Abstract
Non-muscle myosin heavy chain IIA (MYH9), a member of the non-muscle myosin II (NM II) family, is widely expressed in cells. The interaction of MYH9 with actin in the cytoplasm can hydrolyze ATP, completing the conversion of chemical energy to mechanical motion. MYH9 participates in various cellular processes, such as cell adhesion, migration, movement, and even signal transduction. Mutations in MYH9 are often associated with autosomal dominant platelet disorders and kidney diseases. Over the past decade, tumor-related research has gradually revealed a close relationship between MYH9 and the occurrence and development of tumors. This article provides a review of the research progress on the role of MYH9 in cancer regulation. We also discussed the anti-cancer effects of MYH9 under special circumstances, as well as its regulation of T cell function. In addition, given the importance of MYH9 as a key hub in oncogenic signal transduction, we summarize the current therapeutic strategies targeting MYH9 as well as the ongoing challenges.
Graphical Abstract

Similar content being viewed by others
Introduction
Cancer is a leading cause of death worldwide. About one in six women and one in five men develop cancer in their lifetime. In 2018, 9.6 million people died of cancer [1]. Traditional treatments, including surgery, radiotherapy and chemotherapy, serve as the cornerstone of cancer treatment. In contrast, targeted therapy, immunotherapy and gene therapy are emerging cancer treatments that possess high specificity, fewer side effects and long-term effectiveness [2, 3].
Non-muscle myosin heavy chain IIA (MYH9), also known as NMMHC-IIA, is a ubiquitously expressed cytoplasmic myosin, encoded by the MYH9 gene. MYH9 was initially discovered because of platelet disorders caused by mutations in the MYH9 gene, and MYH9-related platelet disorder is a common autosomal dominant inherited disease [4]. MYH9, functions as an intracellular molecular motor that interacts with actin and plays a pivotal role in the generation of chemomechanical forces within the cell and the subsequent reorganization of the actin cytoskeleton. It is indispensable in maintaining signal transduction and cellular morphology and is closely associated with various cellular biological processes, including cell migration, adhesion, polarization, and cytokinesis [5, 6]. Recently, MYH9 has been a key factor in cancer development and progression. Researchers have observed that high MYH9 expression is strongly associated with the metastasis and recurrence of esophageal, colorectal, non-small cell lung, breast, kidney, and gastric cancers [7,8,9,10,11,12]. In acute myeloid leukemia, the expression level of MYH9 can even be used as a prognostic indicator. MYH9 expression has also been shown to be associated with the survival rate of patients with resected non-small cell lung cancer8. However, the regulatory mechanisms of MYH9-related signaling pathways and their roles in cancer development have not yet been elucidated.
The association between MYH9 and malignant progression of cancer, particularly cancer metastasis, has achieved consensus among many researchers. However, most reviews of MYH9 have focused on its role in kidney disease, and a detailed summary of its role in cancer is required. In this review, we describe the regulation of MYH9 by its interacting proteins and non-coding RNAs, as well as the mutual regulation between numerous signaling pathways and MYH9, making MYH9 an important hub in oncogenic signaling. Finally, given the potent oncogenic capability of MYH9, we summarized the current therapeutic strategies targeting MYH9.
Structure, function and role of MYH9
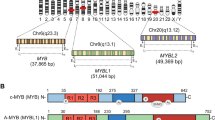
MYH9 is a gene located at the chromosomal locus 22q12.3, boasting a length exceeding 106 kbp and comprising 41 exons. Its open reading frame, extending from the second to the 41st exons, encodes a protein composed of 1 960 amino acids, known as MYH9. Investigations into the promoter region of the MYH9 gene have revealed that it is a quintessential housekeeping gene, devoid of a TATA box but rich in GC content and replete with multiple GC boxes. Furthermore, two enhancer regions were identified within intron 1, downstream of the promoter, located 23–150 kb apart [5].
In line with all type II myosins, non-muscle myosin of class II, isoform A (NM IIA) is a hexameric entity composed of two heavy chains (230 kDa), two regulatory light chains (20 kDa) that modulate myosin function, and two essential light chains (17 kDa) that maintain the structural integrity of the heavy chain. Figure 1 illustrates the structure and function of NM IIA. Each heavy chain, also referred to as MYH9, binds to a regulatory and essential light chain, culminating in the formation of a trimeric complex. Subsequently, two such trimeric complexes amalgamate to give rise to hexameric assemblies. Comprising an N-terminal motor domain and a C-terminal tail domain, each heavy chain is structured such that the motor domain at the N-terminus includes the motor (a spherical head encoded by exons 1–19 of MYH9) and neck (exon 20) regions. The tail domain at the C-terminus features an extended coiled-coil segment (exons 21–40) and a concise, nonhelical tailpiece (exon 41). The head, neck, and tail domains of MYH9 have indispensable and unique functions. The head domain operates as an ATPase capable of hydrolyzing ATP to generate a mechanical force that enables MYH9 to bind to actin and facilitate its movement. The neck domain, which is akin to a lever, amplifies the movement induced by conformational changes in the head domain and provides a binding site for the two light chains. The tail domain, distinguished by its fibrous α-helical structure, plays a pivotal role in orchestrating the assembly of NM IIA functional filaments and promoting the dimerization of MYH9 [13, 14]. The intricate interplay between its structure and function underscores the indispensable role of MYH9 in cellular biology.
The MYH9 gene is pivotal in numerous disease processes and has been linked to a range of autosomal dominant disorders, collectively known as MYH9-related disorders (MYH9-RD). These disorders include the May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian platelet syndrome. All patients with MYH9-RD present with thrombocytopenia, giant platelets, and bleeding. In the early stages of the disease, the patients often exhibit mild symptoms; however, many patients experience a deterioration in their condition, which is directly proportional to time. Typically, patients develop hearing impairment, renal disease, and cataracts after the age of 50 [15, 16]. Studies have suggested that ablation of the MYH9 gene is linked to embryonic lethality in mice, indicating that MYH9 encompasses a multitude of yet-to-be-fully elucidated and irreplaceable functions [17].
MYH9 as an oncogene
MYH9 is a crucial oncogene implicated in various cancers, and its expression levels are elevated and correlated with tumor malignancy and prognosis. MYH9 influences cancer progression through several mechanisms, including protein interactions and regulation by microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). In addition, MYH9 plays a role in signal transduction in cancer cells. This article provides a detailed discussion of the role of MYH9 in cancer progression and its potential as a prospective anticancer target.
MYH9 and interacting proteins
The expression and stability of MYH9 are regulated by a variety of factors in cancer, with protein-protein interactions being of paramount importance. This process also involves post-translational modifications of MYH9 and its target proteins, including ubiquitination, deubiquitination, phosphorylation, and acetylation (Fig. 2). Autophagy-related protein 9 B (ATG9B) serves as a potential target for colorectal cancer metastasis, and its high expression is associated with a poor prognosis. Interestingly, ATG9B promotes colorectal cancer metastasis by interacting with MYH9 rather than through autophagy. The aa368-411 fragment of ATG9B binds to the head domain of MYH9 to antagonize the linkage of the E3 ubiquitin ligase STUB1, thereby preventing ubiquitination degradation and enhancing the stability of both proteins. Increased ATG9B stability facilitates integrin β1 activation and cancer cell invasion [18]. The functional domain of MYH10 interacts with MYH9 to mediate the recruitment of the deubiquitinating enzyme ubiquitin-specific proteases 45, leading to the deubiquitination of the snail and inhibition of its degradation. This process promotes the progression and cisplatin resistance of serous ovarian cancer (SOC). In SOC, the level of coexpression of MYH10+/MYH9 + can be used for prognostic prediction in patients [19]. High-level expression of microtubule-associated protein 7 domain-containing protein 2 (MAP7D2) in microsatellite-stable colorectal cancer (CRC) is negatively correlated with the infiltration of antitumor T lymphocytes. MAP7D2 binds to MYH9 and protects itself from ubiquitination, which can reduce the protein levels of HMGB1 and limit the presence of CD8 cytotoxic T lymphocytes in microsatellite-stable CRC. Targeting MAP7D2 or MYH9 can enhance the efficacy of antitumor immunotherapies [20]. In addition to ubiquitination and deubiquitination, which mediate the pro-carcinogenic effects of MYH9, phosphorylation, dephosphorylation, and acetylation play equally important roles. Protein tyrosine phosphatase 1B (PTP1B), a member of the tyrosine phosphatase family, plays a crucial role in the metastasis of esophageal squamous cell carcinoma (ESCC) and is dependent on its phosphatase activity. PTP1B promotes EGFR protein expression and ESCC metastasis by binding to and dephosphorylating Y1408 of MYH921. Hepatitis B X-interacting protein (HBXIP) also directly interacts with MYH9, and this interaction is enhanced by the phosphorylation of MYH9 mediated by the protein kinase PKCβII, which is recruited by HBXIP. In addition to the protein kinase PKCβII recruited by HBXIP, HBXIP can activate SP1 to promote the transcription of PRKCB (which encodes protein kinase PKCβII). Phosphorylated MYH9 blocks myosin-IIA assembly and promotes breast cancer [22]. Timeless protein, encoded by the clock gene TIMELESS is highly expressed in CRC tissues, and its high expression is associated with H3K27 acetylation in its promoter region. Timeless interacts with MYH9 to maintain its stability and activate the β-catenin signaling pathway. Timeless further regulates CRC tumorigenesis through interaction with MYH9 [23]. In a study, 107 proteins showed upregulated acetylation levels in mice with a histone deacetylase 6 (HDAC6) knockout. Among these, MYH9 acts as a key substrate of HDAC6. HDAC6 influences the ability of MYH9 to bind actin by mediating its deacetylation [24]. In summary, the post-translational modification of MYH9 and its target proteins affects their stability and function, promoting cancer progression.
