Abstract
Cancer cachexia (CC) is a debilitating syndrome that affects 50–80% of cancer patients, varying in incidence by cancer type and significantly diminishing their quality of life. This multifactorial syndrome is characterized by muscle and fat loss, systemic inflammation, and metabolic imbalance. Extracellular vesicles (EVs), including exosomes and microvesicles, play a crucial role in the progression of CC. These vesicles, produced by cancer cells and others within the tumor environment, facilitate intercellular communication by transferring proteins, lipids, and nucleic acids. A comprehensive review of the literature from databases such as PubMed, Scopus, and Web of Science reveals insights into the formation, release, and uptake of EVs in CC, underscoring their potential as diagnostic and prognostic biomarkers. The review also explores therapeutic strategies targeting EVs, which include modifying their release and content, utilizing them for drug delivery, genetically altering their contents, and inhibiting key cachexia pathways. Understanding the role of EVs in CC opens new avenues for diagnostic and therapeutic approaches, potentially mitigating the syndrome’s impact on patient survival and quality of life.
Similar content being viewed by others
Introduction
Cancer, a leading cause of mortality worldwide, is a complex disease marked by the uncontrolled proliferation and spread of abnormal cells [1]. Recent research advances have unraveled the molecular basis of tumor development, paving the way for targeted therapies and personalized medicine [2, 3]. Despite these breakthroughs, challenges persist in early detection, treatment resistance, and metastasis. A prevalent issue in advanced cancer, affecting up to 80% of patients in the terminal stage, particularly those with digestive and respiratory system cancers, is cancer-associated cachexia which directly causes around 20 to 30% of cancer deaths [4]. This complex metabolic syndrome, characterized by irreversible weight loss, muscle wasting, and systemic inflammation, arises from a mix of tumor factors, metabolic disturbances, and immune dysfunction [5]. The resulting imbalance in energy and protein metabolism leads to poorer prognosis, lower survival rates, and diminished quality of life.
Similarly, chemotherapy, a critical treatment modality in cancer care, employs anti-cancer drugs to target rapidly dividing cells [6]. These drugs include alkylating agents that damage DNA, antimetabolites that interfere with the building blocks of DNA and RNA, anti-tumor antibiotics that alter DNA, topoisomerase inhibitors that disrupt enzymes involved in DNA separation, and mitotic inhibitors that hinder structures involved in cell division [7]. Their mechanisms of action involve killing rapidly dividing cells, damaging the DNA of cancer cells, or interfering with their metabolism, effectively halting their growth and spread [7]. Recent advancements in chemotherapy have led to the development of targeted therapies that focus on specific molecules in cancer cells [8], immunotherapies that enhance the immune system’s ability to fight cancer [9], and nanotechnology-based drug delivery systems that aim to improve treatment efficacy while reducing side effects [10]. However, a significant challenge associated with chemotherapy is cachexia, a condition characterized by significant weight loss, muscle wasting, and fatigue, affecting an estimated 20–80% of patients [11, 12]. Cachexia is exacerbated by the direct impact of chemotherapeutic agents on muscle and fat metabolism, which not only diminishes the patient’s quality of life but also affects the tolerability and effectiveness of chemotherapy, increasing the risk of complications.
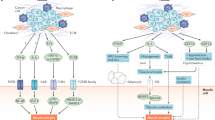
Weight loss and muscle wasting, whether caused by cancer cachexia or chemotherapy-induced cachexia, reduce patients’ tolerance to anti-cancer therapy and increase the risk of post-operative complications [13,14,15], and enervated cardiac and diaphragm muscles can normally be prone to earlier deaths from heart and lung failures [5, 16]. Furthermore, Patients with cancer associated cachexia often suffer from irreversible fatigue, decreased food intake and mobility, leading to reduced physical and emotional activity, impaired daily activity abilities and consequently a reduced quality of life [17, 18]. The complex interplay of tumor-derived factors and host responses orchestrates the development and progression of cachexia [19]. Effective management of cachexia requires early identification and a comprehensive approach, including nutritional support, targeted pharmacotherapy, and tailored exercise interventions, to improve patient outcomes and quality of life. Recently, EVs have emerged as momentous mediators in intercellular communication and have captured considerable attention in cancer studies. These small, membrane-bound particles released by cancer cells can encapsule pro-inflammatory cytokines and bioactive molecules that promote systemic inflammation, metabolic reprogramming, and muscle wasting. They facilitate communication between cancer cells and the tumor microenvironment, exacerbating the cachexia syndrome. Understanding the mechanisms of EVs in cachexia could lead to novel therapeutic strategies for this debilitating condition (Fig. 1).
