Abstract
Background
Physical activity referral schemes (PARS) are composed of various components, such as a written prescription or a person-centered approach. The role of these components in their effectiveness is yet to be understood. Therefore, we aimed to explore the relationships between PARS components and physical activity, scheme uptake, and adherence rate; and to estimate the effect of PARS.
Methods
We searched Scopus, PubMed, Web of Science, CINAHL, ScienceDirect, SpringerLink, HTA, Wiley Online Library, SAGE Journals, Taylor & Francis, Google Scholar, OpenGrey, and CORE. Eligible studies were published between 1990 and November 2023 in English or German, investigated PARS with participants aged ≥ 16 years, and reported physical activity, scheme uptake, or scheme adherence. Separate random-effects meta-analysis by comparison group were conducted for physical activity. Scheme uptake and adherence rates were pooled using proportional meta-analysis. The components were analyzed via univariate meta-regression. We rated the risk of bias using RoB2 and ROBINS-I, and the certainty of evidence using GRADE.
Results
Fifty-two studies were included. PARS were more effective in increasing physical activity than usual care (k = 11, n = 5046, Hedges’ g = 0.18, 95%CI 0.12 to 0.25; high certainty of evidence). When PARS were compared with physical activity advice or enhanced scheme versions, the pooled Hedges’ g values for physical activity were -0.06 (k = 5, n = 1082, 95%CI -0.21 to 0.10; low certainty of evidence), and 0.07 (k = 9, n = 2647, 95%CI -0.03 to 0.18; low certainty of evidence) respectively. Scheme uptake was 87% (95%CI 77% to 94%, k = 14, n = 5000) across experimental studies and 68% (95%CI 51% to 83%, k = 14, n = 25,048) across non-experimental studies. Pooled scheme adherence was 68% (95%CI 55% to 80%, k = 16, n = 3939) and 53% (95%CI 42% to 63%, k = 18, n = 14,605). The meta-regression did not detect any significant relationships between components and physical activity or scheme uptake. A person-centered approach, screening, and brief advice were positively associated with scheme adherence, while physical activity sessions were negatively associated.
Conclusion
PARS are more effective in increasing physical activity than usual care only. We did not identify any components as significant predictors of physical activity and scheme uptake. Four components predicted scheme adherence, indicating that the component-effectiveness relationship warrants further research.
Similar content being viewed by others
Background
The promotion of physical activity (PA) by healthcare professionals has been proposed as a paramount strategy to foster an active society [1]. Physical activity referral schemes (PARS) are a promising intervention that allow healthcare professionals to advocate for PA and integrate its promotion into routine care. Previous seminal evidence syntheses have pointed to favorable but small effects of PARS on PA, and to considerable variation in design and implementation [2,3,4]. In 2014, the National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK) recommended that future research should focus on increasing understanding of what influences effectiveness and cost effectiveness of PARS [5]. Since then, PARS research has mainly focused on participant-level factors (e.g., age, gender and socio-economic status of referrals), system-level factors (e.g., financial reimbursement), and scheme characteristics (e.g., setting, duration and intensity, costs) [6,7,8,9]. However, little attention has been paid to the role of the components that contribute to PARS complexity and heterogeneity. PARS content varies greatly as it can be grounded on various theories or approaches, such as a person-centered approach, and made up of many other standalone interventions, such as brief advice or PA sessions [10]. These separate and potentially active parts of PARS content are referred to as scheme components.
PARS diversity and intricacy are the result of over 30 years of organic development in different countries and healthcare systems. The number of schemes is increasing due to their potential to change PA behavior. For example, the number of European Union member states reporting a national program of healthcare-based PA counseling or prescription increased from approximately 46% to 79% between 2015 and 2018 [11]. Underlying healthcare systems are complex and heterogeneous [12], contributing to the well acknowledged variety and complexity of PARS interventions [13, 14]. Within this complexity, PARS consist of combinations of behavioral support activities (brief advice, counseling session(s), PA sessions) and guiding principles (person-centered approach, individualized content) [10]. These individual components are assumed to contribute to the effectiveness of PARS in varying degrees. Schemes containing the core components of the Swedish model (i.e., patient-centered approach, individually tailored PA recommendations, written prescription, and structured follow-up) have been deemed effective, although it is currently unclear which components are more likely to result in increased PA [15]. In addition, previous research underscores the need to explore the factors that lead to optimal program uptake and adherence, which are necessary to demonstrate the true impact of PARS [9, 16, 17]. What is lacking is a proper understanding of the component-effectiveness relationship [16].
A better understanding of how components may shape scheme effectiveness can help program developers to design PARS that are only as complex as needed [18] or modify existing PARS to increase their effectiveness. Identification of the most effective core components could result in a focus on PARS optimization, more cost-efficient schemes, and improvements in participant outcomes. We have previously identified 19 components [10] and in this study we aimed to examine their effect on PA outcomes, scheme uptake and adherence rates.
Methods
We analyzed the overall effect of 19 PARS components through meta-analysis and then used univariate meta-regression to examine the impact of each component. This analysis builds upon our systematic review [10], which followed the PRISMA guidelines [19], and the review protocol [20].
Literature sources and inclusion criteria
The literature search was performed in Scopus, PubMed, Web of Science, CINAHL, ScienceDirect, SpringerLink, HTA, Wiley Online Library, SAGE Journals, Taylor & Francis, Google Scholar, OpenGrey, and CORE. The time searched in the previously published systematic review was from 1990 to January 2023 [10]. We updated the search in November 2023 (Additional file 1). Two independent reviewers (EM, AB) screened the articles identified from the updated search against the eligibility criteria. Experimental, quasi-experimental, and observational studies published in English or German were included in the systematic review if:
-
Population: The participants were aged ≥ 16 years.
-
Intervention: The study evaluated any intervention labeled as PARS, exercise referral schemes, or exercise on prescription or any similar intervention, such as PA counseling that included at least some form of documentation, such as a prescription or referral form.