MYH9 and its interacting proteins promote cancer progression by regulating post-translational modifications of target proteins, establishing feedback loops that affect their own expression, and controlling other signaling pathways (Created by Biorender.com). MYH9, non-muscle myosin heavy chain IIA; miRNAs, microRNAs; RhoA, Ras homolog family member A; CTNNB1, Catenin Beta 1; MICAL2, Microtubule Associated Monooxygenase, Calponin And LIM Domain Containing 2; GSK3β, Glycogen Synthase Kinase 3 Beta; β-catenin, Catenin Beta 1; c-Jun, Transcription Factor Jun; PI3K, Phosphatidylinositol 3-Kinase; AKT, Protein Kinase B; c-Myc, Myc Proto-Oncogene Protein; P53, Tumor Protein P53; ERK, Extracellular Signal-Regulated Kinases; ETV4, ETS Variant Transcription Factor 4; WNT, Wingless/Integration-1
Beyond the impact of post-translational modifications on its stability and function, MYH9 also engages in protein-protein interactions, establishing a regulatory feedback loop that influences its expression levels. In hepatocellular carcinoma (HCC), MYH9 interacts with GSK3β and induces ubiquitination degradation of GSK3β, which promotes cancer stemness, epithelial-mesenchymal transition (EMT), and c-Jun signaling through the mediation of β-catenin. Interestingly, c-Jun signaling further upregulates MYH9 expression, forming a MYH9/GSK3β/β-catenin/c-Jun/MYH9 regulatory loop. HBX interacts with MYH9 to regulate this loop, and targeting MYH9 is an excellent strategy for inhibiting cancer stemness [25]. Additionally, MYH9 interacts with FOXO1 and downregulates its expression through the PI3K/AKT/c-Myc/P53/miR-133a-3p signaling pathway. The suppression of MYH9 expression concurrently downregulates its interaction with GSK3β, TRAF6 expression, and TRAF6-mediated ubiquitination degradation of GSK3β. These events lead to an increase in GSK3β protein levels and enhance the stemness and EMT levels of nasopharyngeal carcinoma through the β-catenin/TCF4/ZEB1/miR-200b signaling pathway [26]. In another study, a feedback loop was observed between MYH9 and p53. However, instead of promoting the expression of MYH9, this interaction activates p38. Mucin-17, an essential component of the gastric mucosal barrier, plays a pivotal role in inhibiting gastric cancer progression through interaction with MYH9 via the EGF structural domain. Specifically, the interaction between Mucin-17 and MYH9 mediates a feedback loop involving MYH9, RhoA, and p53, which further activates p38 to suppress the NFκB pathway and inflammatory response in gastric cancer cells [27]. Furthermore, these interactions serve to modulate additional signaling pathways, thereby expanding the scope of MYH9’s functional influence. Human tubulin beta class IVa (TUBB4A) is highly expressed only in prostate cancer, and is almost non-existent in many normal tissues. TUBB4A interacts with GSK3β and connects to the N-terminus of MYH9 to regulate GSK3β ubiquitination. This not only protects the cell nucleus during cell migration, but also regulates β-catenin signaling to promote EMT and the progression of prostate cancer [28]. There is evidence that the interplay between CRLF1 and MYH9 promotes the progression of papillary thyroid carcinoma via the ERK/ETV4 signaling pathway. Concurrently, the interaction between FNDC3B and MYH9 fosters the progression of nasopharyngeal carcinoma through the Wnt/β-catenin signaling pathway [29, 30]. In metastatic gastric cancer tissues, MYH9 expression is upregulated and localized to the nuclei of cancer cells through four nuclear localization signals. MYH9 interacts with myosin light chain 9, RNA polymerase II, and β-actin in the nuclei of cancer cells and binds to the CTNNB1 promoter, thereby promoting the transcription of CTNNB1 and metastasis of gastric cancer cells [31]. The interaction of MYH9 with other proteins significantly influences mitochondrial functionality and the quantity of lipid droplets. Microfilament-associated proteins 2 and 3 regulate the recruitment of MYH9 by modulating lipid droplet binding to F-actin. The recruited MYH9, in turn, can bind to microfilament-associated proteins 2 and 3 as well as lipid droplets, thereby regulating the motility and quantity of lipid droplets [32]. Cisplatin, a fundamental therapeutic agent for multiple cancers, induces severe acute kidney injury by inducing mitochondrial fragmentation. This process is mediated through the modulation of the interaction between apyrimidinic endonuclease 2 (APE2) and MYH9 in the mitochondria [33]. In another study, the micropeptide short transmembrane protein 1 (STMP1), located in the inner mitochondrial membrane, was associated with HCC metastasis. STMP1 enhances mitochondrial fission and cancer cell migration by promoting the activation of dynamin-related protein 1. Notably, this process is contingent on interactions between STMP1 and MYH9 [34]. More importantly, the interaction between MYH9 and other proteins can modulate its intracellular localization to mediate cancer cell invasiveness. Alpha-actinin-4 indirectly binds to MYH9 via F-actin, which regulates the invasiveness of cancer cells by modulating the localization of MYH9 [35]. In another study, the protein levels of MYH9 determined the subcellular localization of the nucleocytoplasmic shuttling protein MICAL2, thereby enhancing its role in the growth and invasion of lung adenocarcinoma [36].
In summary, MYH9 plays a crucial role in cancer onset and progression by interacting with various proteins. These interactions encompass a variety of distinct signaling pathways and biological processes. These findings provide a theoretical basis for the development of novel anticancer strategies targeting MYH9. The majority of studies on MYH9 and its target proteins in influencing the behavior of cancer cells have primarily focused on the invasion and metastasis of cancer cells. However, it remains unclear how MYH9 impacts cancer stemness, the cell cycle, apoptosis, and proliferation. On the other hand, tumor immunotherapy has become a significant means of cancer treatment, and MYH9, as an essential cytoskeletal protein, may play a crucial role in tumor immune responses. Yet, related studies have not touched upon the role of MYH9 in tumor immune responses. These issues will contribute to subsequent research by other researchers and better exploration of the mechanisms of tumor development.
