Abstract
The PTEN tumor suppressor is the second most commonly inactivated gene across cancer types. While it’s role in PI3K/AKT and DNA damage pathways are clear, increasing evidences suggest that PTEN may also promote anti-tumor immunity. PTEN-deficient tumors are characterized by (i) reduced levels of cytotoxic T cells, helper T cells and NK cells, (ii) elevated pro-oncogenic inflammatory cytokines like CCL2 and (iii) increased levels of immunosuppressive cells such as MDSCs and Tregs. An intriguing possibility is that link between PTEN and anti-tumor immunity is mediated by the interferon signaling pathway. In this review, we summarize the evidences for the mechanistic link between PTEN deficiency and immunosuppressive tumor microenvironment and the interferon signaling pathway. We further discuss how the link between these pathways can be exploited for development of personalized immunotherapy for patients with PTEN deficient tumors.
Similar content being viewed by others
Background: tumour suppressive functions of PTEN and prevalence of PTEN mutations across cancers
Phosphatase and tensin homolog (PTEN) is a dual phosphatase which has both lipid and protein phosphatase activities in cytoplasm and nucleus respectively. Removing one phosphate group from phosphatidylinositol 3,4,5‑trisphosphate (PIP3) inhibits the activity of the phosphoinositide-3-kinase/AKT serine/threonine kinase (PI3K/AKT) pathway to regulate cell proliferation, metabolism, survival, polarity, migration and angiogenesis [1,2,3,4]. Moreover, protein phosphatase activity of PTEN regulates cell cycle and response to DNA damage in the nucleus [5, 6]. Thus these roles of PTEN suggest that its deficiency could lead to increased genome instability by affecting fidelity of the DNA repair pathway called homologous recombination (HR) [7].
Loss of PTEN functions due to genetic aberration or epigenetic silencing has been related to malignant transformation, progression, chemotherapy response and survival in several cancers [8,9,10,11]. PI3K pathway alterations were identified in 44% of the 60,991 solid tumors and PTEN (9.4%) was the second frequently altered gene after PI3K (13.3%) [12]. Pancancer restricted analyses of different tumors revealed that PTEN alterations, mostly mutations and deep deletions, are frequent in uterine, glioblastoma (GBM), prostate, lung and melanoma cancers (Fig. 1).
Deregulation of PI3K signaling pathway resulting from genetic alterations in the PTEN have been identified in over 50% of GBMs [13]. PTEN mutations are found in 41% of GBM patients and loss of PTEN contributed to impeded DNA repair pathway after ionizing radiation [7, 14]. A recent report highlighted that phosphorylation of PTEN at tyrosine 240 (pY240) by fibroblast growth factor receptor 2 (FGFR2) mediates radiotherapy (RT) resistance in GBM [15]. Homozygous deletions and missense/truncating mutations of PTEN found in 17% of primary prostate cancers [16]. PTEN deletion is also associated with intratumor heterogeneity in prostate cancer [17]. In a large cohort of Non-Small Cell Lung Cancer (NSCLC), PTEN loss was present in half of the squamous cell carcinoma (SCC) and in one-third of adenocarcinoma (AC), and associated with poorer prognosis [18]. In the TCGA melanoma cohort, somatic PTEN alterations were identified in 14% of specimens, consisting of both mutations and focal deletions [19]. Moreover, loss of PTEN has been associated with resistance to BRAF inhibitor and decreased overall survival in melanoma [20, 21].
Evidences for immunosuppressive tumour microenvironment in PTEN deficient tumors
Emerging works suggest that PTEN might have additional functions in the tumor microenvironment including those affecting tumor growth through modulation of the immune response [30, 31]. Host immune response against tumor cells is a tumor suppressor mechanism which provide a barrier to malignant transformation. PTEN signaling influences a broad array of immune cells of both the innate and adaptive compartments (Table 1). Several research groups have reported that PTEN loss tumor cells lead up immunosuppressive infrastructure and break down transformation barrier in the tumor microenvironment (TME).
