Abstract
Background
Cytokine release syndrome (CRS) is a strong immune system response that can occur as a result of the reaction of a cellular immunotherapy with malignant cells. While the frequency and management of CRS in CAR T-cell therapy has been well documented, there is emerging interest in pre-emptive treatment to reduce CRS severity and improve overall outcomes. Accordingly, identification of genomic determinants that contribute to cytokine release may lead to the development of targeted therapies to prevent or abrogate the severity of CRS.
Methods
Forty three clinical CD22 CAR T-cell products were collected for RNA extraction. 100 ng of mRNA was used for Nanostring assay analysis which is based on the nCounter platform. Several public datasets were used for validation purposes.
Results
We found the expression of the PFKFB4 gene and glycolytic pathway activity were upregulated in CD22 CAR T-cells given to patients who developed CRS compared to those who did not experience CRS. Moreover, these results were further validated in cohorts with COVID-19, influenza infections and autoimmune diseases, and in tumor tissues. The findings were similar, except that glycolytic pathway activity was not increased in patients with influenza infections and systemic lupus erythematosus (SLE).
Conclusion
Our data strongly suggests that PFKFB4 acts as a driving factor in mediating cytokine release in vivo by regulating glycolytic activity. Our results suggest that it would beneficial to develop drugs targeting PFKFB4 and the glycolytic pathway for the treatment of CRS.
Similar content being viewed by others
Introduction
Cytokine release is a systemic inflammatory response that occurs when immune cells are activated by foreign substances and release large amounts of cytokines into the body such as: interleukins, interferons, tumor necrosis factors and growth factors [1, 2]. All of these cytokines are small proteins which play a vital role in host defense when regulated normally. However, when the immune response is dysregulated, high levels of cytokines may be produced which causes increased inflammation throughout the body and which results a condition called cytokine release syndrome (CRS). CRS can present with a variety of symptoms ranging from mild to severe and life-threatening manifestations [3, 4]. Mild symptoms of CRS include cough, fever, headache, fatigue, rash, and myalgia [1]. While severe cases are characterized by hypotension and high fever which may progress to an uncontrolled systemic inflammatory response resulting in vascular leakage, circulatory shock, and multi-organ system failure [1]. Thus, effective treatment is required when patients develop severe CRS.
To date, CRS has been observed in infectious diseases (pandemics of influenza [5, 6], SARS-CoV and COVID-19 [7, 8]), certain acquired or inherited autoimmune diseases [9, 10], and following chimeric antigen receptor T-cell (CAR T-cell) therapy [11, 12]. Patients with severe CRS are treated with therapies designed to block specific cytokines, as well as more general immunosuppressive drugs. The anti-cytokine drugs, tocilizumab and siltuximab (anti-IL-6 receptor antibodies) have been widely used to minimize rates of life-threatening CRS in patients receiving CAR T-cell therapy and in patients with severe COVID-19 [13,14,15]. Corticosteroids such as methylprednisolone or dexamethasone, are also used to help mitigate inflammatory and immune responses by providing broad immunosuppression in individuals with autoimmune diseases and COVID-19 [16,17,18]. However, while the usage of tocilizumab may decrease CRS severity, it may increase the risk of neurotoxicity, another common toxicity during CAR T-cell therapy [19, 20]. In addition, not all patients with severe CRS respond well to tocilizumab or corticosteroid treatments [21, 22], thus pre-emptive strategies are being tested [23, 24]. An early clinical trial showed a 69% response rate to tocilizumab in patients with severe or life-threatening CRS [22]. As for corticosteroids, there is still conflicting data as to whether its use compromises CAR T-cell potency [25, 26]. Improved CRS treatment and prevention requires a greater understanding of molecular and cellular determinants contributing to cytokine release. The current understanding of factors that trigger and drive cytokine release remains incomplete.