MYH9 and non-coding RNAs
Non-coding RNAs, including miRNAs, lncRNAs, and circRNAs, regulate the expression and activity of MYH9 (Fig. 3). The intricate regulation of MYH9 by these non-coding RNAs plays a pivotal role in facilitating cancer onset, progression, and metastasis. CircRNAs, derived from the exons of coding genes, are a type of closed-loop RNA that is ubiquitously present within cells and participates in various biological processes. CircRNAs play a significant role in tumorigenesis and can serve as diagnostic markers and therapeutic targets in cancer. Based on research conducted over the past 5 years, it was discovered that circRNAs frequently regulate MYH9 and subsequent cancer progression through the modulation of miRNAs and their protein-coding capabilities. The protein-encoding potential of circRNAs has recently emerged as a burgeoning area of interest. Circ-EIF6 has been correlated with adverse prognostic outcomes and clinicopathological characteristics in triple-negative breast cancer. It harbors a unique open reading frame comprising 675 nucleotides and an internal ribosome entry site, specifically the − 150-bp sequence upstream of the ATG start codon. These attributes confer on circ-EIF6 the capacity to encode the protein EIF6-224aa. Notably, EIF6-224aa impedes the degradation of MYH9 and promotes the progression of triple-negative breast cancer via the Wnt/β-catenin signaling pathway [37]. CircRNAs predominantly modulate the expression of MYH9 and promote cancer progression via their interactions with miRNAs. CircSTX6 exerts dual regulatory effects on MYH9; it modulates MYH9 expression via miR-449b-5p and influences MYH9 transcription by interaction with Cullin 2. Collectively, these actions enable circSTX6 to mediate the upregulation of MYH9, thereby facilitating the proliferation and metastasis of pancreatic ductal adenocarcinoma cells in both in vitro and in vivo settings [38]. In related studies, the circ-NEK6/miR-370-3p/MYH9 axis has been implicated in the development of resistance to 131I radiotherapy in thyroid cancer, while the circ-PRMT5/miR-138-5p/MYH9 axis has been associated with cisplatin resistance in non-small-cell lung cancer [39, 40]. Interestingly, the modulation of MYH9 by circRNAs, mediated by miRNAs, seems to be related to glucose metabolism. Under hypoxic conditions, CircSLAMF6 has been observed to enhance glycolysis in gastric cancer cells. This metabolic shift was concomitant with an increase in cellular migration and invasion, mediated through the regulation of the miR-204-5p/MYH9 axis [41]. Another study conducted in the context of gastric cancer further substantiated that circ-NRIP1 augments glycolysis and promotes disease progression. This is achieved through the modulation of the miR-186-5p/MYH9 axis [42]. The circATP2A2/miR-335-5p/MYH9 axis upregulates glycolysis and cancer progression [43]. In addition to the circRNAs previously discussed, the intron of MYH9 itself can give rise to the circRNAs, circMYH9. This circRNA is highly expressed in CRC, and its expression levels are negatively correlated with both overall survival and recurrence-free survival rates. Overexpression of circMYH9 has been reported to influence tumor growth by mediating serine/glycine metabolism and orchestrating the regulation of reactive oxygen species (ROS) in a manner that is dependent on p53 [44].
CircRNAs, lncRNAs, and miRNAs can individually regulate the expression of MYH9. Additionally, circRNAs and lncRNAs can also regulate the expression of MYH9 through miRNAs. (Created by Biorender.com). MYH9, non-muscle myosin heavy chain IIA; miRNAs, microRNAs; lncRNAs, long non-coding RNAs; circRNAs, circular RNAs; WNT, Wingless/Integration-1; β-catenin, Catenin Beta 1; HIF1α, Hypoxia Inducible Factor 1 Alpha Subunit; FOXE1, Forkhead Box E1
LncRNAs, a category of non-coding RNAs that surpass 200 nucleotides in length, manifest their biological roles through intricate interactions with proteins, DNA, and RNA. They orchestrate cancer progression by modulating the expression of MYH9 via miRNA regulation or through direct interaction with MYH9 itself. In the context of prostate cancer, lncROR, a key oncogenic lncRNA, stabilizes MYH9 via direct interaction, subsequently facilitating the β-catenin/HIF1α pathway. HIF1α, in turn, binds to the promoter region of lncROR, exerting transcriptional activity and establishing a regulatory feedback loop encompassing lncROR, MYH9, and HIF1α. This intricate mechanism has been shown to ultimately amplify the resistance of prostate cancer cells to docetaxel [45]. lncRNA MAFG-AS1 exhibits elevated expression in HCC tissues, particularly in those infected with the hepatitis B virus. This upregulation was attributed to the HBx protein, which enhances the transcription of lncRNA MAFG-AS1. MAFG-AS1 interacts with and stabilizes three subunits of NM IIA, namely, MYH9, MYL12B, and MYL6 [46]. In a separate investigation, the interaction between MYH9 and the lncRNA PTCSC2 was shown to modulate the expression of FOXE1. Notably, FOXE1, which has been implicated in thyroid development, is a substantial risk factor for thyroid cancer [47]. In addition to their direct interactions with MYH9, lncRNAs also significantly regulate MYH9 through an alternative mechanism involving miRNAs. Both the lncRNA HULC/miR-9-5p axis and the lncRNA MRPL23-AS1/miR-30b/Wnt/β-catenin axis enhance the expression of MYH9 and facilitate cancer progression, specifically within the contexts of gastric cancer and osteosarcoma, respectively [48, 49].
miRNAs, small RNA molecules comprising approximately 20–24 nucleotides, can modulate the expression of their target genes via multiple mechanisms. Beyond their regulation by circRNAs and lncRNAs, miRNAs can also independently control the expression of MYH9 and its associated tumorigenic properties. miR-124-3p is known to target and suppress the expression of cytoskeletal genes, including MYH9. In neuroblastoma, reduced expression of miR-124-3p has been implicated in mediating the metastatic capabilities of cancer cells [50]. MiR-6089, a known tumor-suppressive microRNA, directly targets MYH9 and modulates EMT and c-Jun via the Wnt/β-catenin signaling pathway. Interestingly, c-Jun can also inhibit miR-6089 in ovarian cancer, forming a regulatory loop of miR-6089/MYH9/β-catenin/c-Jun [51]. MiRNA-214-3p is highly expressed in various cancers but is significantly downregulated in CRC. It exerts its tumor-suppressive function via the PLAGL2/MYH9 axis [52]. Two studies on miR-let-7f revealed the role of the miR-let-7f/MYH9 axis in promoting the metastasis of gastric cancer and CRC. miR-let-7f directly suppresses MYH9 at both the mRNA and protein levels, thereby inhibiting gastric cancer metastasis. Similarly, another study revealed that FAM222A-AS1 modulates the miR-let-7f/MYH9 axis to facilitate CRC metastasis [53, 54]. In CRC, the overexpression of the precursor miR-124 in cancer cells can inhibit the expression of MYH9 and SOX9, the targets of miR-124, and its tumorigenicity in vivo [55].
In summary, miRNAs, lncRNAs, and circRNAs modulate the transcription, translation, and degradation of MYH9 through various mechanisms. The precise regulation of MYH9 by non-coding RNAs plays a pivotal role in cancer progression. Non-coding RNAs may exhibit diverse functions across different cancer types or disease stages. Current research primarily focuses on specific non-coding RNAs and cancer types, potentially overlooking relationships between other non-coding RNAs and cancers. Future studies should be more comprehensive, exploring a broader range of non-coding RNAs and cancer types to understand and investigate these possible functional differences. Although non-coding RNAs hold potential diagnostic and therapeutic value, translating these research findings into clinical applications presents numerous challenges. These include precisely targeting specific non-coding RNAs and avoiding potential side effects.
MYH9 and signal transduction
Beyond protein interactions and regulation by non-coding RNAs, MYH9 itself can also serve as a component of various oncogenic signaling pathways, notably the Wnt/β-catenin, GSK3β/β-catenin/c-Jun, PI3K/AKT/mTOR, MAPK, and NOTCH pathways. The interplay between MYH9 and the Wnt/β-catenin pathway, as well as the GSK3β/β-catenin/c-Jun pathway, represents a particularly classic and popular combination in current research. In the section on ‘MYH9 and interacting proteins,’ we have repeatedly highlighted the close association of these two pathways with cancer. The PI3K/AKT/mTOR, MAPK, and NOTCH signaling cascades are pivotal in driving cancer progression under the influence of MYH9. MYH9 has been identified as a biomarker for cancer stem cells and facilitates the initiation of esophageal cancer by regulating the PI3K/AKT/mTOR signaling cascade [56]. In parallel, Nucleosome Assembly Protein 1-like 5 governs the progression of HCC through the MYH9/PI3K/AKT/mTOR signaling axis [57]. Intriguingly, MYH9 is capable of independently triggering mTOR or AKT, thereby playing a pro-carcinogenic role in non-small cell lung cancer and renal cell carcinoma [58, 59]. In our exploration of the interplay between MYH9 and MAPK, we found that MYH9 enhances CRC metastasis by activating the MAPK/AKT signaling cascade. Additionally, it reduces ROS levels and promotes radioresistance in head and neck cancer cells through activation of the MAPK-NRF2-GCLC pathway [9, 60]. A recent study on HCC has shed light on the interplay between the NOTCH pathway and MYH9. This study revealed that Transmembrane 4 L six family member 1 could augment the expression of MYH9. This upregulation subsequently triggers the NOTCH pathway, thereby bolstering tumor stemness [61]. Thus, MYH9 serves as a multifaceted signaling hub that orchestrates cancer progression via an array of signaling pathways. Beyond its involvement in extensively researched pathways like the Wnt/β-catenin, GSK3β/β-catenin/c-Jun, PI3K/AKT/mTOR MAPK, and NOTCH pathways, MYH9 has also been implicated in additional signaling trajectories. The S100A4-MYH9 and YY1-FGL1-MYH9 axes both can activate MYH9, which in turn regulates TGF-β-mediated EMT and the secretion of immune-related cytokines, respectively [62, 63]. The interaction between the transcription factors p300 and MRTF-A can upregulate the transcription of MYL9, MYH9, and CYR61, thereby enhancing the migration of breast cancer cells [64]. In prostate cancer, the knockdown of MYH9 promotes the phosphorylation of giantin mediated by polo-like kinase 3 and the dimerization of giantin, mediated by protein disulfide isomerase A3 through the release of Rab6a GTPase. This ultimately restores the morphology of the Golgi apparatus in cancer cells [65].