-
Comparison: The PARS was compared to usual care, PA advice, alternative intervention (scheme versions), or no intervention. When the PARS was compared with PA advice, the comparison group received only advice about PA from the healthcare professional and no further intervention. Some studies compared standard PARS with enhanced versions, typically extending beyond of the standard scheme by incorporating additional components or increasing session frequency. For example, the standard version included a written prescription and counseling support sessions, whereas the enhanced version integrated additional PA sessions.
-
Outcomes: The study reported either PA level, scheme uptake, or adherence rates.
-
Setting: The PARS (or referral to the PARS) was initiated in primary or secondary healthcare, as noted in the included study. Primary healthcare generally includes a general practitioner or practice nurse, and secondary healthcare includes more specialized care, such as a diabetologist, cardiologist, or mental health practitioner.
Risk of bias
We used the Cochrane risk-of-bias tool for randomized trials (RoB2) [21] to assess risk of bias for experimental studies, and Risk of Bias in Non-randomized Studies-of Interventions (ROBINS-I) [22] for quasi-experimental and observational studies. Two authors (EM and AB) assessed studies independently and resolved any disagreements through discussion until consensus was reached. We used the RoB VISualisation (robvis) to create risk of bias graphs in R [23].
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence at outcome level [24]. One author (EM) rated the quality of evidence as very low, low, moderate, or high. For randomized trials contributing to the meta-analysis of PA outcome, the rating started at high quality of evidence. For the uptake and adherence rate meta-analysis the quality rating started as low. We downgraded the quality of evidence if serious or very serious limitations were present in domains of risk of bias, imprecision, inconsistency, indirectness, and publication bias. We present results as GRADE evidence profiles and summary of findings tables [25].
The PARS components
The 19 components investigated in this paper (Table 1) were previously identified by our team through a content analysis of various PARS models [10].
Outcome data extraction
Quantitative data were extracted by one reviewer (EM) using Microsoft Excel spreadsheets. We extracted data related to PA outcome, PARS uptake and adherence rates, as well as study-level characteristics. For the PA outcome, we extracted sample size, reported effect size (ES) if available, and mean and standard deviation (SD) at baseline and follow-ups. Otherwise, we extracted other available statistics that would enable an ES calculation. We contacted authors of 13 primary studies where insufficient data were reported, but only two supplied the requested data. We focused on total PA but when not available, we extracted other reported PA outcomes such as moderate to vigorous PA or walking time. For uptake, the total number of persons offered the PARS and the number entering the scheme were extracted. For adherence, the number who took up and adhered to the PARS were extracted.
Data synthesis
Effect size calculation
The summary statistics and PA instruments differed across studies, thus we used the standardized mean difference Hedges’ g [26, 27] as a uniform measure of effect, using the Cohens’ d interpretation as small (g = 0.2), medium (g = 0.5), and large (g = 0.8) [28]. For independent group comparisons we used the mean difference between groups and the pooled SD at post-scheme. For dependent samples we subtracted pre- from post-scheme mean value and divided by baseline SD. For studies that reported only standard error (SE), we multiplied SE by the square root of the sample size to obtain SD. When only range was reported, in absence of other similar studies to borrow a SD, we adopted a solution proposed by Walter and Yao [29] and used a correction factor f and the sample size. When median and quartile range were reported we followed the formulas from Wan et al. [30]. If only the 95% confidence intervals (CI) were presented, the difference was divided by 3.92 and multiplied with the sample size square root to obtain SD [21]. In the case of dichotomous PA outcomes we transformed the reported odds ratios to Hedges’ g [26]. All the summary statistics transformations were done in Microsoft Excel (Additional file 2).
Meta-analysis
PA data were pooled using separate random-effects meta-analysis by comparison group (usual care, PA advice, and enhanced PARS). Only experimental studies with sufficient data to calculate ES were combined, given that they offer better evidence than other types of studies. We also pooled pre-post studies separately to experimental studies. The first available follow-up post-scheme was pooled. This is because it was the most consistently reported follow-up, mostly ranging from post-scheme to three months. The few cases reporting only six and nine month outcomes were subjected to sensitivity analysis and retained in the analysis if robustness was not compromised. Some studies measured the PA outcome using more than one instrument. As most instruments were self-report, we included self-reported outcomes as a first preferred option in the analysis. In the few studies where this was not available, we included objective measures. We used the DerSimonian-Laird estimator to adjust the weight for each study according to the heterogeneity variance (tau-squared, τ2) [31]. Additionally, the Knapp-Hartung adjustment was applied to CIs of the pooled ES. The results are presented as standardized mean differences (Hedges’ g) and 95% CI. To make the results more tangible for the clinicians and policy makers, we calculated the number needed to treat (NNT) from Hedges’ g using the Kraemer and Kupfer 2006 method [32].
To test the robustness of the pooled ES we searched for outliers and influential cases based on the leave-one-out method [33]. Statistical heterogeneity was investigated using I2 [34] (where 25% low; 50% moderate, 75% substantial). Additionally, we added prediction intervals [35] to the forest plot to show the expected true effects for 95% of similar future studies. We used contour-enhanced funnel plots of Hedges’ g against SE to visually explore publication bias, and in case of more than 10 studies per meta-analysis, we conducted the Egger’s regression test for small-study effects [36].
Data on uptake and adherence rate were pooled using a proportional meta-analysis with the aim of presenting a descriptive analysis of how participants engage with PARS rather than assessing effect. The data were first transformed using Freeman-Tukey double arcsine transformation and back transformed using the inverse logit transformation [37]. The Wilson-Score interval method is used to estimate the 95% CI. We did not assess publication bias for the uptake and adherence meta-analyses given that it is not suggested in these types of data [37].