In recent years, the Nanostring nCounter gene expression platform has emerged and developed quite quickly [27,28,29]. It is a high-fidelity, simple protocol that allows for the detection of 800 mRNA molecules of interest at one time using specific probes. This method has proven to be simpler and more effective compared to real-time qPCR, and time-saving and easier to analyze compared to RNA-seq [30, 31]. Here, in order to explore candidate targets for CRS treatment, we systematically explored the genomic factors found in CAR T-cell clinical products using the Nanostring nCounter platform. In this study, we analyzed 43 pre-infusion CD22 CAR T-cell products and their corresponding clinical CRS grade. After analyzing gene expression using the nSolver software, we compared the differentially expressed genes and pathways among CAR T-cell products associated with different CRS grades. We found that the PFKFB4 gene and its regulated glycolytic pathway activity were gradually upregulated among CAR T-cell products grouped from mild to severe CRS. Moreover, using public datasets we validated our results in several other human diseases where CRS can occur including: COVID-19, influenza, autoimmune diseases and human tumors. These analyses suggest that the PFKFB4 gene may act as a driving factor in triggering the cytokine release process. Drugs targeting PFKFB4 and glycolytic pathway or a combination strategy might be beneficial for the clinical management of cytokine release syndrome.
Materials and methods
CAR T-cell products
CD22 CAR T-cell products were obtained from excess products manufactured for patients enrolled on a phase I trial of CD22 CAR T-cells in B-cell malignancies. (Clinicaltrials.gov NCT02315612). The clinical trial was approved by the National Cancer Institute Institutional Review Board. Products that were analyzed were from patients who consented to additional genomic testing and were enrolled on a companion study for study of biologic correlatives (Clinicaltrials.gov NCT01109394). CRS was graded using ASTCT consensus grading [32].
mRNA extraction
Total RNA was isolated from 43 cryopreserved pre-infusion CD22 CAR T-cell products [33] using miRNeasy Mini Kit (Qiagen). Quantity and Quality were measured by Nanodrop 8000 (Thermo Fisher Scientific) and 2100 Bioanalyzer (Agilent).
Gene expression profiling by Nanostring
Hundred ng of total RNA was hybridized in solution with the nCounter CAR-T Characterization panel (human codeset) at 65 ℃ for 17 h. The hybridized samples were loaded into the nCounter CAR-T cartridge (NanoString Technologies), which was then sealed and placed in the instrument for processing and analysis. RCC files containing raw counts for 780 genes were generated and loaded into nSolver Analysis Software 4.0 for normalization by housekeeping genes and positive controls. The normalized data were imported to Partek Genomics Suite 7.0 to remove any batch effects. Downstream analysis was performed in a Rstudio environment with custom code.
Calculation of differentially expressed genes
The limma R package was used to generate p-values and fold change (FC) for each gene between samples with different CRS grade. Wald’s Test was used to calculate the p value or significance that a gene is differentially expressed. Genes with a p-value ≤ 0.01 and a │log2 (FC)│ ≥ log2(1.5) were identified as differentially expressed genes (Additional file 4: Table S1).
Gene set analysis
Gene set variant analysis (GSVA) was used to look at enrichment scores for custom pathways in order to check pathway activity based on GSVA R package. p-value under 0.05 represents statistical significance.
Public datasets
Publicly available gene-expression profiles were used to validate findings in our studies were downloaded from GEO and TCGA datasets. Among them, cells were collected from whole blood (GSE196822, GSE111368 and GSE72326), peripheral blood mononuclear cells (GSE152418, GSE179627 and GSE114588), and monocytes (GSE147608). PFKFB4 gene expression data and association with immune cells in pan-cancer downloaded from TIMER website.
Statistical analysis
All statistical analysis was performed with GraphPad Prism software and related R package. A p-value less than 0.05 was considered significant.
Results
PFKFB4 gene expression is gradually upregulated among CAR T-cells grouped according to recipient cytokine release syndrome grade
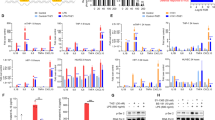
In order to identify gene expression signatures that drive the development of CRS, we collected 43 pre-infusion CD22 CAR T-cell products and analyzed gene expression using the Nanostring nCounter gene profiling system. First, we divided the CAR T-cell samples into four groups based on maximum clinical CRS grade in the associated recipient: Without CRS (n = 3), CRS grade 1 (n = 16), CRS grade 2 (n = 19), CRS grade 3 and 4 (n = 5). Then, we compared differentially expressed genes between 3 combinations of two groups (without CRS vs CRS grade 1, CRS grade 1 vs CRS grade 2, CRS grade 2 vs CRS grade 3 and 4) in order to find genes whose expression gradually changed as CRS great increased. As the volcano plot shows in Fig. 1A–C, we identified some differentially expressed genes based on different comparisons (Additional file 4: Table S1). Interestingly, we found that the PFKFB4 gene was gradually up-regulated as CRS became more severe (Fig. 1D).