In summary, MYH9 serves as a hub protein in cancer, plays a pivotal role in multiple signaling pathways, and mediates various signal transduction pathways.The oncogenic regulatory network of MYH9 is robust and complex. Although the interactions between MYH9 and various oncogenic signaling pathways have been extensively studied, current research may be overly focused on certain specific pathways, such as the Wnt/β-catenin and GSK3β/β-catenin/c-Jun pathways. Future research should more comprehensively explore the role of MYH9 in other potential related pathways and how these pathways function in different types of tumors. In addition, existing studies often focus on describing the role of MYH9 in signaling pathways but lack detailed descriptions of its specific molecular mechanisms and regulatory processes. Future research needs to delve deeper into how MYH9 precisely regulates the behavior of tumor cells, including its intracellular localization, interactions with other proteins, and impact on downstream gene expression. Researchers also need to adopt more advanced technologies, such as single-cell sequencing and proteomic analysis, to reveal the subtle differences and dynamic changes of MYH9 in tumor development.
MYH9 as a tumor suppressor
Although MYH9 is predominantly characterized as an oncogene in most studies, it can paradoxically exhibit tumor-suppressive properties under certain circumstances and inhibit cancer progression. MYH9 enhances p53 post-transcriptional stability and has been identified as a tumor suppressor in squamous cell carcinoma [66]. This was the first study to elucidate the tumor-suppressive role of MYH9 after a significant hiatus from similar research for an extended period. However, the past 3 years have witnessed a resurgence of studies that echo these findings. MYH9 plays a pivotal role in inhibiting melanoma occurrence and metastasis. The knockdown of MYH9 can enhance the invasiveness and migration of cancer cells in vitro by modulating the ERK signaling pathway and EMT. Furthermore, MYH9 regulates the tumor microenvironment by mediating the infiltration of immune cells within the tumor [67]. In ovarian clear cell carcinoma, the robust interaction between MYH9 and ezrin-radixin-moesin-binding phosphoprotein 50 serves as a predictive marker of a favorable prognosis [68]. Another study proposed an intriguing therapeutic approach for breast cancer involving MYH9. By pharmacologically activating the PI3K/AKT signaling pathway in mesenchymal stem cells, the progression and bone metastasis of breast cancer can be inhibited via MYH9 and Hsp90ab1 [69]. It’s worth noting that MYH9 has been reported to inhibit tumor formation in mouse models of mammary cancer, squamous cell skin, tongue carcinoma, and melanoma, all without a universally recognized mechanism [66, 67, 70, 71]. Despite some studies emphasizing the tumor-suppressive role of MYH9, there is a paucity of extensive and in-depth studies providing substantial evidence for this effect. Conversely, research on the oncogenic role of MYH9 has primarily focused on colorectal cancer, esophageal squamous cell carcinoma, nasopharyngeal carcinoma, gastric cancer, lung cancer, and osteosarcoma [20, 26, 27, 30, 36, 48]. Moreover, most researchers have concentrated on a mainstream idea regarding the molecular mechanism of MYH9’s oncogenic role: MYH9 and its interacting proteins enter the nucleus, thereby enhancing the activity of oncogenic signaling pathways or driving changes in cellular states, ultimately mediating cancer progression. However, our understanding of MYH9’s oncogenic and tumor-suppressive roles remains somewhat chaotic and contradictory. In the future, we need to delve deeper into MYH9, including its functions in different types of cells and tissues, and the impact of different genes and signaling pathways on MYH9’s function.
Moreover, an often-overlooked aspect is that MYH9 not only functions in cancer cells but also plays a significant role in immune cells. Specifically, MYH9 may influence the immune response to cancer by modulating T-cell functionality. MYH9 is indispensable for the maturation of T-lymphocyte immune synapses and modulates the scale of the immune synapses. The knockdown of MYH9 in mouse T lymphocytes resulted in an inability to assemble central and peripheral supramolecular activation clusters, and the Src family kinase and its substrate Cas-L cannot be phosphorylated [72]. It was also reported that MYH9 mediates the migratory ability of T lymphocytes. This is partly because MYH9 mediates the de-adhesion of integrin LFA-1 and also because MYH9 co-localizes with the chemokine receptor C-X-C motif chemokine receptor type 4 (CXCR4) at the leading edge of the cell [73, 74]. Notably, miR-34a serves as a central regulator of T lymphocytes and binds to 14 target genes within T lymphocytes, including MYH9. In addition, MYH9 plays a crucial role in the secretion of immune-related cytokines in T cells and the cytotoxic response of CD8+ T cells. In lung adenocarcinoma cells, FGL1 controled the secretion of interleukin-2 in T cells and induced their apoptosis through the YY1-FGL1-MYH9 axis, thereby participating in the immune and proliferative regulation of cancer cells [62]. Regarded as a principal pathogenic determinant for sustained colonization, the urease B subunit (UreB) is deemed a promising vaccine antigen in the battle against Helicobacter pylori infection. MYH9, serving as a direct membrane receptor for UreB, was essential for the upregulation of PD-L1 in bone marrow-derived macrophages and further inhibited the activation of cytotoxic CD8+ T cell responses [75]. In addition to regulating the function of T cells, MYH9 is also indispensable for the migration of neutrophils. A study employing genetic down-regulation of Myh9 in Vav-iCre+/Myh9wt/fl mice revealed a notable reduction in both migration velocity and Euclidean distance during the mechanotactic migration of Vav-iCre+/Myh9wt/fl neutrophils, when contrasted with Vav-iCre2−/Myh9wt/fl control neutrophils [76]. In another study, it was similarly observed that plasma derived from red blood cells prompted the migration of neutrophils and morphological alterations, mediated by MYH9 [77]. In the last three years, the relationship between MYH9 and immune infiltration has been gradually revealed. In human melanoma cells, the circadian clock regulator Bmal1 was observed to play a significant role. Overexpression of either wild-type Bmal1 or a transcriptionally inactive mutant variant led to a non-canonical sequestration of MYH9. This increased MRTF-SRF activity and the AP-1 transcriptional signature, causing a shift in YUMM2.1 cells from a Sox10high state to a Sox9high immune-resistant, mesenchymal state commonly found in human melanomas. Moreover, through the analysis of the immune phenotype of tumors, it was shown that compared to shNC tumors, there was an increase in Monocytic Myeloid-Derived Suppressor Cells, Polymorphonuclear Myeloid-Derived Suppressor Cells, macrophages, and CD4+ T cells in shMyh9 tumors [78]. In another article related to MYH9 and immune infiltration, researchers found that MAP7D2 diminished the infiltration of cytotoxic CD8+ T lymphocytes via the MYH9-HMGB1 axis in colorectal cancer [20]. Even in a recent report from this year, it was highlighted that Quercetin enhances the maturation and proportion of NK cells by interacting with MYH9, leading to improved cognitive functions in aged mice [79]. These studies underscore the significant role of MYH9 in immune function and offer new directions and insights for immunotherapy in cancer. However, these studies are primarily focused on mice and do not address the relationship between human MYH9 and immune cell regulation. Further research is still needed to address these issues in human cancer.
