes by androgens in the rat epididymis. J Endocrinol. 1976;71:355–65.
Brooks DE. Activity and androgenic control of glycolytic enzymes in the epididymis and epididymal spermatozoa of the rat. Biochem J. 1976;156:527–37.
Kohane AC, Garberi JC, Cameo MS, Blaquier JA. Quantitative determination of specific proteins in rat epididymis. J Steroid Biochem. 1979;11:671–4.
Rastogi RK, Milone M, Di Meglio M, Caliendo MF, Chieffi G. Effects of castration, 5 alpha-dihydrotestosterone and cyproterone acetate on enzyme activity in the mouse epididymis. J Reprod Fertil. 1979;57:73–7.
Robaire B. Effects of unilateral orchidectomy on rat epididymal delta 4–5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase. Can J Physiol Pharmacol. 1979;57:998–1003.
Brooks DE. Influence of testicular secretions on tissue weight and on metabolic and enzyme activities in the epididymis of the rat. J Endocrinol. 1979;82:305–13.
Brooks DE. Influence of androgens on the weights of the male accessory reproductive organs and on the activities of mitochondrial enzymes in the epididymis of the rat. J Endocrinol. 1979;82:293–303.
Beck B. Tissue specificity of the epididymal androgen dependent phospholipase A. Int J Androl. 1980;3:349–62.
D’Agostino A, Jones R, White R, Parker MG. Androgenic regulation of messenger RNA in rat epididymis. Biochem J. 1980;190:505–12.
Faye JC, Duguet L, Mazzuca M, Bayard F. Purification, radioimmunoassay, and immunohistochemical localization of a glycoprotein produced by the rat epididymis. Biol Reprod. 1980;23:423–32.
Kirkeby S, Blecher SR. Histochemical studies on genetical control of hormonal enzyme inducibility in the mouse. IV: Cellular localization of androgen sensitive nonspecific esterase in the epididymis. Arch Androl. 1981;6:163–73.
Moore HD. Effects of castration on specific glycoprotein secretions of the epididymis in the rabbit and hamster. J Reprod Fertil. 1981;61:347–54.
Holtz A, Brennan RG, Battista D, Terner C. Androgen control of an inhibitory modulator of phosphodiesterase in rat epididymis and prostate. Endocrinology. 1981;108:1538–44.
Jones R, von Glos KI, Brown CR. Characterization of hormonally regulated secretory proteins from the caput epididymidis of the rabbit. Biochem J. 1981;196:105–14.
Fournier-Delpech S, Pisselet C, Garnier DH, Dubois M, Courot M. Evidence for testosterone induced prealbumin secretion in ram epididymis. C R Seances Acad Sci III. 1981;293:589–94.
Brooks DE. Secretion of proteins and glycoproteins by the rat epididymis: regional differences, androgen-dependence, and effects of protease inhibitors, procaine, and tunicamycin. Biol Reprod. 1981;25:1099–117.
Robaire B, Hales BF. Regulation of epididymal glutathione S-transferases: effects of orchidectomy and androgen replacement. Biol Reprod. 1982;26:559–65.
Blecher SR, Kirkeby S. Histochemical studies on genetical control of hormonal enzyme inducibility in the mouse. V. Histochemical evidence for androgen inducibility of beta-glucuronidase in the epididymis. Acta Histochem. 1982;70:8–21.
González Echeverría FM, Cuasnicú PS, Blaquier JA. Identification of androgen-dependent glycoproteins in the hamster epididymis and their association with spermatozoa. J Reprod Fertil. 1982;64:1–7.
Jones R, Fournier-Delpech S, Willadsen SA. Identification of androgen-dependent proteins synthesized in vitro by the ram epididymis. Reprod Nutr Dev. 1982;22:495–504.
Mayorga LS, Bertini F. Effect of androgens on the activity of acid hydrolases in rat epididymis. Int J Androl. 1982;5:345–52.
Pholpramool C, White RW, Setchell BP. Influence of androgens on inositol secretion and sperm transport in the epididymis of rats. J Reprod Fertil. 1982;66:547–53.
Rukmini V, Reddy PR. Androgen influences on glucosamine 6-phosphate synthase in the epididymis of the rat. Arch Androl. 1983;11:29–31.
Kjaer K, Kirkeby S, Blecher SR. Histochemical studies on genetical control of hormonal enzyme inducibility in the mouse. VI. Effects of short term castration. Arch Androl. 1983;10:51–5.