PFKFB4 gene is gradually up-regulated with the development of cytokine release syndrome in CAR T-cell therapy. A–C Volcano plot of CRS grade-related differentially expressed genes between the different groups. Fold Change > 1.5 and p-value < 0.05 were set as screening criteria. Genes that have both a significant p-value (lower than 0.05) and a fold change (higher than 1.5) are represented as red dots. Genes that either have a significant p-value (lower than 0.05) or a fold change (higher than 1.5) are represented as blue and green dots. Gray dots mean genes neither have a significant p-value nor fold change. PFKFB4 gene expression in CD22 CAR T-cell (D) and CD19 CAR T-cell products (E) based on different CRS grade group
Based on this finding, we sought to explore other CAR T-cell trials. Based on data generated from a CD19 CAR T-cell trial, we found similar results [34]. Based on this study’s supplementary data, we extracted the PFKFB4 gene expression and CRS grade information. As shown in Fig. 1E, PFKFB4 gene expression was up-regulated in patients receiving CD19 CAR T-cells with grade 4 CRS (p = 0.062). Our results indicate that PFKFB4 gene may act as an essential factor in triggering the development of cytokine release syndrome.
PFKFB4 triggers cytokine release through the regulation of glycolytic activity
It has been reported that interleukin and JAK-STAT signaling pathways play a key role in the cytokine release process and blocking/inhibiting these pathways can significantly reduce the severity of CRS induced by CAR T-cells [35]. Consequently, we explored additional key signaling pathways in order to facilitate the development new targeted therapies. As described in Fig. 1, four groups were divided to compare each signaling pathway activity. We selected 18 related signaling pathways, including JAK-STAT and interleukin signaling. First, we recognized the trend that JAK-STAT and interleukin signaling pathway activity was gradually enhanced with higher statistical power during the development of more severe CRS (Fig. 2A and B, Additional file 1: Fig. S1). In addition, a similar and more obvious trend was observed in glycolytic activity (Fig. 2C). We found that the expression of most genes involved in glycolytic signaling pathway were positively correlated with PFKFB4 gene expression (Fig. 2D) including the important regulators of glycolytic process GPI, PGK1, and PKM. Given these results, we speculated that PFKFB4 is a driving factor that plays a vital role in the development of cytokine release possibly through regulation of glycolytic pathway activity.
Glycolytic pathway activity is enhanced in groups with higher CRS grades. A–C Relative signaling pathway activity score was calculated based on GSVA analysis. JAK-STAT, Interleukin, and glycolysis signaling pathways showed higher activity in the high CRS grade group. The x-axis represents different CRS grades. D Correlation between PFKFB4 gene expression and genes involved in glycolysis signaling pathway. Correlation coefficient and p-values are listed at the up-left corner. The x-axis represents relative PFKFB4 gene expression. The y-axis shows the expression of genes that are involved in the glycolysis pathway
PFKFB4 expression and glycolytic pathway activity is enhanced in other diseases associated with cytokine release
Currently, a considerable number of patients with COVID-19 have developed cytokine release syndrome and the resulting CRS severity positively correlated with the pathogenesis and severity of COVID-19. To validate our findings in COVID-19 cohort, we analyzed three public datasets from the GEO platform. In general, PFKFB4 gene expression was significantly up-regulated in COVID-19 patients compared to healthy donors (Fig. 3A). Moreover, its expression was higher in patients with severe syndrome compared to patients with moderate syndrome (Fig. 3B). In addition, we noted that PFKFB4 gene expression in asymptomatic people infected with SARS-CoV-2 and in COVID-19 convalescent people were stable and at the same level as in healthy subjects. While, PFKFB4 expression increased following re-infection by SARS-CoV-2 (Fig. 3C). Concerning glycolytic pathway activity, the results showed that pathway activity also increased during SARS-CoV-2 infection and COVID-19 development (Fig. 3D–F).