The first evidence of PTEN and immune homeostasis was reported that germline deletion of PTEN manifests autoimmune disorders [32]. Type II Interferon (IFN)-γ acts on tumor cells, enhancing their recognition by CD8+ T cells as well as by CD4+ T cells, and unveiling a key role in the promotion of tumor immunogenicity [33]. Therefore, major efforts have been made for the development and establishment of combined clinical therapeutic applications [34,35,36,37]. Src homology-2 domain-containing phosphatase-2 (SHP2), an oncogenic phosphatase, inhibits type II IFN-γ signaling. It was demonstrated that lung adenocarcinoma cells, which express low levels of PTEN, are unresponsive to IFN-γ and restoring PTEN expression reverses cellular unresponsive to IFN-γ [8]. PTEN loss also caused immune escape from IFN-γ-mediated cell proliferation inhibition and cytotoxicity in lung adenocarcinoma cells [8].
Loss of PTEN increased the level of PD-L1 (B7-H1) expression through regulation of translation and it is associated with immunotherapy resistance in patients with GBM [22]. PTEN-null prostate senescent tumors can promote growth of adjacent non-senescent tumor cells and cause chemoresistance through the senescence associated secretory phenotype (SASP) associated mechanism [23]. These tumors are characterized by increased levels of several cytokines, strongly infiltrated by granulocytic myeloid-derived suppressor cells (MDSCs), in absence of CD4+, CD8+, and natural killer (NK) infiltrates. Moreover, tumor-infiltrating MDSC cells suppressed the proliferation of CD8+ T cells and inhibited their cytotoxic functions [23].
PTEN has been reported as a molecular biomarker to predict brisk host response in melanoma cells [24]. According to this, testing PTEN will be useful to identify and recruit melanoma patients that might respond better to immunotherapies [24]. Peng et al. [25] remarked PTEN loss as a resistance marker to T cell-mediated antitumor immune responses in melanoma. PTEN loss was associated with decreased numbers and impaired function of tumor-infiltrating T cells and inferior outcomes with anti-PD-1 treatment. Loss of PTEN in melanomas promoted resistance to immune infiltration of tumors through the production of inhibitory cytokines, C-C motif chemokine ligand 2 (CCL2) and vascular endothelial growth factor A (VEGF) which contributes to the immunosuppressive tumor microenvironment by recruiting suppressive immune cells [25]. Peng’s study delineated the influence of an oncogenic pathway on antitumor immunity and response to immunotherapy [38].
PTEN-mediated mechanism of immune resistance to anti-PD-1 therapy was also confirmed in a case report from a chemotherapy-naïve patient with rapidly-progressive metastatic uterine leiomyosarcoma who experienced complete tumor remission for > 2 years on anti-PD-1 monotherapy [26]. VEGFA expression increased and PD-1+ T cell infiltration reduced in the treatment-resistant mesenchymal tumor with biallelic PTEN loss [26]. It was also suggested that PTEN loss causes prostate cancer initiation and progression by upregulation of inflammatory and cytokine–cytokine receptor signaling pathways and these associate with marked chronic and extensive MDSCs immune cell infiltration [27]. Comparative analysis of prostate cancer models showed that the diverse genetics of prostate cancer with PTEN loss can directly determine the differential infiltration and composition of immune cells in the TME [39]. Major tumor drivers can activate proinflammatory and immunosuppressive programs and at gene-specific intrinsic pathways are at the core of diverse protumoral immune-cell recruitment and infiltration [39].
Diffuse large B-cell lymphoma (DLBCL) patients with low PTEN mRNA levels had significantly poorer overall survival and progression-free survival [11]. Distinct gene expression signatures were identified for low PTEN mRNA expression compared with PTEN mRNAnot low. The spectrum of PTEN-mRNAlow genes showed downregulation of genes involved in immune responses, B-cell receptor (BCR) signaling, gene expression and metabolism [11].
Overexpression of PTEN induced a large number of common differentially expressed genes in the PTEN-null GBM cell line [40]. Several cytokines such as interleukin (IL)-6, IL-8, and IL16 that are highly expressed in GBM were downregulated by PTEN overexpression [40]. It was suggested that downregulation of these proto-oncogenic inflammatory cytokines by PTEN affect not only the GBM cells but also the crosstalk between tumor cells and the microenvironment, both of which are contributing factors in suppressing tumor growth. In a recent study, somatic PTEN mutations were associated with resistance to immune checkpoint inhibitors (ICIs) by altering immunosuppressive environments in GBM [28]. PTEN was significantly more frequently mutated in the non-responsive tumors than in the responsive ones and immunosuppressive signature of GBM was most associated with the CD44+ tumor sub-population of the PTEN-mutated case [28].