As of now, three major subsets of EVs have been authenticated according to their diameter [20, 21]. Exosomes, the most well-studied subtype of EVs, participate in intercellular communication that are secreted by almost all kinds of cell types [22,23,24]. Enclosed in lipid bilayer structures, these EVs transport multifunctional biomolecules, with their bioactive cargo influencing every stage of human cancer development [25, 26], including cancer-induced cachexia (Fig. 2A). They play a role both locally and systemically in regulating a wide array of physiological and pathological processes, particularly in cell-to-cell communication [27, 28]. Recent studies have shed light on the distinct roles of tumor-derived EVs compared to those derived from host tissues, particularly in the context of cancer progression and potential implications for cachexia development [29]. Cancer cell-derived EVs are implicated in various key aspects of tumor biology, such as promoting tumor progression, facilitating immune escape, and contributing to drug resistance [30]. They have been shown to alter metabolic processes, including glycolysis and lipid metabolism, through interactions with different cells within the tumor microenvironment. This includes influencing the behavior of cancer-associated fibroblasts and immune cells in ways that favor tumor growth and metastasis [31]. Furthermore, research tracking tumor cell-derived EVs in vivo has demonstrated that these EVs exhibit a specific distribution pattern and significantly alter the immune cell composition in target organs of metastasis [32]. Such findings emphasize the importance of tumor cell-derived EVs in cancer biology and offer insights into their potential role in cachexia development in cancer patients.
In addition to their role in the cachexia driven by EVs derived from tumors, EVs originated from host tissues, such as skeletal muscle, adipose tissue, and immune cells, have also been implicated in the pathogenesis of cancer-induced wasting. Studies have highlighted the contribution of muscle-derived EVs in promoting muscle wasting and systemic metabolic alterations observed in CC [33, 34]. These EVs have been shown to contain specific miRNAs, long non-coding RNAs, and proteins that can modulate crucial cellular processes, such as dynamic changes, and immune responses [35]. Furthermore, muscle-derived EVs can be taken up by neighboring cells, including adipocytes [36] and macrophages [37]. These interactions indicate a potential role in adipogenesis, influencing the metabolic profile and modulating inflammatory conditions that may be linked to cachexia.
Here, we will explore the emerging role of EVs in CC, highlighting their involvement in the intercellular communication network and their potential as diagnostic and prognostic markers, as well as therapeutic targets. Furthermore, we will discuss recent advancements in EV research, identify key challenges and opportunities in the field, and propose future directions for investigating the complex biology of EVs in the context of CC.
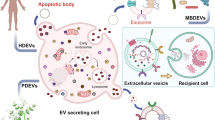
EVs: Composition and origins. EVs are small, membranous structures of varying contents, released by cells into the extracellular space. The composition and formation of EVs vary: (A) Exosomes: These vesicles, ranging from 30 to 100 nm in diameter, originate from the endocytic pathway. Multivesicular bodies (MVBs), which form from early endosomes, have two potential fates. They can fuse with lysosomes for degradation, or merge with the plasma membrane, releasing exosomes into the extracellular space. (B) Microvesicles: These are larger, with diameters between 100 and 1000 nm, and form directly through the outward budding and shedding of the plasma membrane. The figure also illustrates a typical EV cargo system, encompassing diverse components such as proteins (e.g., CD9, CD63, CD81), lipids, nucleic acids, and non-coding RNAs. (C) Apoptotic bodies: These particles, typically ranging in diameter from 1 to 5 micrometers, are produced during the final stages of apoptosis through a complex process involving caspase activation, cellular morphological changes, and blebbing, which culminates in their engulfment and digestion by phagocytes to prevent inflammation and ensure tissue homeostasis.The endoplasmic reticulum (ER) is indicated for context
Biogenesis, release, and uptake of EVs
EVs are diverse and can be categorized into several subsets based on their size and origin. Currently, three primary classes are recognized: exosomes (30–100 nm), microvesicles (100–1000 nm), and apoptotic bodies (1000–5000 nm) [38]. Exosomes, particularly well-researched, are formed through a unique process involving the budding of endosomal multivesicular bodies (MVBs) and the release of intra-luminal vesicles (ILVs) containing exosomes into the extracellular space [39]. This process contrasts with microvesicles, which are generated by the outward budding of the plasma membrane (Fig. 1A) [40]. On the contrary, apoptotic bodies form through the outward blebbing of the plasma membrane in cells undergoing apoptosis [41]. Before being released from parent cells, EVs encapsulate a wide variety of biological molecules, including proteins (e.g., TSG101, CD9, CD63, CD81, HSPs), lipids, nucleic acids, and non-coding RNAs (Fig. 1B). The role of these EVs, especially exosomes [42] and microvesicles [43], is increasingly linked to the development of cachexia in cancer patients. During apoptosis, cells form apoptotic bodies containing cellular components, often leading to anti-inflammatory effects upon uptake by antigen-presenting cells or phagocytes (Fig. 1C) [44].