PFKFB4 expression and glycolytic pathway activity is also enhanced in some human diseases associated with cytokine release syndrome. PFKFB4 gene expression profile in COVID-19 patients vs healthy donor (A), COVID-19 patients with severe symptoms vs moderate symptoms (B), healthy donors vs asymptomatic vs recovered vs re-infected COVID-19 patients (C). Glycolytic activity score in COVID-19 patients vs healthy donors (D), COVID-19 patients with severe symptoms vs moderate symptoms (E), healthy donors vs asymptomatic vs recovered vs re-infection COVID-19 patients (F). PFKFB4 gene expression in people infected with influenza based on early and late stage infection (G–I). PFKFB4 gene expression in different stages of SLE (J) and SJIA (K). HD healthy donor, As asymptomatic, SLE systemic lupus erythematosus, SJIA systemic juvenile idiopathic arthritis
Since people with seasonal influenza infections and autoimmune diseases can also develop CRS, we explored PFKFB4 expression in these diseases. As shown in Fig. 3G–I, we found that PFKFB4 expression was up-regulated in influenza-infected (H1N1, H3N2, Influenza B) individuals in the early stages of infection, and decreased in the recovery stage. Among autoimmune diseases, we selected systemic lupus erythematosus (SLE) and systemic juvenile idiopathic arthritis (SJIA) for further validation. We found that patients with severe or active disease showed higher PFKFB4 gene expression (Fig. 3J and K). However, glycolytic pathway activity showed mixed results. Its activity was lower in early stage of influenza-infected patients and SLE patients with severe status (Additional file 2: Fig. S2A–D), while it was higher in SJIA patients with active disease status (Additional file 2: Fig. S2E).
These results provide further validation of our findings that PFKFB4 gene and glycolytic pathway play a key role in cytokine release.
Up-regulated PFKFB4 gene and glycolytic activity during tumor development
It has been widely accepted that various cytokines are released into the tissue microenvironment during the tumorigenesis and tumor development process. These cytokines may inhibit tumor development but alternatively may contribute to the chronic inflammation that supports tumor growth and has been linked to poor clinical outcomes [36]. In order to investigate whether the PFKFB4 gene is also involved in the cytokine-mediated inflammatory microenvironment and subsequent metastasis process in tumor tissues, we evaluated the correlation of PFKFB4 expression and tumor pathological stages in several types of cancer. First, we used the Tumor Immune Estimation Resource (TIMER2.0) database to explore the expression of PFKFB4 in several cancers. We found its expression was significantly up-regulated in 18 cancer types (i.e., breast invasive carcinoma, liver hepatocellular carcinoma, colon adenocarcinoma, kidney renal clear cell carcinoma, kidney renal papillary cell carcinoma) when compared with corresponding normal tissues (Fig. 4A). Furthermore, we analyzed the correlations between PFKFB4 expression and pathological stages in several tumor types. We selected liver hepatocellular carcinoma and renal cancer, which includes kidney chromophobe, kidney renal clear cell carcinoma, and kidney renal papillary cell carcinoma. We found PFKFB4 gene was significantly up-regulated in patients with higher pathological stage (Fig. 4B and C) and shorter survival time (Additional file 3: Fig. S3A and B). Furthermore, the activity of the glycolytic pathway was also enhanced in patients with late pathological stage (Fig. 4D and E). Moreover, we found PFKFB4 gene expression showed a strong correlation with genes involved in glycolytic pathway in all cancers studied (Additional file 3: Fig. S3C). These data indicate that the PFKFB4 gene regulates glycolytic activity and can promote tumor metastatic process possibly through the induction of a pro-inflammatory microenvironment in tumor tissue by mediating cytokine release.
The relationship between the level of PFKFB4 expression and clinicopathological stages in cancer. A Overview of PFKFB4 gene expression in all cancers. Red represents tumor tissue, blue represent adjacent normal tissue. B, C Upregulation of the PFKFB4 gene in higher pathological stage of liver hepatocellular carcinoma and renal cancer. D, E Enhanced activity of glycolysis in different pathological stage in liver hepatocellular carcinoma and renal cancer. The x-axis represents different pathological stages of the tumors
PFKFB4 induces a pro-inflammatory microenvironment in cancer
To further validate PFKFB4 expression and its role in pro-inflammatory microenvironment in tumors, we analyzed PFKFB4 expression in assorted immune cell infiltration in several algorithms including CIBERSORT, XCELL, EPIC, QUANTISEQ and TIDE. Interestingly, PFKFB4 was significantly negatively correlated with CD8 + T cells (include naïve, central memory, and effector memory), hematopoietic stem cells, and M2 macrophage (anti-inflammatory subsets of macrophage) in most cancer types (Fig. 5A), but it was positively related with neutrophils, cancer-associated fibroblasts (CAF), myeloid-derived suppressor cells (MDSCs), and M0 macrophage in various cancer types (Fig. 5B). These data strongly suggested that PFKFB4 induces a pro-inflammatory microenvironment via mediating cytokine release to recruit neutrophils, CAF, MDSCs and also suppresses CD8 + T cells and M2 macrophages in tumor microenvironment.