Sub-group analysis
In case of low heterogeneity, no further sub-group analysis was made. Subgroup analysis was conducted assuming a common estimate of between-study heterogeneity between subgroups [26]. The potential explanatory characteristics were pre-specified in the review protocol: geographical location, study design, risk of bias, follow-up, population characteristics, and scheme length [20].
Meta-regression
We used univariate meta-regression with a categorical predictor to investigate whether PA, uptake, and adherence rates (as measures of effectiveness) were associated with the presence of specific PARS components (Table 1). Meta-regression was performed only for the components for which 10 or more studies were available (at least five having the component, five not). Components that were not associated with the outcome measure, were excluded (e.g., exit consultations were excluded from components associated with uptake). We conducted additional post-hoc meta-regression analysis using the total number of components as the predictor variable.
All the analysis were done using R studio software (4.3.0) [33, 38]. The analysis scripts are available via R markdown in Open Science Framework (https://osf.io/dv8fb/?view_only=1703f57bd7f74c6ca0786e7093b531ec).
Results
Study selection
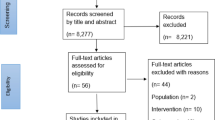
From 57 studies included in our first systematic review [10], six were excluded because of insufficient data to compute ES (Additional file 3). One study was identified from the updated search [39]. In total, 52 studies [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90] were included in the analysis (Fig. 1).
Study selection flow diagram. k number of studies, *for a more detailed description of the flow of studies through the systematic review please see figure 1 in [10]
Study characteristics
The included studies were experimental (k = 28, n = 9730) and non-experimental (k = 24, n = 28,405). The RCTs compared PARS with usual care (k = 11), PA advice (k = 5), and enhanced scheme versions (k = 9). Most studies (k = 50) targeted those with or at risk of non-communicable diseases (physical inactivity in combination with other risk factors such as overweight/obesity, elevated blood pressure, history of myocardial infarction, hypercholesterolemia, impaired glucose tolerance, smoking). PARS length ranged from one day (one-time intervention) to two years. Most studies (65%) were conducted in Europe, and follow-up time ranged from scheme completion to 15 months (Table 2). Of the 41 studies reporting PA, four measured PA via accelerometers or pedometers, 30 via questionnaires, and seven via both methods (Additional file 4). Uptake and adherence were measured via self-report or attendance records. Definitions of uptake and adherence differed slightly between studies (Additional file 5). Two broad categories of PARS were identified; those based on a written prescription (prescription scheme) and those with a referral to another healthcare/PA professional that might include additional prescription (referral scheme). Typically, for prescription schemes, uptake was defined as attendance at the first scheme activity, such as the initial counseling support session. The level of participation in counseling support sessions or other scheme activities was used as a measure of adherence. For referral schemes, uptake was usually defined as attending at least a baseline consultation and/or one PA session. Attendance at PA sessions was the most common measure of adherence.
Risk of bias in included studies
Risk of bias assessment results for each outcome are summarized in Fig. 2. Detailed study-specific traffic light ratings are shown in Additional file 6. Most potential sources of bias in the RCTs were missing data, the measurement of PA outcome through self-report, and lack of pre-specified analysis protocols. The non-experimental studies pooled for uptake and adherence introduced a higher risk of bias.
PARS effectiveness
PARS uptake
On average, 87% (95%CI 77% to 94%) of individuals across 14 randomized trials and 68% (95%CI 51% to 83%) across 14 non-experimental studies that reported uptake opted to enter the offered PARS (Fig. 3). The heterogeneity statistics suggest that there is between-study variability in the true uptake rates. We found sub-group effect only for the analysis of non-experimental studies, where prescription schemes had lower uptake rate than referral schemes. However, unexplained heterogeneity remained extremely high (Additional file 7).
PARS adherence
From those who took up PARS, 68% adhered to it (95%CI 55% to 80%) in experimental studies (Fig. 4). The pooled adherence rate among non-experimental studies was 53% (95%CI 42% to 63%). We found subgroup effects for risk of bias, location, and population only across experimental studies. High risk of bias, UK-based studies, referral schemes, or those including only at-risk populations reported the lowest adherence rates (Additional file 7). However, very high heterogeneity was still present.
Physical activity
The meta-analysis of 11 RCTs (n = 5046) showed that PA improved significantly in participants receiving PARS compared with usual care (Hedges’ g = 0.18, 95%CI 0.12 to 0.25), (Fig. 5). The magnitude of the effect was similar for objective (k = 5, g = 0.21, 95%CI -0.01 to 0.43) and subjective measures of PA (k = 6, g = 0.19, 95%CI 0.09 to 0.28). The NNT-Analysis showed that approximately 10 participants needed to receive PARS instead of usual care for one to increase their PA level. The pooled studies had low heterogeneity but with wide 95%CI (I2 = 8.2%, 95%CI 0.0% to 63.5%) and τ2 = 0.00 (95%CI 0.00 to 0.04). The prediction interval ranged from 0.09 to 0.28. The symmetrical funnel plot and Eggers’ test indicated no evidence of small-study effects (Additional file 8). The pooled effect size of RCTs comparing PARS with PA advice was g = -0.06 (95%CI -0.21 to 0.10). The observed heterogeneity was low (I2 = 0%, 95%CI 0.0% to 79%, τ2 = 0.00, 95%CI 0.00 to 0.17). Enhanced versions of PARS were not more effective than standard less intense models (g = 0.07, 95%CI -0.03 to 0.18). Twenty-four participants needed to follow an enhanced PARS, for one additional participant to increase their PA level compared to those who participated in a less intense version. This difference is not substantial and might be due to chance. The I2 statistic suggests that 4.5% of the observed between-study variability is due to true heterogeneity across the nine included studies (95%CI 0.0% to 66.4%). The study from Isaacs et al. [60] was identified as an outlier. Its inclusion in the analysis lowered the between-group difference to zero (g = 0.01, 95%CI -0.13 to 0.15) and increased heterogeneity to 70.8% (95%CI 44.3% to 84.7%). No publication bias was detected. Due to low heterogeneity no subgroup analysis was undertaken. Results for meta-analysis of specific PA types can be found in Additional file 9 and 10. Pooled non-experimental studies, with substantial heterogeneity and publication bias, showed a small to moderate effect of PARS on PA level (g = 0.40, 95%CI 0.14 to 0.66), (Additional file 11).