Kohane AC, Piñeiro L, Blaquier JA. Androgen-controlled synthesis of specific proteins in the rat epididymis. Endocrinology. 1983;112:1590–6.
Grandmont AM, Chapdelaine P, Tremblay RR. Presence of alpha-glucosidases in the male reproductive system of the rat and hormonal influences. Can J Biochem Cell Biol. 1983;61:764–9.
Mongkolsirikieat S, Chulavatnatol M. Androgenic control of the cyclic AMP-dependent protein kinase isoenzymes of the rat epididymis. J Reprod Fertil. 1983;68:401–5.
Kanai K, Kanamura S, Watanabe J, Asada-Kubota M, Yoshikawa M. Effect of castration and testosterone replacement on high glucose 6-phosphatase activity in principal cells of the mouse epididymis. Anat Rec. 1983;207:289–95.
Goyal HO, Vig MM. Histochemical activity of alkaline phosphatase and acid phosphatase in the epididymis of mature intact and androgen-deprived bulls. Am J Vet Res. 1984;45:444–50.
Mongkolsirikieat S, Chulavatnatol M. Phosphorylated secretory proteins from rat epididymis and their androgenic control. J Reprod Fertil. 1984;72:423–8.
Rajalakshmi M. Hormonal regulation of specific proteins in the rat epididymis. Arch Androl. 1985;14:181–5.
Nemetallah BR, Ellis LC. Prostaglandin dehydrogenase activity of rat and rabbit testicular tissues and accessory glands before and after castration. J Androl. 1985;6:97–101.
Arslan M, Haider MZ, Qazi MH. Characterization and androgen dependence of specific proteins in the epididymis of adult rhesus monkey (Macaca mulatta). Arch Androl. 1986;16:67–74.
Brooks DE, Means AR, Wright EJ, Singh SP, Tiver KK. Molecular cloning of the cDNA for two major androgen-dependent secretory proteins of 18.5 kilodaltons synthesized by the rat epididymis. J Biol Chem. 1986;261:4956–61.
Brooks DE, Means AR, Wright EJ, Singh SP, Tiver KK. Molecular cloning of the cDNA for androgen-dependent sperm-coating glycoproteins secreted by the rat epididymis. Eur J Biochem. 1986;161:13–8.
de las Heras MA, Calandra RS. Androgen-dependence of ornithine decarboxylase in the rat epididymis. J Reprod Fertil. 1987;79:9–14.
Abou-Haïla A, Fain-Maurel MA. Postnatal differentiation and endocrine control of esterase isoenzymes in the mouse epididymis. J Reprod Fertil. 1987;79:437–46.
Klepp O, Magnus K. Some environmental and bodily characteristics of melanoma patients. A case-control study. Int J Cancer. 1979;23:482–6.
Brooks DE. Developmental expression and androgenic regulation of the mRNA for major secretory proteins of the rat epididymis. Mol Cell Endocrinol. 1987;53:59–66.
Mboungou JR, Junera HR, Dadoune JP, Fain-Maurel MA. Characterization and hormonal regulation of tissue and fluid proteins in the mouse epididymis. Reprod Nutr Dev. 1988;28:1275–82.
Holland MK, Orgebin-Crist MC. Characterization and hormonal regulation of protein synthesis by the murine epididymis. Biol Reprod. 1988;38:487–96.
Dacheux F, Dacheux JL. Androgenic control of antagglutinin secretion in the boar epididymal epithelium. An immunocytochemical study. Cell Tissue Res. 1989;255:371–8.
Ghyselinck NB, Jimenez C, Courty Y, Dufaure JP. Androgen-dependent messenger RNA(s) related to secretory proteins in the mouse epididymis. J Reprod Fertil. 1989;85:631–9.
Agrawal YP, Vanha-Perttula T. Gamma-Glutamyl transpeptidase in rat epididymis: effects of castration, hemicastration and efferent duct ligation. Int J Androl. 1989;12:321–8.
Ghyselinck NB, Jimenez C, Lefrançois AM, Dufaure JP. Molecular cloning of a cDNA for androgen-regulated proteins secreted by the mouse epididymis. J Mol Endocrinol. 1990;4:5–12.
Nass SJ, Miller DJ, Winer MA, Ax RL. Male accessory sex glands produce heparin-binding proteins that bind to cauda epididymal spermatozoa and are testosterone dependent. Mol Reprod Dev. 1990;25:237–46.
Grima J, Zwain I, Lockshin RA, Bardin CW, Cheng CY. Diverse secretory patterns of clusterin by epididymis and prostate/seminal vesicles undergoing cell regression after orchiectomy. Endocrinology. 1990;126:2989–97.