Association between PFKFB4 expression and tumor immunity several types of cancer. A Correlations of PFKFB4 gene expression with CD8 + T cells, hematological stem cells, and M2 macrophages. B Correlations of PFKFB4 gene expression with neutrophil, cancer associated fibroblast, myeloid-derived suppressor cells, and M0 macrophages. Purple and red colors represent negative and positive correlations
Discussion
Currently, the primary challenge in the management of CRS is to identify more effective targets for specific therapeutic intervention while maintaining the therapeutic efficacy of CAR T-cells. Our study explored the genomic determinants which trigger cytokine release in immunotherapy and several other diseases. We found that PFKFB4 gene and glycolytic pathway activity were gradually upregulated with the development of increasing severity of cytokine release syndrome in CAR T-cell therapy. Moreover, these results were further validated in cohorts of people with COVID-19, influenza, autoimmune diseases and in tumor tissues although there was no change in glycolytic activity in flu infection and systemic lupus erythematosus (SLE).
Cytokines are regulators of the immune response to infection and inflammation. They function as a double-edged sword in that cytokines commonly alert immune cells to the presence of infections and tissue damage [37], however persistent cytokine production can, in turn, stimulate immune cells to secrete more cytokines that work in both autocrine and paracrine manners leading to a chronic inflammation state and even caused severe cytokine release syndrome when the immune system is hyperactivated. Therefore, it is important to find trigger factors to control cytokine release and maintain normal levels. To date, cytokine release has been increasingly explored in different fields, such as viral infection, autoimmune diseases, and immunotherapy. Most studies report that the JAK-STAT, NF-κB, and type I IFN signaling pathways are the main factors mediating the cytokine release process [38,39,40]. FDA-approved drugs targeting these pathways have already been used for the treatment in patients with cytokine release syndrome in CAR T-cell therapy, SARS-CoV-2 infection, and autoimmune diseases [39, 41,42,43]. In this study, we also found the activity of JAK-STAT signaling pathway was enhanced when patients developed higher grades of CRS, but not that of NF-κB and type I IFN signaling pathways. Furthermore, we found that the glycolytic activity was more noticeably upregulated than the other signaling pathways. Thus, drugs targeting glycolytic pathway may be beneficial for patients who have less/no response to anti-IL6 antibody, JAK inhibitors. Moreover, for the clinical management of cytokine release syndrome a combination of drugs targeting different pathways might be more effective than single-treatment approaches.
To date, most studies of PFKFB4 have focused on its increased expression in cancer tissues and its role in carcinogenesis [44,45,46], there is little knowledge about the biological mechanism on its upregulation under aforementioned situations. This gene encodes a bifunctional enzyme with kinase/phosphatase activity that is the most potent regulator of the PFK-1 gene, which is a key rate-limiting enzyme of glycolysis [47]. It has been reported that immune cells will adapt their metabolism upon infection and become highly glycolytic. For example, the SARS-CoV-2 infection triggers mitochondrial ROS production, which induces stabilization of hypoxia-inducible factor-1α (HIF-1α) and consequently promotes glycolysis [48]. As an enzyme involved in glycolysis, PFKFB4 gene may also change its expression level to deal with the situation. Our study is the first to report that the expression of PFKFB4 increases under these conditions and is a driving force in triggering cytokine release through the involvement of glycolytic pathway activity in CAR T-cells, viral infections and autoimmune diseases. Interestingly, PFKFB4 may even regulate the infiltration of immune cells in tumor tissues, which suggests that PFKFB4 may also be a promising target for the regulation of tumor immunity in some types of cancer. Given the apparent role of PFKFB4 in cytokine release, further studies aimed at developing effective drugs target on PFKFB4 and glycolysis appear particularly promising.