Forest plots indicating PARS effect on physical activity as compared to usual care, PA advice, and scheme intensity determined by random effects meta-analysis. Hedges’ g > 0 favors PARS, PARS physical activity referral scheme, CI confidence intervals, Meta-analysis A Omitting Murphy et al. 2012 as influential case for the PA analysis: g = 0.22, 95%CI 0.16 to 0.29, p-value < 0.0001, I2 = 0% [0.0%; 62.4%], Meta-analysis B Bellanger et al. 2023 included also active participants at baseline
Examination of PARS effectiveness by components
Because of an insufficient number of studies containing some of the components and/or their relevance to the outcome examined, we included 15 components in the meta-regression for PA, eight for uptake and 14 for adherence. No individual components predicted PA level or uptake in experimental studies (Table 3) or non-experimental studies (Additional file 12). Across all studies, PARS based on a person-centered approach or including screening or brief advice, reported 17% to 25% higher adherence rates. In contrast, offering PA sessions was negatively associated with adherence. However, the amount of unexplained heterogeneity remained substantially high.
The number of components in a PARS was identified as a predictor for uptake but not adherence and PA outcome. For any additional increase in number of components, the uptake rate is estimated to increase by around 6% (Table 3). However, the number of components accounts for a very small amount of heterogeneity.
Certainty of evidence
We rated the certainty of evidence under the GRADE criteria for the PA meta-analysis comparing PARS to usual care as high. In contrast, our confidence in the pooled effect estimates for the comparison of PARS with PA advice or alternative PARS versions was limited. The proportional meta-analysis for uptake and adherence rates were descriptive in nature and characterized by very low certainty. See Additional file 13 for GRADE Evidence Profiles and Summary of Findings tables with detailed explanations of the rating decisions.
Discussion
Our results show that PARS are more effective than usual care in increasing PA. We did not find any difference between PARS and PA advice only or various scheme intensities with regard to PA change. This was the first study to examine the potential role of PARS components in effectiveness. PARS components were regressed for their independent effect on PA, scheme uptake, and scheme adherence. Adherence was higher in PARS including a person-centered approach, screening, or brief advice, and lower in schemes offering PA sessions. The meta-regression did not detect a possible relationship between PARS components and PA level or uptake.
Interpretation of the meta-analysis findings
Our study updates previous seminal meta-analyses and reinforces their the PA promoting effect of PARS [2, 16]. In our review, pooled data from 11 RCTs (n = 5046) showed that PARS result in a small increase in PA compared to usual care (high certainty of evidence). Approximately 10 PARS participants are needed for one to become more active. An earlier meta-analysis pooling five RCTs, concluded that 17 people need to participate for one to engage in moderate exercise [3]. However, the analysis pooled together all types of comparison groups [3]. Previous meta-analyses of PARS versus usual care reported that PARS participants had a 12% (95%CI 1.04 to 1.20, k = 5, n = 4504) [16] to 16% (95%CI 1.03 to 1.30, k = 4, n = 2334) [2] higher likelihood of achieving 90 to 150 min of at least moderate PA per week. While these statistics cannot be directly compared, they all confirm that adding PARS to usual care is associated with increased PA. Our meta-analysis adds that 95% of future studies comparing PARS with usual care may expect to have an effect size between 0.09 and 0.28. As with previous meta-analyses [2, 4, 16], we did not find any difference in PA level between PARS with PA advice only (5 RCTs) or with enhanced scheme versions (9 RCTs), (low certainty of evidence).
The lack of difference between the PARS and PA advice may be attributed to two key factors. Firstly, the PA advice demands a lesser commitment from participants compared to PARS. This may lead to lower uptake and adherence rates in PARS, ultimately fewer participants receiving the intervention as intended. Notably, one RCT revealed a significant difference in PA levels between the intervention and comparison groups when adherence rates exceeded 50% [54]. Secondly, participants in the PA advice group might increase their PA levels due to their participation in the study, a phenomenon known as the Hawthorne effect [39, 60]. We encountered similar arguments in the discussion sections of studies comparing standard PARS with enhanced versions, where no difference was detected. Enhanced scheme versions typically incorporate additional components or a higher session frequency, posing greater challenges to implementation by necessitating additional resources. For instance, a study that augmented the standard PARS using the Self-determination Theory reported additional difficulties in training scheme deliverers, potentially influencing the implementation of the enhanced intervention version [47].
As with Pavey et al. [17], experimental studies in our analysis reported significantly higher uptake levels than non-experimental studies and similar adherence in observational studies. However, Pavey et al. [17] reported much lower adherence across RCTs (49%, 95%CI 40% to 59%). Consistent with previous evidence [16, 17], we found considerable heterogeneity in uptake and adherence rates which could not be explained by subgroup analysis. However, the proportional data are known to be inherently highly heterogeneous and so this does not automatically signify data inconsistency [37].
Interpretation of the meta-regression findings
This study is unique in that, to our knowledge, it is the first to examine associations between PARS components and PA, uptake, and adherence. However, the statistical power of the meta-regression was limited. We did not find significant associations between specific PARS components and PA level. Despite this, the regression coefficients indicate a greater effect on PA (g = 0.07 to 0.11) for schemes including a person-centered approach, behavior change techniques, screening, or a written prescription; and lower effect for schemes with exit consultations (g = -0.10). Although these effects appear to be negligible, they might have practical relevance in the context of overall small effect sizes observed in our PARS meta-analysis. Inability to reach statistical significance might be explained by not fulfilling the basic assumption of sufficient heterogeneity to carry out a meta-regression, which in our case was only 33.9% (95%CI 0.0% to 59.4%). Furthermore, we examined the impact of individual components rather than potential combinations. The added value of individual components on PA level was also investigated among some of the included studies. Findings regarding the counseling support session(s) [41, 51, 69] and written prescription [75, 84] were mixed. The inclusion of PA sessions [81] or basing the PARS on the Self-determination Theory [47] did not result in an added impact on PA level.
