Abstract
Background
Hyperkalemia is a common and potentially life-threatening electrolyte disorder in maintenance hemodialysis (MHD) patients. This study aimed to evaluate the efficacy and safety of potassium-lowering regimens during treatment of acute hyperkalemia in MHD patients.
Methods
This retrospective real-world study (RWS) was conducted among 139 MHD patients. They were given different potassium-lowering regimens, viz. the insulin and glucose (IG) intravenous administration group (IG, 46 patients), the sodium polystyrene sulfonate group (SPS, 33 patients), the sodium zirconium cyclosilicate group (SZC, 38 patients), the IG + SZC group (22 patients). The primary efficacy end point was the rate of serum potassium decline at 2 h. The rates of adverse events were also compared.
Results
At 2 h, the mean ± SE change of serum potassium level was − 0.71 ± 0.32 mmol per liter (mmol/L) in IG group, − 0.43 ± 0.38 mmol/L in SPS group, − 0.64 ± 0.36 mmol/L in SZC group, − 1.43 ± 0.38 mmol/L in IG + SZC group (P < 0.01). The serum potassium level in IG + SZC group decreased more than that in the other three groups (P < 0.01), while the serum potassium level in SPS group decreased less than that in the other three groups (P < 0.05). There was no significant difference on the decrease of the serum potassium level between IG group and the SZC group (P = 0.374). The IG group and the IG + SZC group had higher rates of symptomatic hypoglycemia. The SPS group had significant decreases of serum calcium and serum magnesium after treatment.
Conclusions
Among MHD patients with acute hyperkalemia, SZC had similar potassium-lowering efficacy with IG intravenous administration at 2 h and superior on convenience and side-effects.
Similar content being viewed by others
Introduction
Hyperkalemia is a common and potentially life-threatening electrolyte disorder in patients with chronic kidney disease (CKD), especially in those receiving maintenance hemodialysis (MHD) [1,2,3]. It is defined as serum potassium (K+) level > 5.5 mmol/L, regardless of whether patients receive an adequate treatment with hemodialysis (HD) [4, 5]. There occurs a decrease in the transmembrane K+ gradient in hyperkalemia which results in reduced ventricular conduction, cell membrane depolarization, and a decrease in the duration of myocardial action potential [6]. Additional risk factors for hyperkalemia in CKD patients were associated with heart failure, hypertension, diabetes, metabolic acidosis, non-adherence to dietary restrictions, advanced renal impairment and use of renin–angiotensin–aldosterone system inhibitors [1, 2, 7].High serum potassium is usually corrected in a 3–5 h hemodialysis session, consequently the MHD patients regularly experience wide alterations in serum potassium levels, which at the extremes can reach pathologically high or low concentrations. Pre-dialysis hyper-and hypokalemia increases the risk of cardiovascular disease and sudden cardiac death, which is a serious complication endangering the life of MHD patients [7, 8]. Therefore, it is necessary to take emergency measures to reduce serum potassium level to a safe range for the management of acute hyperkalemia.
There are a variety of potassium reduction programs in the acute phase of hyperkalemia, which however are currently lacking controlled trials to guide treatment decisions. The main agents used to correct the serum potassium levels and correcting hyperkalemia include the administration of intravenous insulin and glucose, cation resins such as sodium polystyrene sulfate (SPS), sodium zirconium cyclosilicate (SZC) and the combination of two or more treatment measures [5, 9,10,11]. The clinical studies on agents have reported variable results in reducing serum potassium levels in patients with acute hyperkalemia. However, there is no controlled study on the efficacy and safety of these regimens in the correcting of acute hyperkalemia. Therefore, the objective of this retrospective real-world study (RWS) was to evaluate the efficacy and safety of various regimens for the treatment of acute hyperkalemia in MHD patients (Fig. 1).
Materials and methods
Study population
This was a retrospective, observational, single center, real-world study conducted from June 10, 2020 to February 3, 2021, in which adult MHD patients with hyperkalemia (venous serum potassium > 5.5 mmol/L) were admitted in the Department of Nephrology of the First Affiliated Hospital of Zhengzhou University. We conducted our study using de-identified patient data; This study adhered to the Declaration of Helsinki and informed consent was not required and used anonymized patient data, The approval number of the ethics committee is 2021-KY-0664-001.