Unlike transcription factors, which can regulate gene expression through direct binding to gene promoters, PFKFB4 is an enzyme with kinase and phosphatase activity. The key point is to find the transcription factor to bridge the PFKFB4 enzyme and cytokine gene expression. One study suggests that PFKFB4 ectopic expression elevates lactate levels (synthesized from pyruvate, which is the final product of glycolysis) in the culture medium which initiates NF-κB activation and nuclear translocation. NF-κB within the nucleus binds to the IL-6 promoter region and then enhances IL-6 expression [49]. Another study found that PFKFB4 could interact with ICMT, a post-translational modifier of RAS, and activate RAS/AKT signaling pathway [50]. Several reports have implicated RAS in the ability to promote the production of inflammatory cytokines and chemokines (IL-6, IL-8, GM-CSF et al.) [51,52,53,54]. These results suggest that investigations concerning how PFKFB4 engages in cytokine release in CAR T-cell, primary T cells, and assorted immune cells should be conducted in the future.
Our study has some limitations. First, we only evaluated 43 pre-infusion CD22 CAR T-cell products. Analyzing more samples will not only enhance the statistical power, but will also increase the possibility of finding more candidate driving genes. For this reason we used public datasets to validate our findings. However, the use of these public datasets brings along another limitation,since these public datasets and related background information included in them were out of our control. This limitation makes it difficult to explain some results from these datasets. For example, we saw that the PFKFB4 gene was upregulated in SLE patients with no change in glycolytic activity, but we could not provide a reasonable explanation for this finding since we had no access to additional clinical information concerning these SLE patients. Another limitation is that we did not obtain information about the pro-inflammatory and anti-inflammatory cytokines levels in each patients’ serum after infusion of the CD22 CAR T-cell products or in the patients with the other diseases. This information would have helped to better understand which cytokines were mediated by PFKFB4 gene.
In summary, our results strongly indicate that PFKFB4 is a promising target for controlling cytokine release in immunotherapy and other cytokine release related diseases. More effort should be focused on the identification and development of drugs that target PFKFB4 and glycolysis.
Availability of data and materials
All 43 RCC files from Nanostring assay are available in GEO online dataset (GSE200296) until Dec 31, 2022.
References
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56–56.
Li X, Shao M, Zeng X, Qian P, Huang H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct Target Ther. 2021;6:367.
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96.
Que Y, Hu C, Wan K, Hu P, Wang R, Luo J, Li T, Ping R, Hu Q, Sun Y, Wu X, Tu L, Du Y, Chang C, Xu G. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int Rev Immunol. 2022;41:217–30.
Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MBA, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–91.
Wei F, Gao C, Wang Y. The role of influenza A virus-induced hypercytokinemia. Crit Rev Microbiol. 2022;48:240–56.
Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Investig. 2020;130:2202–5.
Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37–37.
Reynolds JA, McCarthy EM, Haque S, Ngamjanyaporn P, Sergeant JC, Lee E, Lee E, Kilfeather SA, Parker B, Bruce IN. Cytokine profiling in active and quiescent SLE reveals distinct patient subpopulations. Arthritis Res Ther. 2018;20:173–173.
Guan Q, Gao X, Wang J, Sun Y, Shekhar S. Cytokines in autoimmune disease. Mediat Inflamm. 2017;2017:5089815.
Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang H-W, Wang Y, Inglefield J, Dulau-Florea A, Ceppi F, Hermida LC, Stringaris K, Dunham K, Homan P, Jailwala P, Mirazee J, Robinson W, Chisholm KM, Yuan C, Stetler-Stevenson M, Ombrello AK, Jin J, Fry TJ, Taylor N, Highfill SL, Jin P, Gardner RA, Shalabi H, Ruppin E, Stroncek DF, Shah NN. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138:2469–84.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377:2531–44.
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–16.
Remap-Cap Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–502.
Hresko AM, Got CJ, Gil JA. Perioperative management of immunosuppressive medications for rheumatoid arthritis. J Hand Surg Am. 2022. https://doi.org/10.1016/j.jhsa.2021.09.038.
Qiu S, Zhang H, Yu S, Yang Q, Zhang G, Yang H, Li Q, Wang M. Clinical manifestations, prognosis, and treat-to-target assessment of pediatric lupus nephritis. Pediatr Nephrol. 2022;37:367–76.
Mehta J, Rolta R, Mehta BB, Kaushik N, Choi EH, Kaushik NK. Role of dexamethasone and methylprednisolone corticosteroids in coronavirus disease hospitalized patients: a review. Front Microbiol. 2019;13(2022):813358–813358.
Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Reagan PM, Bot A, Rossi JM, Sherman M, Navale L, Jiang Y, Aycock JS, Elias M, Wiezorek JS, Go WY, Miklos DB. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-hodgkin lymphoma (NHL). Blood. 2017;130:1547–1547.
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti–IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64.
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP, Investigators CC-BI. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–16.
Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–7.
Kadauke S, Myers RM, Li Y, Aplenc R, Baniewicz D, Barrett DM, Barz Leahy A, Callahan C, Dolan JG, Fitzgerald JC, Gladney W, Lacey SF, Liu H, Maude SL, McGuire R, Motley LS, Teachey DT, Wertheim GB, Wray L, DiNofia AM, Grupp SA. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39:920–30.
Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, Gust J, Leger KJ, Tarlock K, Cooper TM, Finney OC, Brakke H, Li DH, Park JR, Jensen MC. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134:2149–58.
Topp M, Van Meerten T, Houot R, Minnema MC, Milpied N, Lugtenburg PJ, Thieblemont C, Wermke M, Song K, Avivi I, Kuruvilla J, Dührsen U, Chu R, Zheng L, Plaks V, Kerber A, Kersten MJ. Earlier steroid use with axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory large B cell lymphoma. Blood. 2019;134:243–243.
Strati P, Furqan F, Westin J, Fayad L, Ahmed S, Lee HJ, Iyer SP, Nair R, Nastoupil LJ, Parmar S, Rodriguez MA, Samaniego F, Steiner R, Wang M, Pinnix CC, Flowers C, Horowitz SB, Classen C, Mistry H, Neelapu SS. Prognostic impact of dose, duration, and timing of corticosteroid therapy in patients with large B-cell lymphoma treated with standard of care axicabtagene ciloleucel (Axi-cel). J Clin Oncol. 2020;38:8011–8011.
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–25.
Veldman-Jones MH, Brant R, Rooney C, Geh C, Emery H, Harbron CG, Wappett M, Sharpe A, Dymond M, Barrett JC, Harrington EA, Marshall G. Evaluating robustness and sensitivity of the NanoString technologies nCounter platform to enable multiplexed gene expression analysis of clinical samples. Cancer Res. 2015;75:2587–93.
Scott DW, Chan FC, Hong F, Rogic S, Tan KL, Meissner B, Ben-Neriah S, Boyle M, Kridel R, Telenius A, Woolcock BW, Farinha P, Fisher RI, Rimsza LM, Bartlett NL, Cheson BD, Shepherd LE, Advani RH, Connors JM, Kahl BS, Gordon LI, Horning SJ, Steidl C, Gascoyne RD. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700.
Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, Ferree S, Storhoff J, Schaper C, Cuzick J. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90.
Nielsen T, Wallden B, Schaper C, Ferree S, Liu S, Gao D, Barry G, Dowidar N, Maysuria M, Storhoff J. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14:177.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’Connor RS, Hwang W-T, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71.
Wei S, Gu R, Xu Y, Liu X, Xing Y, Gong X, Zhou C, Liu B, Zhang G, Liu K, Wei H, Mi Y, Wang M, Wang Y, Wang J. Adjuvant ruxolitinib therapy relieves steroid-refractory cytokine-release syndrome without impairing chimeric antigen receptor-modified T-cell function. Immunotherapy. 2020;12:1047–52.
Kartikasari AER, Huertas CS, Mitchell A, Plebanski M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.692142.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00491.
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel T-A, Merkling Sarah H, Treluyer J-M, Veyer D, Mouthon L, Blanc C, Tharaux P-L, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Huarte E, O’Connor RS, Peel MT, Nunez-Cruz S, Leferovich J, Juvekar A, Yang Y-O, Truong L, Huang T, Naim A, Milone MC, Smith PA. Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T-cell therapy. Clin Cancer Res. 2020;26:6299–309.
Moradian N, Gouravani M, Salehi MA, Heidari A, Shafeghat M, Hamblin MR, Rezaei N. Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur Cytokine Netw. 2020;31:81–93.
Luo W, Li Y-X, Jiang L-J, Chen Q, Wang T, Ye D-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41:531–43.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–46.
Skurkovich B, Skurkovich S. Anti-interferon-gamma antibodies in the treatment of autoimmune diseases. Curr Opin Mol Ther. 2003;5:52–7.