Targeting MYH9 for the treatment of cancer
Given the significant role of MYH9 in cancer progression, targeting MYH9 has immense potential in cancer therapies. With the increasing attention given to MYH9 in recent years, numerous therapeutic strategies targeting MYH9 have emerged. We extensively reviewed all studies related to MYH9-targeted cancer therapies and categorized them into two main sections: direct targeting of MYH9 and indirect targeting of MYH9. Among the cancer treatment strategies that directly target MYH9, blebbistatin has been extensively researched. Blebbistatin is a specific ATPase inhibitor that binds to MYH9 and inhibits breast cancer cells [12, 80]. In renal cell carcinoma, pharmacological inhibition of MYH9 by blebbistatin downregulates the nuclear translocation of CXCR4 and inhibits cancer cell metastasis [7]. In another study on non-small cell lung cancer, blebbistatin reduced the interaction between MYH9, β-actin, and EGFR, further inhibiting cancer cell proliferation and promoting cancer cell apoptosis [81]. Amidated fullerene, with significant antitumor effects, inhibits the migration of multiple cancer cells and blocks the G0/G1 cell cycle by directly targeting MYH9 [82, 83]. J13, a natural small molecule isolated from Albizia julibrissin, directly targets the interaction between MYH9 and actin, resulting in the upregulation of mitochondrial fission within cancerous cells. This upregulation induces abnormalities in mitochondrial dynamics. These alterations inhibit the proliferation, migration, and survival of HCC cells, highlighting the potential of J13 as a promising therapeutic agent for HCC [84]. Staurosporine, a naturally occurring non-selective protein kinase inhibitor derived from the bacterium Streptomyces staurosporeus, has been implicated in the regulation of gastric cancer progression. Specifically, staurosporine exerts its anticancer effects by inhibiting the phosphorylation of MYH9 at S1943. This inhibition subsequently downregulates the transcription of CTNNB1 and attenuates Wnt/β-catenin signaling [31].
In addition to direct targeting of MYH9, an increasing number of therapeutic strategies have been developed to indirectly regulate MYH9 expression by targeting upstream proteins. These strategies aim to suppress cancer progression by modulating the activity of proteins that control the expression of MYH9. Cinobufotalin (CB), a bufadienolide derived from toad venom, as well as chemically synthesized CB, exhibits anticancer activity. CB can reduce the expression of MYH9 and the GSK3β/β-catenin pathway regulated by MYH9, thereby inhibiting EMT and tumor stemness in nasopharyngeal carcinoma [26, 85]. Other studies have provided a more specific mechanism by which CB regulates the expression of MYH9. In lung adenocarcinoma and HCC, CB upregulates ENKUR expression by regulating the PI3K/AKT/c-Jun signaling pathway. The upregulated ENKUR can both directly bind to the tail structural domain of MYH9 to regulate its function, and downregulate the transcriptional expression of MYH9 through the β-catenin/c-Jun axis. Furthermore, the expression level of MYH9 can modulate the recruitment of USP7, which mediates the ubiquitination and degradation of c-Myc, ultimately exerting a suppressive effect on EMT signaling and the progression of cancer [86, 87]. In nasopharyngeal carcinoma, CB induces ENKUR expression and inhibits the expression of MYH9 in a similar manner. However, what differs here is that MYH9 inhibits the ubiquitination and degradation of p53 by UBE3A by weakening the recruitment of UBE3A, thereby suppressing the metastasis of nasopharyngeal carcinoma [88]. Apatinib, a cornerstone drug used for chemotherapy in patients with advanced cancer, possesses excellent antiangiogenic capabilities. Apatinib inhibits the interaction between thrombospondin-1 (THBS1) and MYH9 by targeting THBS1, thereby suppressing glioma cells [89]. Bezafibrate, an anti-hyperlipidemic drug, can downregulate PKCβII both in vivo and in vitro and inhibit PKCβII-mediated phosphorylation of MYH9, thereby suppressing the metastasis of breast cancer [22]. In Spitz tumors, DS-6051a, an inhibitor of NTRK1/2/3 and ROS1, suppresses MYH9-NTRK3, ETV6-NTRK3, and MYO5A-NTRK3 fusions, as well as the oncogenic signals activated by these fusions [90]. In gastric cancer, CCG-1423, an inhibitor of the Rho/SRF signaling pathway, suppresses the expression of MYH9, particularly in cells with low SRF expression. CCG-1423 worked synergistically and efficiently with agomir-647 (an engineered miRNA mimic) to inhibit metastasis in gastric cancer [91]. In summary, the overexpression of MYH9 and its association with the malignant progression of cancer have been recognized by many researchers, and drug treatment strategies targeting MYH9 are mainstream research directions. The crucial role and functions of MYH9 in cancer suggest that drugs targeting MYH9 have great potential for cancer treatment.
However, the drugs currently available have disadvantages such as poor specificity, multiple side effects, and immature and unsystematic applications. Although some studies have demonstrated a direct targeting relationship between blebbistatin and MYH9, blebbistatin primarily serves as a widely used and only available specific inhibitor of myosin II, rather than directly targeting MYH9 and exerting anticancer effects. Moreover, issues such as fluorescence interference, low potency, poor water solubility, and easy photodegradation limit the applicability of blebbistatin [92, 93]. Staurosporine, produced by soil microorganisms, is a highly effective protein kinase inhibitor with significant cytotoxic effects on cancer cells. However, its non-specificity, which is its most fatal drawback, has prevented its widespread use in clinical practice [94, 95]. CB, telocinobufagin, bufalin, and marinobufagin are the most common bufadienolides that are naturally found in toad venom. CB has anticancer activity, particularly against nasopharyngeal and liver cancer cells. However, there have been no systematic studies on the proteins targeted by CB or the underlying mechanism. Current research suggests that CB not only indirectly regulates MYH9 but also acts as an inhibitor of SREBP1 to inhibit lipid production and the progression of HCC. It should be noted that CB is heat-sensitive, and CB (0.2 µM) can reduce cell viability to one-third at 44 °C [96,97,98]. Research on other drugs targeting MYH9 is relatively scarce, and the mechanisms are simplistic. Further validation is required to verify the reliability of these results. Therefore, the development of treatments targeting MYH9 remains a significant challenge.
Concluding remarks
In this review, we summarized the pathogenic mechanisms and therapeutic potential of MYH9 in cancer. MYH9 can play either an oncogenic or tumor-suppressive role, depending on the type of tumor, tissue environment, and signaling pathways. However, many researchers are keen to uncover the secrets of MYH9’s powerful oncogenic ability, while overlooking its tumor-suppressive role in certain cancers. Consequently, MYH9 is mostly defined as an oncogene, which is positively correlated with the invasive and metastatic abilities of cancer cells. Nevertheless, the oncogenic and tumor-suppressive roles of MYH9 are like the two sides of a coin. As we strive to reveal the details of one side of the coin, sufficient attention should also be paid to the other side. A comprehensive understanding of the full role of MYH9 is required.