In a metastatic melanoma cohort, higher burden of copy number loss was observed in non-responders compared to responders on cytotoxic T-lymphocyte associated protein 4 (CTLA-4) blockade [41]. PTEN was identified as one of the tumor suppressor genes with recurrent copy number loss from patients with high burden of copy number loss in this study. Copy number loss burden and down-regulation of immune related gene expression was correlated so it was suggested that there may be gene expression sequelae of extensive copy number loss, including PTEN loss [41].
PTEN in colonic smooth muscle cell could modulate cytokines/chemokines production to affect the immune cells recruitment to mucosa of colon [42]. Pancreatic ductal adenocarcinoma (PDAC) genome has frequent deletion of the PTEN as well as loss of expression in primary tumor specimens. The mouse PDAC driven by oncogenic Kras and PTEN loss promotes marked nuclear factor kappa B (NF-κB) activation and its cytokine network, with accompanying robust stromal activation and immune cell infiltration [43]. Recently, PTEN deficiency has been linked to an immunosuppressive state in prostate cancer with distinct changes in the frequency of immune cell types in tumors from different metastatic sites [29]. Forkhead box P3+ (FoxP3+) regulatory T cells (Treg) cells and overexpression of indoleamine 2,3-dioxygenase 1 (IDO1) protein were reported as the source of immunosuppression [29] (Fig. 2).
Immunosuppressive characteristics of PTEN mutant tumors. PTEN-deficient tumors are infiltrated by MDSCs [27] and Tregs [29]. JAK/STAT pathway is activated [23], IDO1 protein [29], PD-1 receptors [22] and inhibitory cytokines [23, 25, 27] are upregulated. CD4+, CD8+ and NK cells exhibited reduced infiltration [23, 25]. Cytotoxic T lymphocytes have reduced lysing activities depending on the granzyme and perforin depletion [25, 26]
Possible mechanisms that link PTEN deficiency with immunosuppressive tumour microenvironment
So far PTEN deficiency has been linked to promoting tumors indirectly through dysregulation of PI3K/AKT and DNA damage. However, mounting evidences suggest that PTEN loss can also directly contribute to immunosuppression of the tumor microenvironment. More specifically, PTEN’s deficiency can lead to immunosuppressive tumor microenvironment due to inability of PTEN-deficient cells to activate the interferon signaling pathway.
Interferons (IFNs), type I, II and III, are pleiotropic immunomodulatory class II cytokines that were discovered as the factors underlying viral interference [44,45,46,47]. During the past decades, the precise role of IFNs in the natural immune response to cancer has begun to be understood [48,49,50]. Immunomodulatory effects of type I IFNs can modify the local immune suppressive tumor microenvironment acting on both innate and adaptive immune components [51, 52]. IFN signaling has been show to promote immunity in multiple ways as follows: (a) stimulating the maturation of dendritic cells (DCs) from monocytes in the presence of IFN-α, enhancing their capacity to process and present dead cell associated antigens, and promoting their migration towards lymph nodes [53], (b) generation of cytotoxic T lymphocytes (CTLs), boosting their immune effector functions by increasing the expression of perforin 1 and granzyme B, and promoting the survival of memory CTLs [54,55,56], (c) activation of NK cells, and also preventing the elimination of antigen-activated CD8+ CTLs by NK cells [57,58,59], (d) inactivation of the suppressive function of Tregs through a pathway that involves the activation of phosphodiesterase-4 and the consequent depletion of cyclic-AMP (cAMP) [60], and (e) stimulating the release of pro-inflammatory cytokines (such as IL-1β and IL-18) by macrophages [61].
Cytosolic DNA sensing pathway (cGAS-STING) is one of the strong inducer of type I IFNs and other inflammatory cytokines in immune and non-immune cells [62, 63]. This strong inflammatory signaling recruits cytotoxic leucocytes and prime T-cell responses, leading to whole tumor regression [64]. PTEN controls the import of interferon regulatory factor 3 (IRF3), a master transcription factor responsible for IFN production, into the nucleus [65, 66]. Thus, deficiency in PTEN can account for the inactivation of several cellular defense pathways simultaneously, which renders cells unable to use interferon production to defend themselves [67].