Inclusion and exclusion criteria
The following inclusion criteria were used to identify our patient population: men or women aged ≥ 18 years on MHD for ≥ 3 months and with serum potassium ≥ 5.5 mmol/L. Patients with erroneous collection method and those using other potassium-lowering drugs within 24 h before enrollment, and with repeated administration were excluded from the study. The case screening process is shown in Fig. 2.
Methods and measurements
Electronic medical records were used to obtain clinical data. The general information of the patients such as age, gender, urine volume, major complications, etc., were collected from the hospital registry. The HD conditions such as function of vascular access, frequency of dialysis session, interval from the last dialysis and duration of the last dialysis were also analyzed. All concomitant medications were recorded such as angiotensin converting enzyme inhibitor (ACEI), angiotensin II receptor antagonist (ARB) or mineralocorticoid receptor antagonist (MRA). The serum levels of electrolytes (potassium, sodium, magnesium, calcium), glucose, and electrocardiogram (ECG) before and 2 h after treatment were also examined.
Patients were given 10 IU insulin plus 60 g glucose by intravenous drip (IG group), 15 g sodium polystyrene sulfonate (SPS group) orally, 10 g sodium zirconium cyclosilicate (SZC group) orally or IG + SZC (IG + SZC group) according to the clinical condition and doctors’ judgment. The serum level of potassium, whether typical findings on ECG or signs of hypervolume or relatively lower serum glucose level existed, were the main concerns (Additional file 1: Fig. S1). Peripheral blood samples, which were drawn on the precise timing point of 2 h after administration of these drugs, were sent for serum electrolyte and glucose test. All enrolled patients were not allowed to take food or medications influencing serum potassium.
Study outcomes
Data extraction was completed by unblinded study investigators. The primary efficacy end point was assessed in terms of the exponential rate of change in the mean serum potassium level, standard rate of serum potassium (intravenous serum potassium < 5.5 mmol/L) and control rate of severe hyperkalemia after 2 h of treatment (from severe hyperkalemia to normal, moderate or mild hyperkalemia).
The main safety endpoints included assessment of adverse events (AEs); changes in laboratory parameters, including assessment of hypoglycemia or low blood sugar and the change rate of serum sodium, calcium and magnesium 2 h after treatment. Severe hyperkalemia refers to venous serum potassium ≥ 6.5 mmol/L with typical ECG changes; moderate hyperkalemia refers to venous serum potassium ≥ 6.0 mmol/L and < 6.5 mmol/l without typical ECG changes [12]. Whereas, mild hyperkalemia refers to venous serum potassium 5.0 < 5.5 mmol/L [13]. Typical ECG changes refer to a symmetrical high sharp T wave, prolonged PR interval, decreased P wave amplitude and widened QRS wave on ECG [14]. Hypocalcemia was defined as corrected serum calcium < 2.1 mmol/L. Hypermagnesemia refers to the serum magnesium level > 1.25 mmol/L. Poor vascular access function refers to the failure of effective HD treatment due to internal fistula thrombosis, severe fistula stenosis and poor dialysis catheter function. Symptomatic hypoglycemia refers to palpitation, pale face, sweating, hunger and other symptoms during the treatment.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 software. Normally distributed data were expressed as mean and standard deviation while non normally distributed data were expressed as median and interquartile range (IQR). before and after treatment, paired Wilcoxon rank sum test (non-normal distribution) or paired t test were used for comparison, and rank sum test (non-normal distribution) or one-way analysis of variance (one-way ANOVA) were used for comparison among multiple groups. Kruskal–Wallis rank sum test (non-normal distribution) or LSD test were used for pairwise comparison. The description of qualitative data was expressed in percentage, and the difference of qualitative data was compared by X2 test or Fisher exact probability method, P < 0.05 was considered as statistically significant.