Under physiological conditions, MYH9, a skeleton-related protein, hydrolyzes ATP to generate mechanical movement and participates in the regulation of cellular contractile forces. In cancer, MYH9 is closely related to numerous interacting proteins, miRNAs, lncRNAs, circRNAs, and signaling pathways. These associations suggest that MYH9 plays a central role in oncogenic signal transduction during the malignant progression of cancer, rather than merely serving as a part of the “cellular engine.” Many researchers are attempting to uncover how MYH9 coordinates the complex oncogenic signal transduction in cancer cells. Because of the powerful oncogenic ability of MYH9, drugs targeting MYH9 are actively being developed. Mainstream strategies for drug development involve direct targeting of MYH9, inhibiting the binding of MYH9 to interacting proteins (especially actin), mediating the phosphorylation of MYH9, or targeting upstream proteins of MYH9 to hinder its role in oncogenic signal transduction. In this review, we have elucidated how MYH9 and its target proteins influence the behavior of tumor cells at the molecular level. Given the current scarcity of drugs targeting MYH9, we propose several promising new directions for the development of drugs targeting MYH9. Firstly, MYH9 interacts with immune cells within the tumor microenvironment, affecting the immune escape mechanisms of the tumor. Therefore, future research could explore how modulating the expression or function of MYH9 could enhance the efficacy of tumor immunotherapy (for example, by targeting MYH9 to activate T cells or enhance antigen presentation). Secondly, the stability and function of MYH9 are strictly regulated by ubiquitination and deubiquitination processes. Researchers could also focus on how to regulate the activity of MYH9 by influencing these processes, such as developing drugs that can intervene in the interaction between MYH9 and ubiquitination or deubiquitination enzymes. Lastly, although drugs targeting MYH9 have shown some therapeutic potential, their effects in combination with traditional chemotherapy and radiotherapy are not yet clear. Researchers should assess the synergistic effects of these drugs when used in conjunction with chemotherapy and radiotherapy, as well as how to optimize such combined treatment strategies. From the perspective of enhancing therapeutic efficacy and reducing side effects, combination therapy strategies may be a promising future direction. MYH9 is a promising therapeutic target for cancer, and drugs that can reasonably balance the advantages and disadvantages of MYH9 will bring new hope to cancer patients.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MYH9:
-
Non-Muscle Myosin Heavy Chain IIA
- NM IIA:
-
Non-Muscle Myosin Of Class II Isoform A
- MYH9-RD:
-
MYH9-Related Disorders
- RhoA:
-
Ras homolog family member A
- miRNAs:
-
microRNAs
- lncRNAs:
-
long non-coding RNAs
- circRNAs:
-
circular RNAs
- ATG9B:
-
Autophagy-Related Protein 9B
- SOC:
-
Serous Ovarian Cancer
- MAP7D2:
-
Microtubule-Associated Protein 7 Domain-Containing Protein 2
- MSS:
-
Microsatellite Stable
- CRC:
-
Colorectal Cancer
- CTLs:
-
Cytotoxic T Lymphocytes
- PTP1B:
-
Protein Tyrosine Phosphatase 1B
- ESCC:
-
Esophageal Squamous Cell Carcinoma
- HBXIP:
-
Hepatitis B X-Interacting Protein
- HDAC6:
-
Histone Deacetylase 6
- HCC:
-
Hepatocellular Carcinoma
- TUBB4A:
-
Tubulin Beta Class Iva
- APE2:
-
Apyrimidinic Endonuclease 2
- STMP1:
-
Short Transmembrane Protein 1
- HMGA1:
-
High Mobility Group AT-Hook 1
- CB:
-
Cinobufotalin
- THBS1:
-
Thrombospondin-1
- CXCR4:
-
C-X-C Motif Chemokine Receptor Type 4
- CTNNB1:
-
Catenin Beta 1
- MICAL2:
-
Microtubule Associated Monooxygenase, Calponin And LIM Domain Containing 2
- GSK3β:
-
Glycogen Synthase Kinase 3 Beta
- β-catenin:
-
Catenin Beta 1
- c-Jun:
-
Transcription Factor Jun
- PI3K:
-
Phosphatidylinositol
- 3-Kinase:
-
AKT, Protein Kinase B
- c-Myc:
-
Myc Proto-Oncogene Protein
- P53:
-
Tumor Protein P53
- ERK:
-
Extracellular Signal-Regulated Kinases
- ETV4:
-
ETS Variant Transcription Factor 4
- WNT:
-
Wingless/Integration-1
- HIF1α:
-
Hypoxia Inducible Factor 1 Alpha Subunit
- FOXE1:
-
Forkhead Box E1
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Urruticoechea A, et al. Recent advances in cancer therapy: an overview. Curr Pharm Des. 2010;16:3–10. https://doi.org/10.2174/138161210789941847.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9. https://doi.org/10.7150/ijms.3635.
Althaus K, Greinacher A. MYH-9 related platelet disorders: strategies for management and diagnosis. Transfus Med Hemother. 2010;37:260–7. https://doi.org/10.1159/000320335.
Pecci A, Ma X, Savoia A, Adelstein RS. MYH9: structure, functions and role of non-muscle myosin IIA in human disease. Gene. 2018;664:152–67. https://doi.org/10.1016/j.gene.2018.04.048.
Asensio-Juárez G, Llorente-González C, Vicente-Manzanares M. Linking the Landscape of MYH9-Related diseases to the Molecular mechanisms that Control Non-muscle myosin II-A function in cells. Cells. 2020;9. https://doi.org/10.3390/cells9061458.
Xu Z, et al. NMMHC-IIA-dependent nuclear location of CXCR4 promotes migration and invasion in renal cell carcinoma. Oncol Rep. 2016;36:2681–8. https://doi.org/10.3892/or.2016.5082.
Xia ZK, et al. Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer. Dis Esophagus. 2012;25:427–36. https://doi.org/10.1111/j.1442-2050.2011.01261.x.
Wang B, et al. MYH9 promotes growth and metastasis via activation of MAPK/AKT signaling in Colorectal Cancer. J Cancer. 2019;10:874–84. https://doi.org/10.7150/jca.27635.
Liu T, et al. Downregulation of non–muscle myosin IIA expression inhibits migration and invasion of gastric cancer cells via the c–Jun N–terminal kinase signaling pathway. Mol Med Rep. 2016;13:1639–44. https://doi.org/10.3892/mmr.2015.4742.
Katono K, et al. Prognostic significance of MYH9 expression in resected non-small cell lung cancer. PLoS ONE. 2015;10:e0121460. https://doi.org/10.1371/journal.pone.0121460.
Derycke L, et al. The role of non-muscle myosin IIA in aggregation and invasion of human MCF-7 breast cancer cells. Int J Dev Biol. 2011;55:835–40. https://doi.org/10.1387/ijdb.113336ld.
Marini M, et al. Non-muscle myosin heavy chain IIA and IIB interact and co-localize in living cells: relevance for MYH9-related disease. Int J Mol Med. 2006;17:729–36.
Franke JD, Dong F, Rickoll WL, Kelley MJ, Kiehart DP. Rod mutations associated with MYH9-related disorders disrupt nonmuscle myosin-IIA assembly. Blood. 2005;105:161–9. https://doi.org/10.1182/blood-2004-06-2067.
Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. https://doi.org/10.1038/nrm2786.
Balduini CL, Pecci A, Savoia A. Recent advances in the understanding and management of MYH9-related inherited thrombocytopenias. Br J Haematol. 2011;154:161–74. https://doi.org/10.1111/j.1365-2141.2011.08716.x.
Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–6. https://doi.org/10.1074/jbc.C400352200.
Zhong Y, et al. MYH9-dependent polarization of ATG9B promotes colorectal cancer metastasis by accelerating focal adhesion assembly. Cell Death Differ. 2021;28:3251–69. https://doi.org/10.1038/s41418-021-00813-z.
Liu L, et al. MYH10 combines with MYH9 to Recruit USP45 by Deubiquitinating snail and promotes serous ovarian Cancer carcinogenesis, progression, and Cisplatin Resistance. Adv Sci (Weinh). 2023;10:e2203423. https://doi.org/10.1002/advs.202203423.
Wu Q, et al. MAP7D2 reduces CD8(+) cytotoxic T lymphocyte infiltration through MYH9-HMGB1 axis in colorectal cancer. Mol Ther. 2023;31:90–104. https://doi.org/10.1016/j.ymthe.2022.09.001.
Pan BQ, et al. PTP1B up-regulates EGFR expression by dephosphorylating MYH9 at Y1408 to promote cell migration and invasion in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2020;522:53–60. https://doi.org/10.1016/j.bbrc.2019.10.168.
Zhang L, et al. HBXIP blocks myosin-IIA assembly by phosphorylating and interacting with NMHC-IIA in breast cancer metastasis. Acta Pharm Sin B. 2023;13:1053–70. https://doi.org/10.1016/j.apsb.2022.11.025.
Cao M, et al. Activation of the clock gene TIMELESS by H3k27 acetylation promotes colorectal cancer tumorigenesis by binding to Myosin-9. J Exp Clin Cancer Res. 2021;40:162. https://doi.org/10.1186/s13046-021-01936-4.
Zhang L, et al. Proteomic identification and functional characterization of MYH9, Hsc70, and DNAJA1 as novel substrates of HDAC6 deacetylase activity. Protein Cell. 2015;6:42–54. https://doi.org/10.1007/s13238-014-0102-8.
Lin X, et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:13. https://doi.org/10.1038/s41392-020-0111-4.
Li Y, et al. Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9. Signal Transduct Target Ther. 2019;4. https://doi.org/10.1038/s41392-019-0084-3.
Yang B, et al. Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop. J Exp Clin Cancer Res. 2019;38:283. https://doi.org/10.1186/s13046-019-1279-8.
Gao S, et al. TUBB4A interacts with MYH9 to protect the nucleus during cell migration and promotes prostate cancer via GSK3β/β-catenin signalling. Nat Commun. 2022;13:2792. https://doi.org/10.1038/s41467-022-30409-1.
Yu ST, et al. CRLF1-MYH9 Interaction regulates proliferation and metastasis of papillary thyroid carcinoma through the ERK/ETV4 Axis. Front Endocrinol (Lausanne). 2020;11:535. https://doi.org/10.3389/fendo.2020.00535.