Vreeburg JT, Holland MK, Cornwall GA, Orgebin-Crist MC. Secretion and transport of mouse epididymal proteins after injection of 35S-methionine. Biol Reprod. 1990;43:113–20.
Walker JE, Jones R, Moore A, Hamilton DW, Hall L. Analysis of major androgen-regulated cDNA clones from the rat epididymis. Mol Cell Endocrinol. 1990;74:61–8.
Faure J, Ghyselinck NB, Jimenez C, Dufaure JP. Specific distribution of messenger ribonucleic acids for 24-kilodalton proteins in the mouse epididymis as revealed by in situ hybridization: developmental expression and regulation in the adult. Biol Reprod. 1991;44:13–22.
de las Heras MA, Calandra RS. S-adenosyl-L-methionine decarboxylase activity in the rat epididymis: ontogeny and androgenic control. J Androl. 1991;12:209–13.
Viger RS, Robaire B. Differential regulation of steady state 4-ene steroid 5 alpha-reductase messenger ribonucleic acid levels along the rat epididymis. Endocrinology. 1991;128:2407–14.
Abou-Haïla A, Fain-Maurel MA. Selective action of androgens on the molecular forms of esterases characterized by two-dimensional gel electrophoresis in the epididymis and vas deferens of the mouse. Int J Androl. 1991;14:209–22.
Carles C, Fournier-Delpech S, Ribadeau-Dumas B. Purification of an ovine, androgen-dependent epididymal protein. Evidence for a strong amino acid sequence homology with serum albumin. Reprod Nutr Dev. 1992;32:277–84.
Cyr DG, Hermo L, Blaschuk OW, Robaire B. Distribution and regulation of epithelial cadherin messenger ribonucleic acid and immunocytochemical localization of epithelial cadherin in the rat epididymis. Endocrinology. 1992;130:353–63.
Girotti M, Jones R, Emery DC, Chia W, Hall L. Structure and expression of the rat epididymal secretory protein I gene. An androgen-regulated member of the lipocalin superfamily with a rare splice donor site. Biochem J. 1992;281(Pt 1):203–10.
Cyr DG, Robaire B. Regulation of sulfated glycoprotein-2 (clusterin) messenger ribonucleic acid in the rat epididymis. Endocrinology. 1992;130:2160–6.
Holland MK, Vreeburg JT, Orgebin-Crist MC. Testicular regulation of epididymal protein secretion. J Androl. 1992;13:266–73.
Jimenez C, Lefrancois AM, Ghyselinck NB, Dufaure JP. Characterization and hormonal regulation of 24 kDa protein synthesis by the adult murine epididymis. J Endocrinol. 1992;133:197–203.
Cornwall GA, Orgebin-Crist MC, Hann SR. Differential expression of the mouse mitochondrial genes and the mitochondrial RNA-processing endoribonuclease RNA by androgens. Mol Endocrinol. 1992;6:1032–42.
Liu HW, Sun GH, Shy SR, Shyu HY. Postnatal development and testosterone-dependence of GP-83 and GP-49, two sperm maturation-related glycoproteins in BALB/c mouse epididymis. Cell Tissue Res. 1992;269:189–94.
Zwain IH, Grima J, Cheng CY. Rat epididymal retinoic acid-binding protein: development of a radioimmunoassay, its tissue distribution, and its changes in selected androgen-dependent organs after orchiectomy. Endocrinology. 1992;131:1511–26.
Cornwall GA, Orgebin-Crist MC, Hann SR. The CRES gene: a unique testis-regulated gene related to the cystatin family is highly restricted in its expression to the proximal region of the mouse epididymis. Mol Endocrinol. 1992;6:1653–64.
Rigaudière N, Ghyselinck NB, Faure J, Dufaure JP. Regulation of the epididymal glutathione peroxidase-like protein in the mouse: dependence upon androgens and testicular factors. Mol Cell Endocrinol. 1992;89:67–77.
Lefrançois AM, Jimenez C, Dùfaure JP. Developmental expression and androgen regulation of 24 kDa secretory proteins by the murine epididymis. Int J Androl. 1993;16:147–54.
Regalado F, Esponda P, Nieto A. Temperature and androgens regulate the biosynthesis of secretory proteins from rabbit cauda epididymidis. Mol Reprod Dev. 1993;36:448–53.
Gupta G, Srivastava A, Setty BS. Activities and androgenic regulation of kreb cycle enzymes in the epididymis and vas deferens of rhesus monkey. Endocr Res. 1994;20:275–90.