Different cell types in the tumor microenvironment, including cancer cells, stromal cells, and immune cells, secrete EVs [20, 45]. Their release is influenced by conditions common in cancer cachexia, such as hypoxia, cellular stress, oncogenic signaling, and cell proliferation, as well as interactions within the tumor microenvironment [45,46,47,48,49,50]. For instance, hypoxia can trigger endoplasmic reticulum (ER) stress, leading to the activation of the unfolded protein response (UPR). This response enhances EV secretion as a means of removing misfolded proteins from the cell [51]. It can also activate oncogenic signaling pathways, including PI3K/Akt, MAPK, and mTOR, known to regulate EV secretion in cancer cells [51, 52]. EVs harbor molecules that can enhance tumor progression, metastasis, and drug resistance, and modulate the behavior of adjacent cells and the immune response.
Once released, EVs can be taken up by recipient cells through mechanisms such as endocytosis, micropinocytosis [53], and direct fusion with the plasma membrane [54]. The uptake is influenced by the EVs’ cargo composition, surface proteins, and the type of recipient cell. In CC, EVs from cancer cells can be absorbed by different cell types, including skeletal muscle cells, adipocytes, and immune cells, delivering bioactive molecules that affect their function and phenotype. For example, cancer cell-derived EVs can induce muscle wasting by affecting muscle physiology, including myofibrillar protein degradation [55, 56]. and mitochondrial function in muscle cells [57]. Another study underscores the importance of the cargo carried by different types of tumor-derived EVs, as they can mediate various pathways of internalization into recipient cells and induce similar phenotypes through different mechanisms [58].They relatively contribute to adipose tissue dysfunction, such as lipolysis [58] and adipocyte atrophy [59], and can reach organs like the liver to induce metabolic changes [60] and inflammation [61], exacerbating cachexia. Overall, while it’s clear that EVs play a role in cancer progression and potentially in the development of cachexia, the specific differences in EVs between cachectic and non-cachectic cancers remain an area for further investigation.
Impact of cancer cell-derived extracellular vesicles on recipient cells. This figure illustrates the role of EVs released by cancer cells in interacting with various recipient cells, affecting their functions and behaviors: (A) Intercellular Communication: EVs from cancer cells can travel to adjacent or distant cells. These EVs deliver a range of molecules, thereby modulating the behavior of recipient cells. (B) Muscle Cell Interaction: EVs originating from cancer cells carry specific cargoes that can trigger signaling pathways in muscle cells. This interaction influences muscle homeostasis and functionality. (C) Adipose Tissue Effects: Cancer-derived EVs can exert a direct or indirect impact on adipose tissue, contributing to adipose atrophy. (D) Metabolic Alterations: Tumor cell-derived EVs can transport bioactive molecules that affect metabolism, leading to decreased energy intake and increased energy expenditure, consequently causing body weight loss. (E) Appetite and Inflammation: Cancer cells may release EVs containing hormones, neurotransmitters, and pro-inflammatory factors. These contents can lead to diminished appetite or anorexia
EVs and pathogenesis of CC
Although research on EVs in relation to CC is still in its early stages, there has been a significant enhancement in the depth and breadth of related studies. These studies offer new insights into the potential mechanisms driving the onset and progression of cancer-associated cachexia. The impact of tumor-derived EVs and their contents on cancer progression and the development of CC has become a focal point of considerable scientific interest and concern. Pitzer and colleagues have extensively reviewed the direct effects of tumor-derived exosomes on skeletal muscle and adipose tissue, as well as their intercellular communication, which are principal factors in the weight loss associated with cancer cachexia [62]. In the following section, we will delve into the interactions between EVs and cancer-induced cachexia, focusing on skeletal muscle wasting, loss of adipose tissue, systemic inflammation, metabolic alterations, and the regulation of central nervous system (CNS) homeostasis. Our aim is to provide a more comprehensive overview of the intricate interplay among these factors. Please refer to the specific role by which EVs contribute to cachexia in Fig. 2.
EVs and skeletal muscle wasting
Muscle wasting and atrophy are significant characteristics of CC [63, 64]. Recent evidence indicates that EVs play a crucial role in the muscle wasting associated with CC [65]. Research has demonstrated that EVs originating from cancer cells directly influence skeletal muscle cells, leading to muscle wasting (Fig. 2A, B) [55, 56]. Furthermore, research has shown that EVs derived from cancer cells contain specific molecular cargo that can impact various cellular processes, especially those relevant to muscle tissue.