Results
Characteristics of study participants
139 patients were treated by potassium lowering drugs, among which 90.7% were unable to receive HD treatment due to vascular access dysfunction. Among the 139 patients, 81 were males and 58 were females, with an average age of 53.63 ± 14.62 years, 129 patients had access dysfunction, 47 patients had diabetes mellitus and 65 patients had heart failure. There were no significant differences in age, gender, urine output, dialysis frequency, complications and drug combination among the groups (P > 0.05 for all the groups). There were significant differences in the time from the last HD between IG group and SPS group (P = 0.016) (Table 1).
Baseline serum potassium level
There was a significant difference in the baseline serum potassium level of each group before potassium lowering treatment (H = 27.32, P < 0.01). IG + SZC group had significantly higher serum potassium level than that of the other three groups (Fig. 3). Among the other three groups, the serum potassium level of SZC group was the highest, but there was no significant difference compared with the other two groups (H = 2.90, P = 0.235). In the IG + SZC group, 63.6% of patients had serum potassium ≥ 6.5 mmol/L and 85.7% of them had severe hyperkalemia, which was significantly higher than those in the other three groups (Table 1).
Primary efficacy outcome: the rate of serum potassium decline
After treatment, there were statistical differences in serum potassium levels among groups (H = 10.28, P = 0.016) (Fig. 4 and Table 2). However, the differences between any two groups were not statistically significant except a significantly lower serum potassium level in the IG group than that in the SPS group (P = 0.017). Although the serum potassium level of SZC group was lower than that of SPS group after treatment, there was no statistical significance (P > 0.05) (Table 2).
The serum potassium level of each group decreased significantly after the treatment (P < 0.01) (Fig. 5). Figure 6 shows the change range (t = 36.94, P < 0.01) and change rate of serum potassium (t = 28.25, P < 0.01) in each group after treatment that exhibited significant differences. The serum potassium in the IG + SZC group decreased by 1.43 ± 0.38 mmol/L (21.51 ± 5.74%), significantly higher than the remaining groups (P < 0.01). The decrease of serum potassium of SPS group was the lowest i.e., 0.43 ± 0.38 mmol/L (7.12 ± 6.32%), which was significantly lower than the other three groups (P < 0.05), and there was no statistical significance between IG group and SZC group in the changes of serum potassium level (P = 0.374). The SPS group had the largest CV (89.3%) compared with other groups (Table 2).
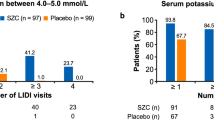
Secondary efficacy outcome: the standard-reaching rate of serum potassium level
After potassium lowering treatment, there was no significant difference in the rate of reaching the standard of serum potassium among groups (t2 = 4.95, P = 0.176) (Fig. 7). The control rate of severe hyperkalemia in SZC group was higher than that in SPS group, but the difference was not statistically significant perhaps due to the number of cases (P = 0.135). (Table 2).
Associated adverse events
There was no difference in the changes of serum sodium level during potassium lowering treatment among groups (Table 3). Serum calcium levels in IG group, SPS group and IG + SZC group decreased significantly after treatment (P < 0.01). Serum magnesium levels of SPS group and IG + SZC group were significantly decreased after treatment (P < 0.01). However, there was no significant difference in the changes of serum sodium, calcium and magnesium levels in SZC group (P > 0.05).
During potassium-lowering treatment, 6 cases (13.0%) of the hypoglycemia reactions occurred in the IG group, of which 4 cases (8.7%) were non-diabetic patients, 2 cases (4.3%) were diabetic patients. Also, there were 2 cases (9.0%) with symptomatic hypoglycemia in the IG + SZC group. There were no evident adverse reactions in observed in the SZC and SPS groups.
Discussion
Hyperkalemia is a common complication in patients undergoing MHD treatment, which may result in malignant arrhythmias and sudden death [2, 15]. Moreover, acute hyperkalemia increases the risk of cardiovascular death, emergency hospitalization and the overall medical burden of MHD patients [15, 16]. The incidence of hyperkalemia is higher in patients with long interval dialysis, inadequate dialysis or interrupted dialysis is referred when the hemodialysis treatment is temporarily paused to allow the patient to attend the bathroom. Vascular access dysfunction is one of the most common reasons for discontinuing dialysis in MHD patients. Therefore, the retrospective RWS mainly included MHD patients who had to discontinue dialysis due to vascular access dysfunction.