Palladino MA, Hinton BT. Expression of multiple gamma-glutamyl transpeptidase messenger ribonucleic acid transcripts in the adult rat epididymis is differentially regulated by androgens and testicular factors in a region-specific manner. Endocrinology. 1994;135:1146–56.
Hermo L, Barin K, Oko R. Developmental expression of immobilin in the rat epididymis. Anat Rec. 1994;240:86–103.
Hermo L, Barin K, Oko R. Developmental expression of sulfated glycoprotein-2 in the epididymis of the rat. Anat Rec. 1994;240:327–44.
Winer MA, Wolgemuth DJ. The segment-specific pattern of A-raf expression in the mouse epididymis is regulated by testicular factors. Endocrinology. 1995;136:2561–72.
Uchendu CN. Renin-like activity in the rat epididymis. Indian J Physiol Pharmacol. 1995;39:204–8.
Gupta G, Setty BS. Activities and androgenic regulation of lysosomal enzymes in the epididymis of rhesus monkey. Endocr Res. 1995;21:733–41.
Viger RS, Robaire B. The mRNAs for the steroid 5 alpha-reductase isozymes, types 1 and 2, are differentially regulated in the rat epididymis. J Androl. 1996;17:27–34.
Hermo L, Papp S. Effects of ligation, orchidectomy, and hypophysectomy on expression of the Yf subunit of GST-P in principal and basal cells of the adult rat epididymis and on basal cell shape and overall arrangement. Anat Rec. 1996;244:59–69.
Bérubé B, Lefièvre L, Coutu L, Sullivan R. Regulation of the epididymal synthesis of P26h, a hamster sperm protein. J Androl. 1996;17:104–10.
Cyr DG, Hermo L, Laird DW. Immunocytochemical localization and regulation of connexin43 in the adult rat epididymis. Endocrinology. 1996;137:1474–84.
Abou-Haila A, Tulsiani DR, Skudlarek MD, Orgebin-Crist MC. Androgen regulation of molecular forms of beta-D-glucuronidase in the mouse epididymis: comparison with liver and kidney. J Androl. 1996;17:194–207.
Kalla NR, Kaur S, Ujwal N, Mehta U, Joos H, Frick J. Alpha-glucosidase activity in the rat epididymis under different physiological conditions. Int J Androl. 1997;20:92–5.
Cornwall GA, Hsia N. ADAM7, a member of the ADAM (a disintegrin and metalloprotease) gene family is specifically expressed in the mouse anterior pituitary and epididymis. Endocrinology. 1997;138:4262–72.
Rudolph DB, Hinton BT. Stability and transcriptional regulation of gamma-glutamyl transpeptidase mRNA expression in the initial segment of the rat epididymis. J Androl. 1997;18:501–12.
Pera I, Derr P, Yeung CH, Cooper TG, Kirchhoff C. Regionalized expression of CD52 in rat epididymis is related to mRNA poly(A) tail length. Mol Reprod Dev. 1997;48:433–41.
Abou-Haila A, Orgebin-Crist MC, Skudlarek MD, Tulsiani DR. Identification and androgen regulation of egasyn in the mouse epididymis. Biochim Biophys Acta. 1998;1401:177–86.
Sorrentino C, Silvestrini B, Braghiroli L, Chung SS, Giacomelli S, Leone MG, et al. Rat prostaglandin D2 synthetase: its tissue distribution, changes during maturation, and regulation in the testis and epididymis. Biol Reprod. 1998;59:843–53.
Schwaab V, Faure J, Dufaure JP, Drevet JR. GPx3: the plasma-type glutathione peroxidase is expressed under androgenic control in the mouse epididymis and vas deferens. Mol Reprod Dev. 1998;51:362–72.
Fouchécourt S, Dacheux F, Dacheux JL. Glutathione-independent prostaglandin D2 synthase in ram and stallion epididymal fluids: origin and regulation. Biol Reprod. 1999;60:558–66.
Sivashanmugam P, Richardson RT, Hall S, Hamil KG, French FS, O’Rand MG. Cloning and characterization of an androgen-dependent acidic epididymal glycoprotein/CRISP1-like protein from the monkey. J Androl. 1999;20:384–93.
Kaunisto K, Fleming RE, Kneer J, Sly WS, Rajaniemi H. Regional expression and androgen regulation of carbonic anhydrase IV and II in the adult rat epididymis. Biol Reprod. 1999;61:1521–6.