Wang F, Wu X, Li Y, Cao X, Zhang C, Gao Y. PFKFB4 as a promising biomarker to predict a poor prognosis in patients with gastric cancer. Oncol Lett. 2021;21:296.
Feng C, Li Y, Li K, Lyu Y, Zhu W, Jiang H, Wen H. PFKFB4 is overexpressed in clear-cell renal cell carcinoma promoting pentose phosphate pathway that mediates Sunitinib resistance. J Exp Clin Cancer Res. 2021;40:308.
Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, Fenga C, Stratakis CA, Spandidos DA, Tsatsakis AM. Inflammation and metabolism in cancer cell—mitochondria key player. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00348.
Bartrons R, Simon-Molas H, Rodríguez-García A, Castaño E, Navarro-Sabaté À, Manzano A, Martinez-Outschoorn UE. Fructose 2,6-bisphosphate in cancer cell metabolism. Front Oncol. 2018;8:331–331.
Codo AC, Davanzo GG, Monteiro LDB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437-446.e5.
Li D, Tang J, Gao R, Lan J, Shen W, Liu Y, Chen Y, Sun H, Yan J, Nie Y, Luo N. PFKFB4 promotes angiogenesis via IL-6/STAT5A/P-STAT5 signaling in breast cancer. J Cancer. 2022;13:212–24.
Sittewelle M, Kappès V, Lécuyer D, Monsoro-Burq AH. The glycolysis regulator PFKFB4 interacts with ICMT and activates RAS/AKT signaling-dependent cell migration in melanoma. bioRxiv. 2021. https://doi.org/10.1101/2020.03.23.004119.
Catanzaro JM, Sheshadri N, Pan J-A, Sun Y, Shi C, Li J, Powers RS, Crawford HC, Zong W-X. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat Commun. 2014;5:3729.
Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58.
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–47.
Hossain MA, Larrous F, Rawlinson SM, Zhan J, Sethi A, Ibrahim Y, Aloi M, Lieu KG, Mok YF, Griffin MDW, Ito N, Ose T, Bourhy H, Moseley GW, Gooley PR. Structural Elucidation of Viral Antagonism of Innate Immunity at the STAT1 Interface. Cell Rep. 2019;29:1934-1945 e8.
Acknowledgements
This work utilized the computational resources of the NIH HPC Biowulf cluster.
Funding
Open Access funding provided by the National Institutes of Health (NIH). This research was supported by the intramural research program of the National Institutes of Health, NIH Clinical Center.
Author information
Authors and Affiliations
Contributions
LS, AP, PJ and DS designed the article structure and wrote the manuscript. LS, AP, RS, YC, JM, HL were responsible for collecting samples and analyzing data. YZ contributed to data analysis and statistical methods. NNS, RS, MP and SP contributed to data interpretation and manuscript revision. All authors contributed to the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The CD22 CAR T-cell products in clinical trial was approved by the National Cancer Institute Institutional Review Board. Products that were analyzed were from patients who consented to additional genomic testing and were enrolled on a companion study for study of biologic correlatives (Clinicaltrials.gov NCT01109394).
Consent for publication
Not applicable.
Competing interests
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Signaling pathway activity in different CRS grade groups. Associated signaling pathway score was calculated based on GSVA analysis. No obvious trend or statistical significance in these pathways. x-axis represent different CRS grade.
Additional file 2: Figure S2.
Glycolytic activity in influenza infection and autoimmune disease. Glycolytic activity score in influenza infected diseases based on early and late stages (A–C). Glycolytic activity score in different stages of SLE (D) and SJIA (E).
Additional file 3: Figure S3.
Association between PFKFB4 expression and survival time in cancer. Correlation between PFKFB4 expression and overall survival time in patients with liver hepatocellular carcinoma (A) and renal cancer (B). The x-axis the represents overall survival time. The y-axis represents survival probability. The different colors indicate the expression level of PFKFB4. (C). Expression correlations between PFKFB4 and genes involved in the glycolytic pathway.
Additional file 4: Table S1.
Differentially expressed genes based on different CRS grade group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, L., Pelayo, A., Shi, R. et al. Identification of genomic determinants contributing to cytokine release in immunotherapies and human diseases. J Transl Med 20, 338 (2022). https://doi.org/10.1186/s12967-022-03531-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03531-3