No component significantly predicted variation in PARS uptake. Prescription schemes reported approximately 6% higher uptake rates, but this relationship did not reach statistical significance and the amount of unaccounted heterogeneity remained substantially high. PARS including a person-centered approach, screening, or brief advice achieved higher adherence rates. In contrast, including PA sessions was associated with decreased adherence. While this is counterintuitive, Pavey et al. [17] also suggested that a higher number of sessions might be related to lower adherence. There are several potential explanations for this. PA session attendance provides a tangible measure of adherence that does not exist in PARS offering counseling only. Additionally, participants asked to attend PA sessions might face barriers related to transportation, accessibility, inconvenient timings, poor supervisory experiences, inadequate/inappropriate content, and lack of enjoyment, individualization, or relatable peers [3, 8, 56, 91]. PARS to date are based mainly on Social Cognitive Theory, Self-determination Theory, and the Transtheoretical Model [10], which give limited attention to the affective determinants of PA behavior such as enjoyment [92]. Future PARS could consider using the lens of affective science [93] to provide PA sessions that increase positive experiences and consequently engagement. PARS could intensify efforts towards increasing individual competencies needed for independent PA (e.g., PA-related Health Competence [94].
Limitations and strengths
There are several caveats to this review. First, the results could be affected by the coding of components from scheme content reported in individual studies in our previous review [10]. To avoid subjective assumptions, we suggest authors of future studies identify and report PARS components based on our classification [10] and use the PARS taxonomy [95] to report characteristics. Second, not all components were investigated due to the limited number of studies available. Third, the relationship between components and PARS outcomes investigated through the meta-regression is not causal but observational [96]. The results might be misleading because of biases and confounding by other factors not related to scheme design (e.g., healthcare system characteristics) [97]. Fourth, we addressed only one aspect of PARS complexity: the components and a simplified linear relationship with scheme outcomes. Other characteristics of PARS and the causal pathway, such as between-components and scheme by context interactions, healthcare and societal ecosystems in which PARS are delivered, and characteristics of PARS delivers and receivers were not considered [18]. However, a focused question and simple analysis is suggested to be a good start for understanding complexity [18]. Fifth, the component content might be as important as whether the scheme includes it or not. For example, behavior change techniques may be implemented in varying degrees and combinations. Finally, only English and German publications were included, and the certainty of the evidence was assessed by one reviewer. This may introduce some uncertainties regarding the inclusion of all relevant studies and the confidence level of the pooled effect estimates.
The review also has several strengths. The methods were pre-registered and published to reduce bias or change of research question based on identified evidence. To avoid data dredging [96], meta-regression variables were prespecified in advance and we adhered to the prespecified question in the protocol [20]. All extracted data and analyses are transparent and reproducible. We included observational studies to provide naturalistic comparisons and rated the certainty of evidence for each meta-analysis outcome.
Implications for practice and policy and future research
This study reinforces the potential of PARS as a strategy to support an active society by promoting PA in healthcare settings [1]. We highlight well-defined components that can guide PARS design. Consideration might be given to adding a person-centered approach, screening, or brief advice to existing schemes for improving the adherence rate. Future research should focus on understanding the role of components in PARS effectiveness. High quality experimental studies manipulating the use of components, such as factorial RCTs, could provide evidence about the effect of individual or combined components [98]. For example, in a two-by-two factorial experiment, two components, e.g., PA sessions and counseling support sessions, can be used as factors with two levels (present or absent). This results in four possible combinations to which participants can be randomly assigned. Additionally, research should compare the effect of components and their implementation cost. This could help optimize PARS by highlighting components that have a small effect but high implementation cost to help decision-makers find a balance between cost and effect. This is important to create sustainable PARS and increase their public health impact. Qualitative research exploring experiences of PARS participants and deliverers with the components could be valuable in contributing to wider understanding. Thus, mixed-methods designs are essential in evaluating PARS. The example of PARS and their complexity highlights that research about PA promotion in healthcare settings might benefit from the theories and methods used in complexity research [99] and systems thinking [100].
Our findings are hypothesis generating and not final conclusions. We encourage future studies to test the effect of the identified components. Further research is needed to confirm or establish new associations between PARS components and outcomes by using more sophisticated statistical methods such as component network meta-analysis models, and component individual participant data meta-analysis.
Conclusions
Implementing PARS within healthcare settings might be valuable for effectively increasing PA on a broader scale. Findings from the meta-regression increase our understanding of the role of scheme components on PA, uptake and adherence. PARS may have higher adherence rates if they include a person-centered approach, screening, or brief advice. PARS including PA sessions reported lower adherence rates but as these are a promising source of PA experience, schemes should optimize the content of PA sessions and consider paying special attention to the affective response and enjoyment. No association was found between components and PA level or scheme uptake. However, components should not be disregarded because of statistical significance, but rather further investigated. Taken together, the findings indicate that scheme components can contribute to a better understanding of PARS effectiveness.
Availability of data and materials
The dataset and the R script used to generate the results in this article is available in the Open Science Framework repository at https://osf.io/dv8fb/?view_only=1703f57bd7f74c6ca0786e7093b531ec.
Abbreviations
- CI:
-
Confidence interval
- ES:
-
Effect size
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- NNT:
-
Number needed to treat
- PA:
-
Physical activity
- PARS:
-
Physical activity referral scheme(s)
- RCT:
-
Randomized controlled trial
- RoB2:
-
Cochrane risk-of-bias tool for randomized trials
- ROBINS-I:
-
Risk of Bias in Non-randomized Studies-of Interventions
- SD:
-
Standard deviation
- SE:
-
Standard error
References
World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healtier world. Geneva: World Health Organization; 2018.