Syntin P, Dacheux JL, Dacheux F. Postnatal development and regulation of proteins secreted in the boar epididymis. Biol Reprod. 1999;61:1622–35.
Hermo L, Xiaohong S, Morales CR. Circulating and luminal testicular factors affect LRP-2 and apo J expression in the epididymis following efferent duct ligation. J Androl. 2000;21:122–44.
Leung PS, Wong TP, Lam SY, Chan HC, Wong PY. Testicular hormonal regulation of the renin-angiotensin system in the rat epididymis. Life Sci. 2000;66:1317–24.
Hamil KG, Sivashanmugam P, Richardson RT, Grossman G, Ruben SM, Mohler JL, et al. HE2beta and HE2gamma, new members of an epididymis-specific family of androgen-regulated proteins in the human. Endocrinology. 2000;141:1245–53.
Mathur PP, Marshall A, Cheng CY. Protein profiles in various epididymal segments of normal and castrated rats. Asian J Androl. 2000;2:57–64.
Desai KV, Kondaiah P. Androgen ablation results in differential regulation of transforming growth factor-beta isoforms in rat male accessory sex organs and epididymis. J Mol Endocrinol. 2000;24:253–60.
Cheuk BL, Leung PS, Lo AC, Wong PY. Androgen control of cyclooxygenase expression in the rat epididymis. Biol Reprod. 2000;63:775–80.
Cyr DG, Dufresne J, Pillet S, Alfieri TJ, Hermo L. Expression and regulation of metallothioneins in the rat epididymis. J Androl. 2001;22:124–35.
Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–63.
Lareyre JJ, Winfrey VP, Kasper S, Ong DE, Matusik RJ, Olson GE, et al. Gene duplication gives rise to a new 17-kilodalton lipocalin that shows epididymal region-specific expression and testicular factor(s) regulation. Endocrinology. 2001;142:1296–308.
Ezer NN, Robaire B. Gene expression is selectively affected along the epididymis after Orchidectomy. ScientificWorldJournal. 2001;1:56.
Andonian S, Adamali H, Hermo L. Expression and regulation of H + K + ATPase in lysosomes of epithelial cells of the adult rat epididymis. Mol Reprod Dev. 2001;58:398–410.
Nonaka MI, Wang G, Mori T, Okada H, Nonaka M. Novel androgen-dependent promoters direct expression of the C4b-binding protein alpha-chain gene in epididymis. J Immunol. 2001;166:4570–7.
Yang G, Gregory CW, Shang Q, O’Brien DA, Zhang YL. Differential expression of CCAAT/enhancer-binding protein-delta (c/EBPdelta) in rat androgen-dependent tissues and human prostate cancer. J Androl. 2001;22:471–80.
Cornwall GA, Collis R, Xiao Q, Hsia N, Hann SR. B-Myc, a proximal caput epididymal protein, is dependent on androgens and testicular factors for expression. Biol Reprod. 2001;64:1600–7.
Ibrahim NM, Young LG, Fröhlich O. Epididymal specificity and androgen regulation of rat EP2. Biol Reprod. 2001;65:575–80.
Nixon B, Hardy CM, Jones RC, Andrews JB, Holland MK. Rabbit epididymal secretory proteins. III. Molecular cloning and characterization of the complementary DNA for REP38. Biol Reprod. 2002;67:147–53.
Leung PS, Wong TP, Chung YW, Chan HC. Androgen dependent expression of AT1 receptor and its regulation of anion secretion in rat epididymis. Cell Biol Int. 2002;26:117–22.
Turner TT, Bomgardner D. On the regulation of Crisp-1 mRNA expression and protein secretion by luminal factors presented in vivo by microperfusion of the rat proximal caput epididymidis. Mol Reprod Dev. 2002;61:437–44.
Luedtke CC, McKee MD, Cyr DG, Gregory M, Kaartinen MT, Mui J, et al. Osteopontin expression and regulation in the testis, efferent ducts, and epididymis of rats during postnatal development through to adulthood. Biol Reprod. 2002;66:1437–48.
Badran HH, Hermo LS. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl. 2002;23:358–73.
Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van Hoek A, Brown D, et al. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod. 2002;66:1716–22.
Nixon B, Jones RC, Hansen LA, Holland MK. Rabbit epididymal secretory proteins. I. characterization and hormonal regulation. Biol Reprod. 2002;67:133–9.
Tong MH, Song W-C. Estrogen sulfotransferase: discrete and androgen-dependent expression in the male reproductive tract and demonstration of an in vivo function in the mouse epididymis. Endocrinology. 2002;143:3144–51.