IFNs can be activated through intra- and extra tumor mechanisms to induce immune cells to effectively eliminate tumors and overcome the immunosuppressive tumor microenvironment.
i. Intra-tumor mechanisms
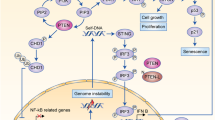
In the tumor cells, cytosolic DNA sensing pathway is induced by various forms of genotoxic stress; DNA damaging drugs, ionizing radiation, oxidative stress, replicative stress, oncogenic signaling, and chromosomal missegregation [68]. Nuclear DNA damage generates cytoplasmic DNA by missegregated chromosomes in subsequent cell divisions which will form micronuclei [64]. Cytoplasmic DNA binds to cGAS in a sequence independent manner and trigger the production of cGAMP which acts as a second messenger to activate stimulator of interferon gene (STING) on the endoplasmic reticulum surface [69]. STING then activates transcription factors IRF3 and NF-KB through the protein phosphatase activity of PTEN to elicit the IFNs and cytokines (Fig. 3) [63]. Mitochondria has extensive overlapping transcriptional units and stress associated perturbation of transcript processing can lead to the accumulation of dsRNAs leading to MDA5/RIG1 mediated activation of IFN signaling [70].
The potential mechanism of PTEN in type I interferon mediated immunogenicity. Cytosolic DNA sensing can be activated by intra or extra tumor mechanisms. In the tumor cells, cytosolic genomic or mitochondrial DNA binds to cGAS to trigger the production of cGAMP. cGAMP activates STING and then transcription factors IRF3 and NF-KB. PTEN dephosphorylates IRF3 and activates its import to the nucleus and starts the transcription of type I IFN and interferon stimulated genes (ISGs). In the macrophages and DCs, phagocytosed tumors genomic or mitochondrial DNA also activates cGAS/STING pathway
ii. Extra-tumor mechanisms
Necrotic or apoptotic tumor cells can release free or vesicle-protected DNA which likely be phagocytosed by macrophages and DCs. Tumor-derived nucleic acids are taken up by host antigen presenting cells (APCs), translocate into cytosol, trigger the cGAS/STING pathway and contribute to the antitumor immune responses [71, 72]. Phagocytosed tumor derived mtDNA was also recognized by cGAS in the DC cytosol, contributing to type I IFN production and antitumor adaptive immunity [73]. Intratumoral injection of cGAMP transiently induced migration of macrophages into tumor site in a STING-dependent manner and these cells exhibit phagocytosis and tumor necrosis factor α (TNFα) production [74].
Exploiting immunotherapies in PTEN deficient cancers
PTEN loss cause immunosuppressive microenvironment through; disruption of lymphocyte infiltration dynamics, upregulation of inhibitory cytokines, decreasing the lysing activities of cytotoxic T lymphocytes depending on the granzyme and perforin depletion. Cancer types such as GBM and prostate, in which PTEN-deficiency is common, have low to moderate level of mutations so they would not have many neoantigens which correlates with resistance to ICIs. Thus, determining and considering of PTEN status and selection of patients to recovery of the immunogenicity before the immunotherapy may increase the success of immunotherapy.
PTEN deficient tumors do not necessarily have a better response to immune checkpoint inhibitors
The effects of the PTEN loss on the PD-L1 expression have been studied in several cancers. Some clinical data indicates that loss of PTEN is associated with elevated PD-L1 levels. However, some studies do not support the role of PTEN in regulation of PD-L1.
PTEN loss did not show correlation with PD-L1 expression in prostate and breast cancers, high grade neuroendocrine carcinoma of the lung, pulmonary squamous cell, adenocarcinoma, pulmonary sarcomatoid and endometrial carcinoma [89,90,91,92,93,94]. Although PD-L1 expression was significantly correlated with tumor grade with all PD-L1+ cases, mutations of PTEN did not correlated with increased intratumoral expression of either PD1+TIL or PD-L1 in GBM [75]. Expression of PD-L1 was investigated in a panel of 51 melanoma cell lines and similarly no association was found between the level of PD-L1 expression and mutations in PTEN [76] which was confirmed by Peng et al. [25]. TCGA data showed that basal-like tumors, the majority of which were triple-negative breast cancers (TNBCs) showed PTEN mutation or loss in 35% of tumors, which also correlated with PI3K pathway activation [77]. However, homozygote deletion of PTEN or activating mutation in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) was not associated with increased expression of PD-L1 in TNBCs [78]. In the diffuse large B-cell lymphoma, loss of cytoplasmic PTEN was associated with TP53 mutations higher PTEN-targeting microRNA expression and lower mean level of PD-L1 expression whereas PTEN deletion/mutation and expression of p-AKT, PI3K, or nucleoplasmic-PTEN had no association with PD-L1 expression [11].