Increasing the excretion of potassium is the fundamental measure of emergency potassium-lowing strategy, including diuretics, emergency dialysis and intestinal potassium excretion drugs. Generally, diuretics was inefficacious in excreting potassium in most MHD patients due to poor renal function and insufficient potassium excretion capacity [17]. Some MHD patients with hyperkalemia could not obtain dialysis timely due to dysfunctional access or other reasons. Therefore, the use of intestinal potassium excreting drugs or glucose plus insulin to promote the transfer of potassium ions into cells have gained much attention for the treatment of hyperkalemia in most MHD patients. When serum potassium drops to the safe range, receiving HD after repairing procedures become the common treatment measure for most MHD patients with access dysfunction. During the treatment of hyperkalemia, the commonly used drugs include IG, SPS, SZC or their combinations. However, to the best of our knowledge, there is no reported comparative study on the efficacy and safety of these regimens in MHD patients with acute hyperkalemia.
In this study, 139 MHD patients with acute hyperkalemia were enrolled, most of them with poor vascular access function. The four different kinds of potassium reduction regimens were applied i.e., IG, SPS, SZC or IG + SZC. The baseline serum potassium level and the proportion of patients with serum potassium > 6.5 mmol/L in the IG + SZC group was significantly higher than those in the other three groups with the great apprehension that severe hyperkalemia may not be corrected by a single potassium lowering regimen, and for the sake of safety, two drugs were combined to strengthen the reduction of serum potassium.
The mean decrease of serum potassium in MHD patients after 2 h of application of SZC was 0.64 ± 0.36 mmol/L, which is consistent with the previously reported studies [18], indicating that SZC can rapidly reduce serum potassium levels in MHD patients during the correction period of acute hyperkalemia. SZC is an inorganic cation exchanger whose unique structure enables it to effectively simulate physiological potassium channels in vivo, highly select serum potassium and accurately capture serum potassium. Its selectivity to serum potassium ions is about twenty-five times more to that of serum calcium and magnesium ions [18, 19]. In an environment that simulates the human gastrointestinal tract, SZC works in combination with serum potassium in the stomach and continues to function throughout the gastrointestinal tract, allowing a drop in serum potassium levels to be observed within 1 h of taking the drug [19]. After 2 h of treatment, there were no significant differences between SZC group and IG group in terms of the rate of serum potassium decline, the standard-reaching rate of serum potassium and the control rate of severe hyperkalemia. However, the IG group requires intravenous infusion, and there is a risk of hypoglycemic reaction. The IG + SZC group had the largest decrease in serum potassium of all the groups. Although the basal potassium level was the highest, the IG + SZC group had a relatively high decrease in serum potassium, the standard-reaching rate of serum potassium, and the control rate of severe hyperkalemia, indicating that SZC combined with IG application can exert a greater potassium-lowering effect. The standard-reaching rate of serum potassium in SPS group was similar to that in SZC group, but the decrease of serum potassium and the control rate of severe hyperkalemia in SPS group were significantly lower than those in SZC group. In addition, the CV of serum potassium decline in SPS group was the largest, suggesting that the efficacy of SPS in the correction period of acute hyperkalemia in MHD patients has great individual differences.
After taking SZC, there were no significant changes in serum sodium, calcium and magnesium, and no serious adverse events in MHD patients in this study, which was consistent with the results of previous clinical trials [11, 20]. However, there were 6 (13.0%) and 2 (9.0%) patients in IG group and IG + SZC groups, respectively with symptomatic hypoglycemia. In SPS group, serum calcium and magnesium decreased significantly, which was related to the non-selective cation exchange of polymer exchange resins.
There are some limitations in this study. Firstly, this study is a retrospective RWS with few selected cases. Secondly, considering the serious consequences of acute hyperkalemia and the safety of patients, all the subjects were not randomly grouped, but treatment options were chosen according to the clinical judgement. In addition, this study was of relatively short duration for it only compared the effect of 2 h after administration in MHD patients. Therefore, additional studies need to be included for rigorous, multi-center prospective intervention study in patients receiving MHD, although previous studies in the non-dialysis population have demonstrated that the efficacy and safety of SZC.