Palladino MA, Mallonga TA, Mishra MS. Messenger RNA (mRNA) expression for the antimicrobial peptides beta-defensin-1 and beta-defensin-2 in the male rat reproductive tract: beta-defensin-1 mRNA in initial segment and caput epididymidis is regulated by androgens and not bacterial lipopolysaccharides. Biol Reprod. 2003;68:509–15.
Fouchécourt S, Lareyre J-J, Chaurand P, DaGue BB, Suzuki K, Ong DE, et al. Identification, immunolocalization, regulation, and postnatal development of the lipocalin EP17 (epididymal protein of 17 kilodaltons) in the mouse and rat epididymis. Endocrinology. 2003;144:887–900.
Hsia N, Cornwall GA. Cres2 and Cres3: new members of the cystatin-related epididymal spermatogenic subgroup of family 2 cystatins. Endocrinology. 2003;144:909–15.
Ezer N, Robaire B. Gene expression is differentially regulated in the epididymis after orchidectomy. Endocrinology. 2003;144:975–88.
Hu Y, Zhou Z, Xu C, Shang Q, Zhang Y-D, Zhang Y-L. Androgen down-regulated and region-specific expression of germ cell nuclear factor in mouse epididymis. Endocrinology. 2003;144:1612–9.
Joshi SA, Shaikh S, Ranpura S, Khole VV. Postnatal development and testosterone dependence of a rat epididymal protein identified by neonatal tolerization. Reproduction. 2003;125:495–507.
Li Y, Friel PJ, McLean DJ, Griswold MD. Cystatin E1 and E2, new members of male reproductive tract subgroup within cystatin type 2 family. Biol Reprod. 2003;69:489–500.
Andonian S, Hermo L. Immunolocalization of the Yb1 subunit of glutathione S-transferase in the adult rat epididymis following orchidectomy and efferent duct ligation. J Androl. 2003;24:577–87.
Hermo L, Adamali HI, Trasler JM. Postnatal development and regulation of beta-hexosaminidase in epithelial cells of the rat epididymis. J Androl. 2004;25:69–81.
Zhu H, Ma H, Ni H, Ma X-H, Mills N, Yang Z-M. Expression and regulation of lipocalin-type prostaglandin d synthase in rat testis and epididymis. Biol Reprod. 2004;70:1088–95.
Kappler-Hanno K, Kirchhoff C. Rodent epididymal cDNAs identified by sequence homology to human and canine counterparts. Asian J Androl. 2003;5:277–86.
Zhang H, Jones R, Martin-DeLeon PA. Expression and secretion of rat SPAM1(2B1 or PH-20) in the epididymis: role of testicular lumicrine factors. Matrix Biol. 2004;22:653–61.
Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505.
Turner TT, Bomgardner D, Jacobs JP. Sonic hedgehog pathway genes are expressed and transcribed in the adult mouse epididymis. J Androl. 2004;25:514–22.
Zhu H, Ma H, Ni H, Ma X-H, Mills N, Yang Z-M. L-prostaglandin D synthase expression and regulation in mouse testis and epididymis during sexual maturation and testosterone treatment after castration. Endocrine. 2004;24:39–45.
Suzuki K, Lareyre J-J, Sánchez D, Gutierrez G, Araki Y, Matusik RJ, et al. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene. 2004;339:49–59.
Maróstica E, Avellar MCW, Porto CS. Effects of testosterone on muscarinic acetylcholine receptors in the rat epididymis. Life Sci. 2005;77:656–69.
Li Y, Putnam-Lawson CA, Knapp-Hoch H, Friel PJ, Mitchell D, Hively R, et al. Immunolocalization and regulation of cystatin 12 in mouse testis and epididymis. Biol Reprod. 2005;73:872–80.
Carroll M, Hamzeh M, Robaire B. Expression, localization, and regulation of inhibitor of DNA binding (id) proteins in the rat epididymis. J Androl. 2006;27:212–24.
Yuan H, Liu A, Zhang L, Zhou H, Wang Y, Zhang H, et al. Proteomic profiling of regionalized proteins in rat epididymis indicates consistency between specialized distribution and protein functions. J Proteome Res. 2006;5:299–307.
Yenugu S, Chintalgattu V, Wingard CJ, Radhakrishnan Y, French FS, Hall SH. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus). Reprod Biol Endocrinol. 2006;4:7.
Shayu D, Rao AJ. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol Cell Endocrinol. 2006;249:40–50.