Low PTEN mRNA expression was associated with down-regulation of a group of genes involved in immune responses and B-cell development/differentiation, and poorer survival in DLBCL independent of AKT activation [11]. PD-L1 expression levels and PTEN were significantly associated with glandular component of adenosquamous cell carcinoma, whereas there were no associations for the adenocarcinoma and squamous components of lung squamous cell carcinoma [79]. Biallelic inactivation of serine/threonine kinase 11 (Lkb1) and PTEN in the mouse lung activated the Akt and mTor pathways and lead to squamous phenotype with elevated PD-L1 expression [80]. PTEN loss with increased PD-L1 was reported by Parsa and colleagues in GBM cell lines and they also suggested the involvement of the PI3K pathway [22]. It was confirmed in the breast and prostate cancer cell lines that PTEN loss significantly associated to increased PD-L1 expression levels [81]. Likewise, PTEN loss led to upregulation of the PD-L1 expression in TNBC and colorectal cancers [82, 83].
The disagreement in the results of these studies may be due to the differences in signaling context of cancers or in association with other genes highlighting that multiple mechanisms may be involved in PD-L1 regulation in tumors. Further clinical studies applying precision genomics and well annotated clinical samples are needed to define the role of PTEN on the PD-L1 expression.
Activating the IFN pathway for treatment of PTEN deficient tumors
Since macrophage polarization is major mechanism of escape from immune control of cancer growth, targeting of tumor-associated macrophages (TAMs) as a promising therapeutic strategy for cancer [84, 85]. Therapies such as anti-CSF1R and anti-CD47 that deplete to M2 myeloid cells are undergoing clinical trials. After CSF1R inhibition, TAMs lose M2 polarization and show enhanced phagocytosis, providing a molecular corollary for their impaired tumor-promoting functions [86]. PLX3397, an inhibitor of CSF1R, blocked glioma progression, markedly suppressed tumor cell proliferation and reduced tumor grade in proneural glioma mouse model [87]. After anti-CD47 blockade, tumor-associated microglia was able to effectively phagocytize tumor cells [88]. However, interfering with these receptors can have severe side effects such as toxicity or autoimmunity as they are also present in non-tumor compartment as well.
An alternative approach that may have benefit is exploiting the IFN signaling pathway [89]. RT increased intratumoral production of IFNβ and enhanced the cross-priming capacity of tumor infiltrating DC from wild type mice but not type I IFN receptor deficient mice [90]. Delivery of exogenous IFNβ into the tumor tissue in the absence of RT is also sufficient to selectively expand antigen-specific T cells leading to complete tumor regression [90]. IFN-β/Temozolomide (TMZ) combination therapy provided suppression of further tumor growth and prolonged survival were achieved in the majority of the malignant gliomas refractory to TMZ [91].
STING was required for type I IFN-dependent antitumor effects of radiation and radiation-induced adaptive immune responses [71]. Combination treatment with the cancer vaccine STINGVAX, a STING agonists, and immune checkpoint inhibitors produces synergistic antitumor effects, which indicates that the cGAS–STING pathway is important for the sensing of tumors by the innate immune system and has a critical role in intrinsic antitumor immunity [92, 93]. STING significantly contributed to antiglioma immunity via enhancement of type I IFN signaling in the tumor microenvironment and suggested a potential use of STING agonists for the development of effective immunotherapy [94]. However, we do not know yet how PTEN mutations affect cGAS/STING activity and IFN release. Therefore, further studies are needed to better understand PTEN’s role in modulating interferon pathway and cytokine signaling to the tumor microenvironment to develop effective immunotherapy targets.
After the new function for the PTEN in regulating IFN responses to viral infection was reported, it was speculated that disruption of PTEN function might define the opportunity for viruses to kill cancer [67]. Oncoviral immunotherapies are rising as a novel therapeutic class which has a markedly lower rate of serious adverse effects and greater specificity to target tumor cells [95]. PTEN expression by an oncolytic herpesvirus lysed the bulk tumor mass while creating an ATP-rich immune stimulating microenvironment during infection and decreased PD-L1 expression on the surface of tumor cells after treatment, in a murine model of breast cancer with brain metastases and intracranial human GBM tumors in nude mice [96]. Reconstitution of PTEN expression during oncolysis can enhance the antitumor immunity and overcome tumor immune escape. However, more work is needed on safety and efficacy evaluation of arming oncolytic herpesviruses with PTEN.