Pavey TG, Taylor AH, Fox KR, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ. 2011;343:d6462.
Williams N, Hendry M, France B, et al. Effectiveness of exercise-referral schemes to promote physical activity in adults: systematic review. Br J Gen Pract. 2007;57(545):979–86.
Orrow G, Kinmonth A-L, Sanderson S, et al. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389.
National Institute for Health and Care Excellence. Physical activity: exercise referral schemes (PH54). London: NICE; 2014.
Calonge Pascual S, Casajús Mallén JA, González-Gross M. Adherence factors related to exercise prescriptions in healthcare settings: a review of the scientific literature. Res Q Exerc Sport. 2020;93(1):1–10.
Eynon M, Foad J, Downey J, et al. Assessing the psychosocial factors associated with adherence to exercise referral schemes: a systematic review. Scand J Med Sci Sports. 2019;29(5):638–50.
Morgan F, Battersby A, Weightman AL, et al. Adherence to exercise referral schemes by participants - what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health. 2016;16:227.
Rowley N, Mann S, Steele J, et al. The effects of exercise referral schemes in the United Kingdom in those with cardiovascular, mental health, and musculoskeletal disorders: a preliminary systematic review. BMC Public Health. 2018;18(1):949.
Mino E, Hanson C, Naber I, et al. A systematic review and narrative synthesis of physical activity referral schemes’ components. Int J Behav Nutr Phys Act. 2023;20(1):140.
Whiting S, Mendes R, Morais ST, et al. Promoting health-enhancing physical activity in Europe: surveillance, policy development and implementation 2015–2018. Health Policy. 2021;125(8):1023–30.
Gaeta M, Campanella F, Capasso L, et al. An overview of different health indicators used in the European Health Systems. J Prev Med Hyg. 2017;58(2):E114–20.
Dugdill L, Graham RC, McNair F. Exercise referral: the public health panacea for physical activity promotion? A critical perspective of exercise referral schemes; their development and evaluation. Ergonomics. 2005;48(11–14):1390–410.
Kallings L. The Swedish approach on physical activity on prescription. Clin Health Promot. 2016;6(2):31–3 http://www.clinhp.org/ifile/Vol6_Supplement2_HEPA_p31_p33.pdf.
Onerup A, Arvidsson D, Blomqvist Å, et al. Physical activity on prescription in accordance with the Swedish model increases physical activity: a systematic review. Br J Sports Med. 2019;53(6):383–8.
Campbell F, Holmes M, Everson-Hock E, et al. A systematic review and economic evaluation of exercise referral schemes in primary care: a short report. Health Technol Assess. 2015;19(60):1–110.
Pavey T, Taylor A, Hillsdon M, et al. Levels and predictors of exercise referral scheme uptake and adherence: a systematic review. J Epidemiol Community Health. 2012;66(8):737–44.
Petticrew M, Anderson L, Elder R, et al. Complex interventions and their implications for systematic reviews: a pragmatic approach. J Clin Epidemiol. 2013;66(11):1209–14.
Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Mino E, Geidl W, Naber I, et al. Physical activity referral scheme components: a study protocol for systematic review and meta-regression. BMJ Open. 2021;11(6):e049549.
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.3 (updated August 2022). Cochrane; 2022. Available from www.training.cochrane.org/handbook.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020;12(1):1–7.
Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated December 2015. The GRADE Working Group; 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html.
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime; 2024. Available from gradepro.org. (https://www.gradepro.org/terms/cite).
Borenstein M. Introduction to meta-analysis. Oxford: Wiley; 2009.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Erlbaum; 1988.
Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol. 2007;60(8):849–52.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–6.
Harrer M, Cuijpers P, Furukawa TA, et al. Doing meta-analysis with R: a hands-on guide. 1st ed. Boca Raton and London: Chapman & Hall/CRC Press; 2021.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane handbook for systematic reviews of interventions version 6.3 (updated August 2022). Cochrane; 2022. Available from https://gdt.gradepro.org/app/handbook/handbook.html.
Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1):189.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Bellanger W, Peurois M, Connan L, et al. Comparing physical activity prescription with verbal advice for general practice patients with cardiovascular risk factors: results from the PEPPER randomised controlled trial. BMC Public Health. 2023;23(1):1402.
Aittasalo M, Miilunpalo S, Kukkonen-Harjula K, et al. A randomized intervention of physical activity promotion and patient self-monitoring in primary health care. Prev Med. 2006;42(1):40–6.
Andersen P, Holmberg S, Årestedt K, et al. Physical activity on prescription in routine health care: 1-year follow-up of patients with and without counsellor support. Int J Environ Res Public Health. 2020;17(16):5679.
Bredahl T, Singhammer J, Roessler K. “Is intensity decisive?” Changes in levels of self-efficacy, stages of change and physical activity for two different forms of prescribed exercise. Sport Sci Rev. 2011;20(3–4):85.
Buckley B, Thijssen DH, Murphy RC, et al. Pragmatic evaluation of a coproduced physical activity referral scheme: a UK quasi-experimental study. BMJ Open. 2020;10(10):e034580.
Crone D, Johnston LH, Gidlow C, et al. Uptake and participation in physical activity referral schemes in the UK: an investigation of patients referred with mental health problems. Issues Ment Health Nurs. 2008;29(10):1088–97.
Dinan S, Lenihan P, Tenn T, et al. Is the promotion of physical activity in vulnerable older people feasible and effective in general practice? Br J Gen Pract. 2006;56(531):791–3.
Dodd-Reynolds CJ, Vallis D, Kasim A, et al. The Northumberland Exercise Referral Scheme as a universal community weight management programme: a mixed methods exploration of outcomes, expectations and experiences across a social gradient. Int J Environ Res Public Health. 2020;17(15):5297.