Sipilä P, Pujianto DA, Shariatmadari R, Nikkilä J, Lehtoranta M, Huhtaniemi IT, et al. Differential endocrine regulation of genes enriched in initial segment and distal caput of the mouse epididymis as revealed by genome-wide expression profiling. Biol Reprod. 2006;75:240–51.
Yenugu S, Hamil KG, Grossman G, Petrusz P, French FS, Hall SH. Identification, cloning and functional characterization of novel sperm associated antigen 11 (SPAG11) isoforms in the rat. Reprod Biol Endocrinol. 2006;4:23.
Yamazaki K, Adachi T, Sato K, Yanagisawa Y, Fukata H, Seki N, et al. Identification and characterization of novel and unknown mouse epididymis-specific genes by complementary DNA microarray technology. Biol Reprod. 2006;75:462–8.
Jalkanen J, Kotimäki M, Huhtaniemi I, Poutanen M. Novel epididymal protease inhibitors with Kazal or WAP family domain. Biochem Biophys Res Commun. 2006;349:245–54.
Davies B, Behnen M, Cappallo-Obermann H, Spiess A-N, Theuring F, Kirchhoff C. Novel epididymis-specific mRNAs downregulated by HE6/Gpr64 receptor gene disruption. Mol Reprod Dev. 2007;74:539–53.
Primiani N, Gregory M, Dufresne J, Smith CE, Liu YL, Bartles JR, et al. Microvillar size and espin expression in principal cells of the adult rat epididymis are regulated by androgens. J Androl. 2007;28:659–69.
Prabagaran E, Hegde UC, Moodbidri SB, Bandivdekar AH, Raghavan VP. Postnatal expression and androgen regulation of HOXBES2 homeoprotein in rat epididymis. J Androl. 2007;28:755–71.
Hamzeh M, Robaire B. Identification of early response genes and pathway activated by androgens in the initial segment and caput regions of the regressed rat epididymis. Endocrinology. 2010;151:4504–14.
Ding N-Z, He M, He C-Q, Hu J-S, Teng J, Chen J. Expression and regulation of FAAP in the mouse epididymis. Endocrine. 2010;38:188–93.
Ma L, Li W, Zhu H-P, Li Z, Sun Z-J, Liu X-P, et al. Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis. PLoS ONE. 2010;5:e15439.
Rajesh A, Madhubabu G, Yenugu S. Identification and characterization of Wfdc gene expression in the male reproductive tract of the rat. Mol Reprod Dev. 2011;78:633–41.
Suryawanshi AR, Khan SA, Joshi CS, Khole VV. Epididymosome-mediated acquisition of MMSDH, an androgen-dependent and developmentally regulated epididymal sperm protein. J Androl. 2012;33:963–74.
Li X, Zhan X, Liu S, Hu S, Zhu C, Hall SH, et al. Cloning and primary characterizations of rLcn9, a new member of epididymal lipocalins in rat. Acta Biochim Biophys Sin (Shanghai). 2012;44:876–85.
Silva EJR, Patrão MTCC, Tsuruta JK, O’Rand MG, Avellar MCW. Epididymal protease inhibitor (EPPIN) is differentially expressed in the male rat reproductive tract and immunolocalized in maturing spermatozoa. Mol Reprod Dev. 2012;79:832–42.
Carvelli L, Bannoud N, Aguilera AC, Sartor T, Malossi E, Sosa MA. Testosterone influences the expression and distribution of the cation-dependent mannose-6-phosphate receptor in rat epididymis. Implications in the distribution of enzymes. Andrologia. 2014;46:224–30.
Li K, Liu Y, Xia X, Wang L, Lu M, Hu Y, et al. Bactericidal/permeability-increasing protein in the reproductive system of male mice may be involved in the sperm-oocyte fusion. Reproduction. 2013;146:135–44.
Pujianto DA, Loanda E, Sari P, Midoen YH, Soeharso P. Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod Biol Endocrinol. 2013;11:59.
Rengaraj D, Hwang YS, Liang XH, Deng WB, Yang ZM, Han JY. Comparative expression and regulation of TMSB4X in male reproductive tissues of rats and chickens. J Exp Zool Ecol Genet Physiol. 2013;319:584–95.
Wang C-M, Hu S-G, Ru Y-F, Yao G-X, Ma W-B, Gu Y-H, et al. Different effects of androgen on the expression of Fut1, Fut2, Fut4 and Fut9 in male mouse reproductive tract. Int J Mol Sci. 2013;14:23188–202.