Li YQ, et al. FNDC3B 3’-UTR shortening escapes from microRNA-mediated gene repression and promotes nasopharyngeal carcinoma progression. Cancer Sci. 2020;111:1991–2003. https://doi.org/10.1111/cas.14394.
Ye G, et al. Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis. Theranostics. 2020;10:7545–60. https://doi.org/10.7150/thno.46001.
Zhao P, Han H, Wu X, Wu J, Ren Z. ARP2/3 Regulates Fatty Acid Synthesis by Modulating Lipid Droplets’ Motility. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23158730.
Hu Y, et al. Cisplatin-mediated upregulation of APE2 binding to MYH9 provokes mitochondrial fragmentation and acute kidney Injury. Cancer Res. 2021;81:713–23. https://doi.org/10.1158/0008-5472.Can-20-1010.
Xie C, et al. Mitochondrial micropeptide STMP1 enhances mitochondrial fission to promote Tumor Metastasis. Cancer Res. 2022;82:2431–43. https://doi.org/10.1158/0008-5472.Can-21-3910.
Barai A, Mukherjee A, Das A, Saxena N, Sen S. α-Actinin-4 drives invasiveness by regulating myosin IIB expression and myosin IIA localization. J Cell Sci. 2021;134. https://doi.org/10.1242/jcs.258581.
Zhou W, et al. MICAL2 is a novel nucleocytoplasmic shuttling protein promoting cancer invasion and growth of lung adenocarcinoma. Cancer Lett. 2020;483:75–86. https://doi.org/10.1016/j.canlet.2020.04.019.
Li Y, et al. circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol Ther. 2022;30:415–30. https://doi.org/10.1016/j.ymthe.2021.08.026.
Meng L, et al. CircSTX6 promotes pancreatic ductal adenocarcinoma progression by sponging miR-449b-5p and interacting with CUL2. Mol Cancer. 2022;21:121. https://doi.org/10.1186/s12943-022-01599-5.
Xu Y, et al. Circular RNA PRMT5 knockdown enhances cisplatin sensitivity and immune response in non-small cell lung cancer by regulating miR-138-5p/MYH9 axis. J buon. 2021;26:1850–61.
Chen F, et al. Knockdown of circ_NEK6 decreased (131)I resistance of differentiated thyroid carcinoma via regulating miR-370-3p/MYH9 Axis. Technol Cancer Res Treat. 2021;20:15330338211004950. https://doi.org/10.1177/15330338211004950.
Fang X, Bai Y, Zhang L, Ding S. Silencing circSLAMF6 represses cell glycolysis, migration, and invasion by regulating the miR-204-5p/MYH9 axis in gastric cancer under hypoxia. Biosci Rep. 2020;40. https://doi.org/10.1042/bsr20201275.
Liu Y, et al. circ-NRIP1 promotes glycolysis and tumor progression by regulating miR-186-5p/MYH9 Axis in Gastric Cancer. Cancer Manag Res. 2020;12:5945–56. https://doi.org/10.2147/cmar.S245941.
Cao X, et al. circATP2A2 promotes osteosarcoma progression by upregulating MYH9. Open Med (Wars). 2021;16:1749–61. https://doi.org/10.1515/med-2021-0370.
Liu X, et al. CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol Cancer. 2021;20:114. https://doi.org/10.1186/s12943-021-01412-9.
Jiang X, Xu Y, Liu R, Guo S. Exosomal lincROR promotes Docetaxel Resistance in prostate Cancer through a β-catenin/HIF1α positive feedback Loop. Mol Cancer Res. 2023;21:472–82. https://doi.org/10.1158/1541-7786.Mcr-22-0458.
Zhang F, et al. HBx-upregulated MAFG-AS1 promotes cell proliferation and migration of hepatoma cells by enhancing MAFG expression and stabilizing nonmuscle myosin IIA. Faseb j. 2021;35:e21529. https://doi.org/10.1096/fj.202002374R.
Wang Y, et al. MYH9 binds to lncRNA gene PTCSC2 and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc Natl Acad Sci U S A. 2017;114:474–9. https://doi.org/10.1073/pnas.1619917114.
Zhang H, Liu S, Tang L, Ge J, Lu X. Long non-coding RNA (LncRNA) MRPL23-AS1 promotes tumor progression and carcinogenesis in osteosarcoma by activating Wnt/β-catenin signaling via inhibiting microRNA miR-30b and upregulating myosin heavy chain 9 (MYH9). Bioengineered 12, 162–171, https://doi.org/10.1080/21655979.2020.1863014 (2021).
Liu T, et al. LncRNA HULC promotes the progression of gastric cancer by regulating miR-9-5p/MYH9 axis. Biomed Pharmacother. 2020;121:109607. https://doi.org/10.1016/j.biopha.2019.109607.
Nolan JC, et al. A context-dependent role for MiR-124-3p on cell phenotype, viability and Chemosensitivity in Neuroblastoma in vitro. Front Cell Dev Biol. 2020;8:559553. https://doi.org/10.3389/fcell.2020.559553.
Liu L, et al. miR-6089/MYH9/β-catenin/c-Jun negative feedback loop inhibits ovarian cancer carcinogenesis and progression. Biomed Pharmacother. 2020;125:109865. https://doi.org/10.1016/j.biopha.2020.109865.
Zhou Z, et al. MicroRNA-214-3p targets the PLAGL2-MYH9 axis to suppress tumor proliferation and metastasis in human colorectal cancer. Aging. 2020;12:9633–57. https://doi.org/10.18632/aging.103233.
Song M, et al. The long non-coding RNA FAM222A-AS1 negatively modulates MiR-Let-7f to promote Colorectal Cancer Progression. Front Oncol. 2022;12:764621. https://doi.org/10.3389/fonc.2022.764621.
Liang S, et al. MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS ONE. 2011;6:e18409. https://doi.org/10.1371/journal.pone.0018409.
Park SY, et al. KITENIN-targeting microRNA-124 suppresses colorectal cancer cell motility and tumorigenesis. Mol Ther. 2014;22:1653–64. https://doi.org/10.1038/mt.2014.105.
Kai JD, et al. MYH9 is a novel cancer stem cell marker and prognostic indicator in esophageal cancer that promotes oncogenesis through the PI3K/AKT/mTOR axis. Cell Biol Int. 2022;46:2085–94. https://doi.org/10.1002/cbin.11894.
Zhao R, et al. NAP1L5 targeting combined with MYH9 inhibit HCC progression through PI3K/AKT/mTOR signaling pathway. Aging. 2022;14:9000–19. https://doi.org/10.18632/aging.204377.
Xu Z, et al. Single-cell RNA-sequencing analysis reveals MYH9 promotes renal cell carcinoma development and sunitinib resistance via AKT signaling pathway. Cell Death Discov. 2022;8:125. https://doi.org/10.1038/s41420-022-00933-6.
Chen M, et al. MYH9 is crucial for stem cell-like properties in non-small cell lung cancer by activating mTOR signaling. Cell Death Discov. 2021;7:282. https://doi.org/10.1038/s41420-021-00681-z.
You GR et al. MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway. Cells 11, https://doi.org/10.3390/cells11182855 (2022).
Yang SB, et al. TM4SF1 upregulates MYH9 to activate the NOTCH pathway to promote cancer stemness and lenvatinib resistance in HCC. Biol Direct. 2023;18:18. https://doi.org/10.1186/s13062-023-00376-8.
Tang XY, et al. Dual immunological and proliferative regulation of immune checkpoint FGL1 in lung adenocarcinoma: the pivotal role of the YY1-FGL1-MYH9 axis. Front Immunol. 2022;13:1014053. https://doi.org/10.3389/fimmu.2022.1014053.
Li F, et al. S100A4-MYH9 Axis Promote Migration and Invasion of Gastric Cancer cells by inducing TGF-β-Mediated epithelial-mesenchymal transition. J Cancer. 2018;9:3839–49. https://doi.org/10.7150/jca.25469.
He H, et al. Transcriptional factors p300 and MRTF-A synergistically enhance the expression of migration-related genes in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2015;467:813–20. https://doi.org/10.1016/j.bbrc.2015.10.060.
Petrosyan A, Holzapfel MS, Muirhead DE, Cheng PW. Restoration of compact golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin O-glycan synthesis. Mol Cancer Res. 2014;12:1704–16. https://doi.org/10.1158/1541-7786.Mcr-14-0291-t.
Schramek D, et al. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science. 2014;343:309–13. https://doi.org/10.1126/science.1248627.