Conclusion
To conclude, in our opinion this retrospective RWS suggested that in the correction of acute hyperkalemia among MHD patients, SZC had the similar efficacy as IG intravenous potassium-lowering, and had more advantages on convenience and safety. Combined SZC and IG increased the efficacy of potassium-lowering and the standard-reaching rate of serum potassium.
Availability of data and materials
All data will be made available upon request.
Abbreviations
- MHD:
-
Maintenance hemodialysis
- IG:
-
Insulin and glucose
- SPS:
-
Sodium polystyrene sulfonate
- SZC:
-
Sodium zirconium cyclosilicate
- CKD:
-
Chronic kidney disease
- HD:
-
Hemodialysis
- RWS:
-
Real-world study
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin II receptor antagonist
- MRA:
-
Mineralocorticoid receptor antagonist
References
Palmer BF. Potassium binders for hyperkalemia in chronic kidney disease-diet, renin-angiotensin-aldosterone system inhibitor therapy, and hemodialysis. Mayo Clin Proc. 2020;95(2):339–54.
Kovesdy CP, et al. Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019;4(2):301–9.
Peacock WF, et al. Real world evidence for treatment of hyperkalemia in the Emergency Department (REVEAL-ED): a multicenter, prospective, observational study. J Emerg Med. 2018;55(6):741–50.
Lindner G, et al. Acute hyperkalemia in the emergency department: a summary from a kidney disease: improving global outcomes conference. Eur J Emerg Med. 2020;27(5):329–37.
Takkar C, Nassar T, Qunibi W. An evaluation of sodium zirconium cyclosilicate as a treatment option for hyperkalemia. Expert Opin Pharmacother. 2021;22(1):19–28.
Long B, Warix JR, Koyfman A. Controversies in management of hyperkalemia. J Emerg Med. 2018;55(2):192–205.
Yusuf AA, et al. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44(3):179–86.
Hoppe LK, et al. Association of abnormal serum potassium levels with arrhythmias and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Cardiovasc Drugs Ther. 2018;32(2):197–212.
Pirklbauer M. Hemodialysis treatment in patients with severe electrolyte disorders: management of hyperkalemia and hyponatremia. Hemodial Int. 2020;24(3):282–9.
Beccari MV, Meaney CJ. Clinical utility of patiromer, sodium zirconium cyclosilicate, and sodium polystyrene sulfonate for the treatment of hyperkalemia: an evidence-based review. Core Evid. 2017;12:11–24.
Kosiborod M, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–33.
Hammer F, et al. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95(4):983–91.
Collins AJ, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21.
Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546–54.
Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28(11):3155–65.
Gilligan S, Raphael KL. Hyperkalemia and hypokalemia in CKD: prevalence, risk factors, and clinical outcomes. Adv Chronic Kidney Dis. 2017;24(5):315–8.
Bianchi S, et al. Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol. 2019;32(4):499–516.
Packham DK, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31.
Stavros F, et al. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS ONE. 2014;9(12): e114686.
Spinowitz BS, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809.
Acknowledgements
The authors thank all the patients and researchers who participated in this study. The authors are also thankful to the medical team of Department of Nephrology and Blood Purification Center of the First Affiliated Hospital of Zhengzhou University for their assistance and support.
Funding
National Natural Science Foundation of China (81873612).
Author information
Authors and Affiliations
Contributions
WP designed the study; YL, XXY, LYB, ZFX and LP collected data; YL and XXY conducted statistical analysis and drafted the initial manuscript, LXH and WP reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The approval number of the ethics committee of the First Affiliated Hospital of Zhengzhou University is 2021-KY-0664-001.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Scheme of selection of potassium-lowering regimens for enrolled patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, L., Xing, X., Li, Y. et al. Effects of different potassium-lowering regimens on acute hyperkalemia in hemodialysis patients: a real-world, retrospective study. J Transl Med 20, 333 (2022). https://doi.org/10.1186/s12967-022-03530-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03530-4