Rengaraj D, Gao F, Liang X-H, Yang Z-M. Expression and regulation of type II integral membrane protein family members in mouse male reproductive tissues. Endocrine. 2007;31:193–201.
Rengaraj D, Liang X-H, Gao F, Deng W-B, Mills N, Yang Z-M. Differential expression and regulation of integral membrane protein 2b in rat male reproductive tissues. Asian J Androl. 2008;10:503–11.
Hu S-G, Zou M, Yao G-X, Ma W-B, Zhu Q-L, Li X-Q, et al. Androgenic regulation of beta-defensins in the mouse epididymis. Reprod Biol Endocrinol. 2014;12:76.
Liu X, Wang W, Liu F. New insight into the castrated mouse epididymis based on comparative proteomics. Reprod Fertil Dev. 2015;27:551–6.
Ribeiro CM, Queiróz DBC, Patrão MTCC, Denadai-Souza A, Romano RM, Silva EJR, et al. Dynamic changes in the spatio-temporal expression of the β-defensin SPAG11C in the developing rat epididymis and its regulation by androgens. Mol Cell Endocrinol. 2015;404:141–50.
Xin A, Zhao Y, Yu H, Shi H, Diao H, Zhang Y. Characterization of β-defensin 42 expressed in principal cells at the initial segment of the rat epididymis. Acta Biochim Biophys Sin (Shanghai). 2015;47:861–9.
Li Y, Wang H, Qin Y, Liu J, Li N, Ji Z, et al. Deep sequencing reveals microRNA signature is altered in the rat epididymis following bilateral castration. Genes Genomics. 2019;41:757–66.
Pujianto DA, Muliawati D, Rizki MD, Parisudha A, Hardiyanto L. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod Biol. 2020;20:536–40.
Carvelli L, Aguilera AC, Zyla L, Pereyra LL, Morales CR, Hermo L, et al. Castration causes an increase in lysosomal size and upregulation of cathepsin D expression in principal cells along with increased secretion of procathepsin D and prosaposin oligomers in adult rat epididymis. PLoS ONE. 2021;16:e0250454.
Pujianto DA, Permatasari S. Mouse CD52 is predominantly expressed in the Cauda Epididymis, regulated by Androgen and lumicrine factors. J Hum Reprod Sci. 2021;14:350–5.
Wijayarathna R, Genovese R, Meinhardt A, Loveland KL, Groome NP, Hinton BT, et al. Examination of testicular lumicrine regulation of activins and immunoregulatory genes in the epididymal caput. Andrology. 2022;10:190–201.
Jones RC, Stone GM, Hinds LA, Setchell BP. Distribution of 5 alpha-reductase in the epididymis of the tammar wallaby (Macropus eugenii) and dependence of the epididymis on systemic testosterone and luminal fluids from the testis. J Reprod Fertil. 1988;83:779–83.
Chu C, Zhang YL, Yu L, Sharma S, Fei ZL, Drevet JR. Epididymal small non-coding RNA studies: progress over the past decade. Andrology. 2019;7:681–9.
Yang L, Fox SA, Kirby JL, Troan BV, Hinton BT. Putative regulation of expression of members of the ets variant 4 transcription factor family and their downstream targets in the rat epididymis. Biol Reprod. 2006;74:714–20.
Jones RC. Evolution of the vertebrate epididymis. J Reprod Fertil Suppl. 1998;53:163–81.
Acknowledgements
The author acknowledges the NGS core facility at the Research Institute for Microbial Diseases of Osaka University for the sequencing and data analysis.
Funding
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP21H02487, JP21H00231, and JP21K19263), Japan Science and Technology Agency (JPMJPR2143), the Japan Foundation for Applied Enzymology (2023-10), the Chugai Foundation for Innovative Drug Discovery Science (2022-I-05), the UBE Foundation, and the Uehara Memorial Foundation to DK.
Author information
Authors and Affiliations
Contributions
D.K. designed and performed experiments. D.K. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All experiments involving animals were approved by the Institutional Animal Care and Use Committees of Osaka University (Osaka, Japan) and were conducted in compliance with the university guidelines and regulations for animal experimentation.
Consent for publication
Not applicable.
Declaration of generative AI in scientific writing
The authors declare that no Generative AI was used in preparing the manuscript.
Submission declaration and verification
The authors declare that this manuscript has not been published previously and is not under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kiyozumi, D. Distinct actions of testicular endocrine and lumicrine signaling on the proximal epididymal transcriptome. Reprod Biol Endocrinol 22, 40 (2024). https://doi.org/10.1186/s12958-024-01213-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-024-01213-x