Singh SK, et al. MYH9 suppresses melanoma tumorigenesis, metastasis and regulates tumor microenvironment. Med Oncol. 2020;37:88. https://doi.org/10.1007/s12032-020-01413-6.
Nakagawa M, et al. Interaction between membranous EBP50 and myosin 9 as a favorable prognostic factor in ovarian clear cell carcinoma. Mol Oncol. 2023;17:2168–82. https://doi.org/10.1002/1878-0261.13503.
Sun X, Li K, Aryal UK, Li BY, Yokota H. PI3K-activated MSC proteomes inhibit mammary tumors via Hsp90ab1 and Myh9. Mol Ther Oncolytics. 2022;26:360–71. https://doi.org/10.1016/j.omto.2022.08.003.
Conti MA, et al. Conditional deletion of nonmuscle myosin II-A in mouse tongue epithelium results in squamous cell carcinoma. Sci Rep. 2015;5:14068. https://doi.org/10.1038/srep14068.
Kas SM, et al. Insertional mutagenesis identifies drivers of a novel oncogenic pathway in invasive lobular breast carcinoma. Nat Genet. 2017;49:1219–30. https://doi.org/10.1038/ng.3905.
Kumari S, et al. T lymphocyte myosin IIA is required for maturation of the immunological synapse. Front Immunol. 2012;3:230. https://doi.org/10.3389/fimmu.2012.00230.
Rey M, et al. Cutting edge: association of the motor protein nonmuscle myosin heavy chain-IIA with the C terminus of the chemokine receptor CXCR4 in T lymphocytes. J Immunol. 2002;169:5410–4. https://doi.org/10.4049/jimmunol.169.10.5410.
Morin NA, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205:195–205. https://doi.org/10.1084/jem.20071543.
Wu J, et al. Helicobacter urease suppresses cytotoxic CD8 + T-cell responses through activating Myh9-dependent induction of PD-L1. Int Immunol. 2021;33:491–504. https://doi.org/10.1093/intimm/dxab044.
Zehrer A, et al. A fundamental role of Myh9 for Neutrophil Migration in Innate Immunity. J Immunol. 2018;201:1748–64. https://doi.org/10.4049/jimmunol.1701400.
Yu C, et al. PRBC-derived plasma induces non-muscle myosin type IIA-mediated neutrophil migration and morphologic change. Immunopharmacol Immunotoxicol. 2013;35:71–9. https://doi.org/10.3109/08923973.2012.677046.
Zhang X, et al. Cell state dependent effects of Bmal1 on melanoma immunity and tumorigenicity. Nat Commun. 2024;15:633. https://doi.org/10.1038/s41467-024-44778-2.
Su T, et al. Quercetin promotes the proportion and maturation of NK cells by binding to MYH9 and improves cognitive functions in aged mice. Immun Ageing. 2024;21:29. https://doi.org/10.1186/s12979-024-00436-1.
Lv Y, Lu S, Lu T, Kou J, Yu B. Homology model of nonmuscle myosin heavy chain IIA and binding mode analysis with its inhibitor blebbistatin. J Mol Model. 2013;19:1801–10. https://doi.org/10.1007/s00894-012-1750-3.
Chiu HC, Chang TY, Huang CT, Chao YS, Hsu JT. EGFR and myosin II inhibitors cooperate to suppress EGFR-T790M-mutant NSCLC cells. Mol Oncol. 2012;6:299–310. https://doi.org/10.1016/j.molonc.2012.02.001.
Zhou W, et al. Aminated Fullerene abrogates Cancer Cell Migration by directly targeting myosin heavy chain 9. ACS Appl Mater Interfaces. 2020;12:56862–73. https://doi.org/10.1021/acsami.0c18785.
Huo J, et al. Amphiphilic aminated derivatives of [60]Fullerene as potent inhibitors of Tumor Growth and Metastasis. Adv Sci (Weinh). 2022;9:e2201541. https://doi.org/10.1002/advs.202201541.
Qian Y, et al. Pharmacologically targeting molecular motor promotes mitochondrial fission for anti-cancer. Acta Pharm Sin B. 2021;11:1853–66. https://doi.org/10.1016/j.apsb.2021.01.011.
Liu Y, et al. Cinobufotalin powerfully reversed EBV-miR-BART22-induced cisplatin resistance via stimulating MAP2K4 to antagonize non-muscle myosin heavy chain IIA/glycogen synthase 3β/β-catenin signaling pathway. EBioMedicine. 2019;48:386–404. https://doi.org/10.1016/j.ebiom.2019.08.040.
Hou R, et al. ENKUR expression induced by chemically synthesized cinobufotalin suppresses malignant activities of hepatocellular carcinoma by modulating β-catenin/c-Jun/MYH9/USP7/c-Myc axis. Int J Biol Sci. 2022;18:2553–67. https://doi.org/10.7150/ijbs.67476.
Liu JH, et al. The small molecule chemical compound cinobufotalin attenuates resistance to DDP by inducing ENKUR expression to suppress MYH9-mediated c-Myc deubiquitination in lung adenocarcinoma. Acta Pharmacol Sin. 2022;43:2687–95. https://doi.org/10.1038/s41401-022-00890-x.
Hou R, et al. Chemically synthesized cinobufagin suppresses nasopharyngeal carcinoma metastasis by inducing ENKUR to stabilize p53 expression. Cancer Lett. 2022;531:57–70. https://doi.org/10.1016/j.canlet.2022.01.025.
Yao H, et al. Apatinib inhibits glioma cell malignancy in patient-derived orthotopic xenograft mouse model by targeting thrombospondin 1/myosin heavy chain 9 axis. Cell Death Dis. 2021;12:927. https://doi.org/10.1038/s41419-021-04225-2.
Yeh I, et al. NTRK3 kinase fusions in Spitz tumours. J Pathol. 2016;240:282–90. https://doi.org/10.1002/path.4775.
Ye G, et al. MicroRNA-647 targets SRF-MYH9 Axis to Suppress Invasion and Metastasis of Gastric Cancer. Theranostics. 2017;7:3338–53. https://doi.org/10.7150/thno.20512.
Roman BI, Verhasselt S, Stevens CV. Medicinal Chemistry and Use of myosin II inhibitor (S)-Blebbistatin and its derivatives. J Med Chem. 2018;61:9410–28. https://doi.org/10.1021/acs.jmedchem.8b00503.
Rauscher A, Gyimesi M, Kovács M, Málnási-Csizmadia A. Targeting myosin by blebbistatin derivatives: optimization and pharmacological potential. Trends Biochem Sci. 2018;43:700–13. https://doi.org/10.1016/j.tibs.2018.06.006.
Ōmura S, Asami Y, Crump A. Staurosporine: new lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J Antibiot (Tokyo). 2018;71:688–701. https://doi.org/10.1038/s41429-018-0029-z.
Lopez MS, et al. Staurosporine-derived inhibitors broaden the scope of analog-sensitive kinase technology. J Am Chem Soc. 2013;135:18153–9. https://doi.org/10.1021/ja408704u.
Meng H, et al. Novel SREBP1 inhibitor cinobufotalin suppresses proliferation of hepatocellular carcinoma by targeting lipogenesis. Eur J Pharmacol. 2021;906:174280. https://doi.org/10.1016/j.ejphar.2021.174280.
Jia J, Li J, Zheng Q, Li D. A research update on the antitumor effects of active components of Chinese medicine ChanSu. Front Oncol. 2022;12:1014637. https://doi.org/10.3389/fonc.2022.1014637.
Asrorov AM, et al. Toad venom bufadienolides and bufotoxins: an updated review. Drug Dev Res. 2023;84:815–38. https://doi.org/10.1002/ddr.22072.
Acknowledgements
We would like to thank the Editeg (https://www.editeg.com/) for the language service. Pictures were designed by the BioRender (https://biorender.com/) and gained the copyright for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82270785) and Natural Science Foundation of Jilin Province (project no. YDZJ202301ZYTS046).
Author information
Authors and Affiliations
Contributions
Conceptualisation: HZ, YiW, and YL; drafting of manuscript: YP and YiangZ; revising of manuscript: XG and FL; searching the literature: YuW and BL; designing the figures and tables: YangZ.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Pan, Y., Yang, X. et al. Unveiling the enigmatic role of MYH9 in tumor biology: a comprehensive review. Cell Commun Signal 22, 417 (2024). https://doi.org/10.1186/s12964-024-01781-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01781-w