Conclusion
Several functions ensure PTEN the master regulator of physiological processes such as cell metabolism, motility, polarity, genome integrity, proliferation and viability. This review highlights the effects of PTEN deficiency on immunosuppressive TME and exploiting immunotherapies in PTEN deficient tumors (Table 2). PTEN loss can directly determine the differential infiltration and composition of immune cells in the TME and response to immunotherapy. In this case how could immunotherapy apply to PTEN deficient tumors? Considering of PTEN status and selection of patients to recovery of the immunogenicity before the immunotherapy may increase the success of immunotherapy. PTEN’s role in the interferon signaling suggests that tumors from tissues such as brain, breast, ovarian and prostate which poorly respond to existing checkpoint inhibitors, may benefit from activating interferon signaling particularly in PTEN deficient tumors where this pathway is expected to have been suppressed.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available in the Genomic Data Commons Data Portal repository, https://portal.gdc.cancer.gov/.
Abbreviations
- AC:
-
adenocarcinoma
- AKT:
-
AKT serine/threonine kinase
- APCs:
-
antigen presenting cells
- BCR:
-
B cell receptor
- cAMP:
-
cyclic-AMP
- CCL2:
-
C-C motif chemokine ligand-2
- CTLs:
-
cytotoxic T lymphocytes
- CTLA-4:
-
cytotoxic T-lymphocyte associated protein 4
- DCs:
-
dendritic cells
- DLBCL:
-
diffuse large B-cell lymphoma
- FGFR2:
-
fibroblast growth factor receptor 2
- FoxP3:
-
Forkhead box P3
- GBM:
-
glioblastoma
- HR:
-
homologous recombination
- ICIs:
-
immune checkpoint inhibitors
- IDO1:
-
indoleamine 2,3-dioxygenase 1
- IFN:
-
interferon
- IL:
-
interleukin
- IRF3:
-
interferon regulatory factor 3
- ISGs:
-
interferon stimulated genes
- MDSCs:
-
myeloid-derived suppressor cells
- NF-κB:
-
nuclear factor kappa-B
- NK:
-
natural killer
- PDAC:
-
pancreatic ductal adenocarcinoma
- PIK3CA:
-
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
- PI3K:
-
phosphoinositide-3-kinase
- PIK3:
-
phosphatidyl inositol 3-kinase
- PTEN :
-
phosphatase and tensin homolog
- PIP3:
-
phosphatidylinositol 3,4,5‑trisphosphate
- RT:
-
radiotherapy
- SASP:
-
senescence associated secretory phenotype
- SCC:
-
squamous cell carcinoma
- SHP2:
-
Src homology-2 domain-containing phosphatase-2
- STING:
-
stimulator of interferon gene
- NSCLC:
-
non-small cell lung cancer
- TAMs:
-
tumor-associated macrophages
- TME:
-
tumor microenvironment
- TMZ:
-
temozolomide
- TNBCs:
-
triple-negative breast cancers
- TNFα:
-
tumor necrosis factor-α
- Tregs:
-
regulatory T cells
- VEGF:
-
vascular endothelial growth factor-A
References
Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–8.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74.
Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y, et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10(7):401–4.
Serra H, Chivite I, Angulo-Urarte A, Soler A, Sutherland JD, Arruabarrena-Aristorena A, et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat Commun. 2015;6:7935.
Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7(2):193–204.
Brandmaier A, Hou SQ, Shen WH. Cell cycle control by PTEN. J Mol Biol. 2017;429(15):2265–77.
Mansour WY, Tennstedt P, Volquardsen J, Oing C, Kluth M, Hube-Magg C, et al. Loss of PTEN-assisted G2/M checkpoint impedes homologous recombination repair and enhances radio-curability and PARP inhibitor treatment response in prostate cancer. Sci Rep. 2018;8(1):3947.
Chen CL, Chiang TH, Tseng PC, Wang YC, Lin CF. Loss of PTEN causes SHP2 activation, making lung cancer cells unresponsive to IFN-gamma. Biochem Biophys Res Commun. 2015;466(3):578–84.
Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, et al. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67(9):4467–73.
Raffone A, Travaglino A, Saccone G, Campanino MR, Mollo A, De Placido G, et al. Loss of PTEN expression as diagnostic marker of endometrial precancer: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2019;98(3):275–86.