Duda JL, Williams GC, Ntoumanis N, et al. Effects of a standard provision versus an autonomy supportive exercise referral programme on physical activity, quality of life and well-being indicators: a cluster randomised controlled trial. Int J Behav Nutr Phys Act. 2014;11(1):10.
Edmunds J, Ntoumanis N, Duda JL. Adherence and well-being in overweight and obese patients referred to an exercise on prescription scheme: a self-determination theory perspective. Psychol Sport Exerc. 2007;8(5):722–40.
Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326(7393):793.
Foley L, Maddison R, Jones Z, et al. Comparison of two modes of delivery of an exercise prescription scheme. N Z Med J. 2011;124(1338):44–54.
Fortier MS, Hogg W, O’Sullivan TL, et al. Impact of integrating a physical activity counsellor into the primary health care team: physical activity and health outcomes of the Physical Activity Counselling randomized controlled trial. Appl Physiol Nutr Metab. 2011;36(4):503–14.
Gademan MGJ, Deutekom M, Hosper K, et al. The effect of exercise on prescription on physical activity and wellbeing in a multi-ethnic female population: a controlled trial. BMC Public Health. 2012;12:758.
Galaviz K, Lévesque L, Kotecha J. Evaluating the effectiveness of a physical activity referral scheme among women. J Prim Care Community Health. 2013;4(3):167–71.
Gallegos-Carrillo K, García-Peña C, Salmerón J, et al. Brief counseling and exercise referral scheme: a pragmatic trial in Mexico. Am J Prev Med. 2017;52(2):249–59.
Hanson CL, Allin LJ, Ellis JG, et al. An evaluation of the efficacy of the exercise on referral scheme in Northumberland, UK: association with physical activity and predictors of engagement. A naturalistic observation study. BMJ Open. 2013;3(8):e002849.
Hanson CL, Neubeck L, Kyle RG, et al. Gender differences in uptake, adherence and experiences: a longitudinal, mixed-methods study of a physical activity referral scheme in Scotland, UK. Int J Environ Res Public Health. 2021;18(4):1700.
Harrison RA, McNair F, Dugdill L. Access to exercise referral schemes – a population based analysis. J Public Health (Oxf). 2005;27(4):326–30.
Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health (Oxf). 2005;27(1):25–32.
Hesketh K, Jones H, Kinnafick F, et al. Home-Based HIIT and traditional MICT prescriptions improve cardiorespiratory fitness to a similar extent within an exercise referral scheme for at-risk individuals. Front Physiol. 2021;12:750283.
Isaacs AJ, Critchley JA, Tai SS, et al. Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol Assess. 2007;11(10):1–165, iii−iv.
James EL, Ewald BD, Johnson NA, et al. Referral for expert physical activity counseling: a pragmatic RCT. Am J Prev Med. 2017;53(4):490–9.
Kallings LV, Johnson JS, Fisher RM, et al. Beneficial effects of individualized physical activity on prescription on body composition and cardiometabolic risk factors: results from a randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2009;16(1):80–4.
Kallings LV, Leijon ME, Kowalski J, et al. Self-reported adherence: a method for evaluating prescribed physical activity in primary health care patients. J Phys Act Health. 2009;6(4):483–92.
Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10(3):206–12.
Lawton BA, Rose SB, Elley CR, et al. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BMJ. 2008;337:a2509.
Leijon ME, Bendtsen P, Ståhle A, et al. Factors associated with patients self-reported adherence to prescribed physical activity in routine primary health care. BMC Fam Pract. 2010;11:38.
Livingston PM, Craike MJ, Salmon J, et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: a multicenter cluster randomized controlled trial (ENGAGE). Cancer. 2015;121(15):2646–54.
Lord JC, Green F. Exercise on prescription: does it work? Health Educ J. 1995;54(4):453–64.
Lundqvist S, Börjesson M, Cider Å, et al. Long-term physical activity on prescription intervention for patients with insufficient physical activity level—a randomized controlled trial. Trials. 2020;21(1):793.
Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8(3):e017211.
Morén C, Welmer A-K, Hagströmer M, et al. The effects of “physical activity on prescription” in persons with transient ischemic attack: a randomized controlled study. J Neurol Phys Ther. 2016;40(3):176–83.
Murphy SM, Edwards RT, Williams N, et al. An evaluation of the effectiveness and cost effectiveness of the National Exercise Referral Scheme in Wales, UK: a randomised controlled trial of a public health policy initiative. J Epidemiol Community Health. 2012;66(8):745–53.
Pardo A, Violán M, Cabezas C, et al. Effectiveness of a supervised physical activity programme on physical activity adherence in patients with cardiovascular risk factors. Apunts Med Esport. 2014;49(182):37–44.
Petrella RJ, Lattanzio CN, Shapiro S, et al. Improving aerobic fitness in older adults: effects of a physician-based exercise counseling and prescription program. Can Fam Physician. 2010;56(5):e191–200.
Pfeiffer BA, Clay SW, Conatser JRRR. A green prescription study: does written exercise prescribed by a physician result in increased physical activity among older adults? J Aging Health. 2001;13(4):527–38.
Prior F, Coffey M, Robins A, et al. Long-term health outcomes associated with an exercise referral scheme: an observational longitudinal follow-up study. J Phys Act Health. 2019;16(4):288–93.
Riera-Sampol A, Bennasar-Veny M, Tauler P, et al. Effectiveness of physical activity prescription by primary care nurses using health assets: a randomized controlled trial. J Adv Nurs. 2020;77(3):1518–32.
Romé A, Persson U, Ekdahl C, et al. Physical activity on prescription (PAP): costs and consequences of a randomized, controlled trial in primary healthcare. Scand J Prim Health Care. 2009;27(4):216–22.
Samdal GB, Meland E, Eide GE, et al. The Norwegian Healthy Life Centre Study: a pragmatic RCT of physical activity in primary care. Scand J Public Health. 2019;47(1):18–27.