Wang X, Cao X, Sun R, Tang C, Tzankov A, Zhang J, et al. Clinical significance of PTEN deletion, mutation, and loss of PTEN expression in de novo diffuse large B-cell lymphoma. Neoplasia. 2018;20(6):574–93.
Millis SZ, Jardim DL, Albacker L, Ross JS, Miller VA, Ali SM, et al. Phosphatidylinositol 3-kinase pathway genomic alterations in 60,991 diverse solid tumors informs targeted therapy opportunities. Cancer. 2019;125(7):1185–99.
Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. 2008;7(9):1321–5.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
Ma J, Benitez JA, Li J, Miki S, de Ponte de Albuquerque C, Galatro T, et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;35(3):504.e7–518.e7.
Cancer Genome Atlas Research N. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–25.
Yun JW, Lee S, Ryu D, Park S, Park WY, Joung JG, et al. Biomarkers associated with tumor heterogeneity in prostate cancer. Transl Oncol. 2019;12(1):43–8.
Kerr KM, Dafni U, Schulze K, Thunnissen E, Bubendorf L, Hager H, et al. Prevalence and clinical association of gene mutations through multiplex mutation testing in patients with NSCLC: results from the ETOP Lungscape Project. Ann Oncol. 2018;29(1):200–8.
Cancer Genome Atlas N. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–96.
Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–60.
Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20(21):5527–36.
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8.
Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9(1):75–89.
Dong Y, Richards JA, Gupta R, Aung PP, Emley A, Kluger Y, et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene. 2014;33(38):4632–42.
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–16.
George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic Uterine leiomyosarcoma. Immunity. 2017;46(2):197–204.
Garcia AJ, Ruscetti M, Arenzana TL, Tran LM, Bianci-Frias D, Sybert E, et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol Cell Biol. 2014;34(11):2017–28.
Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–9.
Vidotto T, Saggioro FP, Jamaspishvili T, Chesca DL, Picanco de Albuquerque CG, Reis RB, et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate. 2019;79(9):969–79.
Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547–62.
Brandmaier A, Hou SQ, Demaria S, Formenti SC, Shen WH. PTEN at the interface of immune tolerance and tumor suppression. Front Biol. 2017;12(3):163–74.
Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten ± mice. Science. 1999;285(5436):2122–5.
Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847.
Miller CH, Maher SG, Young HA. Clinical use of interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79.
Windbichler GH, Hausmaninger H, Stummvoll W, Graf AH, Kainz C, Lahodny J, et al. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82(6):1138–44.
Giannopoulos A, Constantinides C, Fokaeas E, Stravodimos C, Giannopoulou M, Kyroudi A, et al. The immunomodulating effect of interferon-gamma intravesical instillations in preventing bladder cancer recurrence. Clin Cancer Res. 2003;9(15):5550–8.
Khammari A, Nguyen JM, Saint-Jean M, Knol AC, Pandolfino MC, Quereux G, et al. Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-gamma) in metastatic melanoma patients. Cancer Immunol Immunother. 2015;64(7):805–15.
Rizvi NA, Chan TA. Immunotherapy and oncogenic pathways: the PTEN connection. Cancer Discov. 2016;6(2):128–9.
Bezzi M, Seitzer N, Ishikawa T, Reschke M, Chen M, Wang G, et al. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat Med. 2018;24(2):165–75.
Wang Y, Wong CW, Yan M, Li L, Liu T, Or PM, et al. Differential regulation of the pro-inflammatory biomarker, YKL-40/CHI3L1, by PTEN/Phosphoinositide 3-kinase and JAK2/STAT3 pathways in glioblastoma. Cancer Lett. 2018;429:54–65.
Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9(379):eaah3560.
Qi X, Xu J, Gu P, Yang X, Gao X. PTEN in smooth muscle cells is essential for colonic immune homeostasis. Int J Biochem Cell Biol. 2014;53:108–14.
Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1(2):158–69.
Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–67.
Barral PM, Sarkar D, Su ZZ, Barber GN, DeSalle R, Racaniello VR, et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol Ther. 2009;124(2):219–34.
Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86.
Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32.
Medrano RFV, Hunger A, Mendonca SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017;8(41):71249–84.
Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–14.
Cheon H, Borden EC, Stark GR. Interferons and their stimulated genes in the tumor microenvironment. Semin Oncol. 2014;41(2):156–73.
Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14(4):432–6.
Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16(3):131–44.
Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol. 2008;180(3):1462–70.
Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189(3):521–30.
Guillot B, Portales P, Thanh AD, Merlet S, Dereure O, Clot J, et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br J Dermatol. 2005;152(4):690–6.
Ilander M, Kreutzman A, Rohon P, Melo T, Faber E, Porkka K, et al. Enlarged memory T-cell pool and enhanced Th1-type responses in chronic myeloid leukemia patients who have successfully discontinued IFN-alpha monotherapy. PLoS ONE. 2014;9(1):e87794.
Muller L, Aigner P, Stoiber D. Type I interferons and natural killer cell regulation in cancer. Front Immunol. 2017;8:304.
Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, et al. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40(6):961–73.
Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40(6):949–60.
Bacher N, Raker V, Hofmann C, Graulich E, Schwenk M, Baumgrass R, et al. Interferon-alpha suppresses cAMP to disarm human regulatory T cells. Cancer Res. 2013;73(18):5647–56.
Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187(5):2540–7.
Lemos H, Huang L, McGaha TL, Mellor AL. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur J Immunol. 2014;44(10):2847–53.
Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med. 2018;215(5):1287–99.
Mitchison TJ, Pineda J, Shi J, Florian S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017;7(11):17018.
Li S, Zhu M, Pan R, Fang T, Cao YY, Chen S, et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat Immunol. 2016;17(3):241–9.
Chen L, Guo D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol Immunol. 2017;14(7):581–9.
Champion BR, Fisher K, Seymour L. A PTENtial cause for the selectivity of oncolytic viruses? Nat Immunol. 2016;17(3):225–6.
Vanpouille-Box C, Demaria S, Formenti SC, Galluzzi L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell. 2018;34(3):361–78.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91.
Linder A, Hornung V. Mitochondrial dsRNA: a new DAMP for MDA5. Dev Cell. 2018;46(5):530–2.
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–52.
Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154(6):1822.e2–1835.e2.
Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H, et al. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47(2):363.e5–373.e5.
Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K, et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother. 2017;66(6):705–16.
Garber ST, Hashimoto Y, Weathers SP, Xiu J, Gatalica Z, Verhaak RG, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. 2016;18(10):1357–66.
Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20(13):3446–57.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Barrett MT, Lenkiewicz E, Malasi S, Basu A, Yearley JH, Annamalai L, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20(1):71.
Hlaing AM, Furusato B, Udo E, Kitamura Y, Souda M, Masutani M, et al. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem Biophys Res Commun. 2018;503(4):2764–9.
Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25(5):590–604.
Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28(2):306–12.
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70.
Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE. 2013;8(6):e65821.
Dehne N, Mora J, Namgaladze D, Weigert A, Brune B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol. 2017;35:12–9.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72.
Yan D, Kowal J, Akkari L, Schuhmacher AJ, Huse JT, West BL, et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene. 2017;36(43):6049–58.
Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, et al. Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci USA. 2019;116(3):997–1006.
Kane A, Yang I. Interferon-gamma in brain tumor immunotherapy. Neurosurg Clin N Am. 2010;21(1):77–86.
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–96.
Kawaji H, Tokuyama T, Yamasaki T, Amano S, Sakai N, Namba H. Interferon-beta and temozolomide combination therapy for temozolomide monotherapy-refractory malignant gliomas. Mol Clin Oncol. 2015;3(4):909–13.
Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283):283ra52.
Corrales L, Gajewski TF. Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. Cytokine. 2016;77:245–7.
Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–208.
Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6(1):140.
Russell L, Swanner J, Jaime-Ramirez AC, Wang Y, Sprague A, Banasavadi-Siddegowda Y, et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat Commun. 2018;9(1):5006.
Acknowledgements
Not applicable.
Funding
Nizar Batada is funded by the University of Edinburgh’s Chancellor’s Fellowship and the Wellcome Trust Seed Award (206077/Z/17/Z). Vildan Bozok Centintas is supported by TUBITAK—Science Fellowships and Grant Programs Department.
Author information
Authors and Affiliations
Contributions
VBC performed the literature review, wrote the manuscript and generated the figures. NB performed bioinformatics analysis, wrote the manuscript and generated the figures. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cetintas, V.B., Batada, N.N. Is there a causal link between PTEN deficient tumors and immunosuppressive tumor microenvironment?. J Transl Med 18, 45 (2020). https://doi.org/10.1186/s12967-020-02219-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-020-02219-w