Sjöling M, Lundberg K, Englund E, et al. Effectiveness of motivational interviewing and physical activity on prescription on leisure exercise time in subjects suffering from mild to moderate hypertension. BMC Res Notes. 2011;4(1):1–7.
Sørensen JB, Kragstrup J, Skovgaard T, et al. Exercise on prescription: a randomized study on the effect of counseling vs counseling and supervised exercise. Scand J Med Sci Sports. 2008;18(3):288–97.
Sørensen J, Sørensen JB, Skovgaard T, et al. Exercise on prescription: changes in physical activity and health-related quality of life in five Danish programmes. Eur J Public Health. 2011;21(1):56–62.
Stewart L, Dolan E, Carver P, et al. Per-protocol investigation of a best practice exercise referral scheme. Public Health. 2017;150:26–33.
Swinburn BA, Walter LG, Arroll B, et al. The green prescription study: a randomized controlled trial of written exercise advice provided by general practitioners. Am J Public Health. 1998;88(2):288–91.
Taylor AH, Doust J, Webborn N. Randomised controlled trial to examine the effects of a GP exercise referral programme in Hailsham, East Sussex, on modifiable coronary heart disease risk factors. J Epidemiol Community Health. 1998;52(9):595–601.
Taylor AH, Taylor RS, Ingram WM, et al. Adding web-based behavioural support to exercise referral schemes for inactive adults with chronic health conditions: the e-coachER RCT. Health Technol Assess. 2020;24(63):1–106.
van de Vijver PL, Schalkwijk FH, Numans ME, et al. Linking a peer coach physical activity intervention for older adults to a primary care referral scheme. BMC Prim Care. 2022;23(1):118.
Ward M, Phillips CJ, Farr A, et al. Heartlinks—a real world approach to effective exercise referral. Int J Health Promot Educ. 2010;48(1):20–7.
Webb R, Thompson JES, Ruffino J-S, et al. Evaluation of cardiovascular risk-lowering health benefits accruing from laboratory-based, community-based and exercise-referral exercise programmes. BMJ Open Sport Exerc Med. 2016;2(1):e000089.
Williams MH, Cairns SP, Simmons D, et al. Face-to-face versus telephone delivery of the green prescription for Maori and New Zealand Europeans with type-2 diabetes mellitus: influence on participation and health outcomes. N Z Med J. 2017;130:71–9.
Hanson CL, Oliver EJ, Dodd-Reynolds CJ, et al. How do participant experiences and characteristics influence engagement in exercise referral? A qualitative longitudinal study of a scheme in Northumberland, UK. BMJ Open. 2019;9(2):e024370.
Rhodes RE, Fiala B, Conner M. A review and meta-analysis of affective judgments and physical activity in adult populations. Ann Behav Med. 2009;38(3):180–204.
Ekkekakis P, Russell JA. The measurement of affect, mood, and emotion: a guide for health-behavioral research. New York: Cambridge University Press; 2013.
Carl J, Sudeck G, Pfeifer K. Competencies for a healthy physically active lifestyle-reflections on the model of physical activity-related health competence. J Phys Act Health. 2020;17(7):688–97.
Hanson CL, Oliver EJ, Dodd-Reynolds CJ, et al. A modified Delphi study to gain consensus for a taxonomy to report and classify physical activity referral schemes (PARS). Int J Behav Nutr Phys Act. 2020;17(1):158.
Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73.
Deeks JJ, Higgins JPT, Altman DG, eds. Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of Interventions version 6.1 (updated September 2020). Cochrane; 2020. Available from www.training.cochrane.org/handbook.
Collins LM, Dziak JJ, Kugler KC, et al. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47(4):498–504.
Kannampallil TG, Schauer GF, Cohen T, et al. Considering complexity in healthcare systems. J Biomed Inform. 2011;44(6):943–7.
Peters DH. The application of systems thinking in health: why use systems thinking? Health Res Policy Sys. 2014;12:51.
Acknowledgements
Not applicable.
Disclaimer
SW, KW and GG are staff members of the World Health Organization. The views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views, decisions, or policies of the institutions to which they are affiliated.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study took place within the BewegtVersorgt project, which is supported by the Federal Ministry of Health based on a resolution of the German Bundestag by the Federal Government (ZMV I 1—2519FSB109). The funder was not involved in any of this study activities, and the expressed views are solely those of the authors.
Author information
Authors and Affiliations
Contributions
EM rated the quality of evidence assessment, and conducted data extraction, cleaning, statistical analyses, wrote the first draft, and takes full responsibility for the accuracy of the data analysis. WG and KP supervised EM during the study process. EM and AB assessed the risk of bias. MS assisted in decision-making regarding data-analysis. CLH edited the manuscript. All authors (CLH, WG, KP, MS, SK, IN, AB, AW, SW, SM, KW, GG) critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12966_2024_1623_MOESM2_ESM.xlsx
Additional file 2. Microsoft Excel file with the formulas used to transform and harmonize the summary statistics reported across the included studies.
12966_2024_1623_MOESM9_ESM.docx
Additional file 9. Forest and funnel plots of PARS effect on various physical activity outcomes as compared to usual care or physical activity advice (RCTs).
12966_2024_1623_MOESM10_ESM.docx
Additional file 10. Meta-analysis of randomized trials comparing enhanced with standard PARS for specific physical activity outcomes.
12966_2024_1623_MOESM12_ESM.docx
Additional file 12. The relationship between PARS components and effect on physical activity level from 12 pre-post studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mino, E., Pfeifer, K., Hanson, C.L. et al. Are physical activity referral scheme components associated with increased physical activity, scheme uptake, and adherence rate? A meta-analysis and meta-regression. Int J Behav Nutr Phys Act 21, 82 (2024). https://doi.org/10.1186/s12966-024-01623-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-024-01623-5