Cancer cell-derived EVs may contribute to muscle wasting through the transfer of specific molecules, such as non-coding RNAs. Notably, several studies have identified particular microRNAs (miRNAs) in EVs from cancer cells that are closely linked to muscle wasting [42, 43, 66,67,68,69,70]. Mechanistically, miRNAs transferred to skeletal muscle cells via EVs play a pivotal role in regulating gene expression linked to muscle protein synthesis and degradation. They influence key pathways, including TLR7 signaling [43, 66], Forkhead Box O (FoxO) Transcription Factors [70], the ubiquitin-proteasome system, and autophagy-lysosome pathways [71]. Additionally, EV-associated miRNAs impact essential signaling routes critical for muscle growth and atrophy, notably the insulin-like growth factor-1 (IGF-1) and mTOR pathways [72], which are vital for muscle protein synthesis. The specific roles and mechanisms of EV-encapsulated microRNAs in these processes are detailed in Table 1, highlighting their integral part in muscular function and health. Additionally, recent research has explored the role of EV-based circular RNAs (circRNAs) derived from tumor cells in contributing to muscle wasting [73].
In addition to non-coding RNAs, EVs from cancer cells can induce pro-inflammatory responses and activate inflammatory pathways. Two key pro-inflammatory cytokines that can be upregulated are tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) [74]. The activation of the NF-kB (Nuclear Factor-kappa B) pathway, a key event in this process, leads to the upregulation of pro-inflammatory genes [75]. Additionally, the IL-6-mediated STAT3 (signal transducer and activator of transcription 3) pathway is implicated in muscle inflammation and wasting [75, 76]. These EVs also impact other cell types within the muscle microenvironment, such as fibroblasts and adipocytes, leading to tissue remodeling and metabolic changes that further contribute to muscle wasting [77]. Moreover, cancer cell-derived EVs interfere with muscle regeneration by affecting the activation and differentiation of muscle cells [57]. These EVs can transport bioactive molecules like oxidized proteins, which, when internalized by muscle cells, disrupt the cellular redox balance, leading to oxidative stress and the degradation of contractile proteins [47, 78]. Furthermore, cancer cell-derived EVs may influence the contractile properties of muscle cells. These vesicles can carry factors that modify the expression and activity of proteins crucial for muscle contraction and force generation [58, 79, 80]. Collectively, these studies shed light on the molecular mechanisms by which tumor cell-released EVs mediate muscle wasting and affect muscle function in distally located muscles.
EVs and adipose atrophy
Cancer cachexia frequently encompasses not only muscle wasting but also the loss of adipose tissue [80]. EVs released by cancer cells can have a direct or indirect impact on adipose tissue metabolism, accelerating the wasting of adipose tissue (Fig. 2C). Additionally, EVs secreted by cancer cells may carry pro-inflammatory factors that disrupt the normal functioning of adipose tissue, contributing to its degradation [59, 76]. This process can result in adipocyte atrophy and a significant loss of fat tissue, which is a hallmark of cancer cachexia.
Tumor-derived EVs may contribute to adipose wasting through the transfer of non-coding RNAs (ncRNAs), particularly microRNAs (miRNAs). Research has shown that tumor EVs can deliver specific miRNAs to adipose tissue, impacting various functions such as adipocyte differentiation, the conversion of white adipose tissues (WATs) to a more metabolically active state, lipolysis, lipid metabolism, and insulin sensitivity (Table 2) [81,82,83,84,85,86,87]. In addition to ncRNAs, tumor EVs may also carry proteins that directly influence adipose tissue biology. For instance, EVs from certain tumor types can contain factors that promote lipolysis, the process of breaking down stored fats [88, 89]. This activity can lead to an increased release of fatty acids from adipose tissue, contributing to its wasting.
EVs and systemic inflammation
EVs have been recognized as significant mediators of inflammation in various diseases, including cancer [90]. In CC, EVs released by either tumor cells or immune cells play a role in exacerbating inflammatory processes. Research has linked EVs to the promotion of tumor-associated inflammation, muscle wasting, and systemic metabolic imbalances in CC [76, 91]. EVs originating from tumor cells are known to carry a range of pro-inflammatory molecules (Fig. 3A), including cytokines such as IL-6, interleukin-1 beta (IL-1β), Interleukin-10 (IL-10), interferon-γ (IFNγ), and Tumor Necrosis Factor-alpha (TNF-α) [92]; chemokines like CXCL1, CXCL8, and CCL2 [93], and growth factors such as Vascular Endothelial Growth Factor (VEGF) and Transforming Growth Factor-beta (TGF-β) [94]. These molecules not only promote cancer progression but also accelerate the onset and development of cancer-related cachexia (Fig. 3B) [95,96,97,98].
Recent research has also illuminated the crucial immune cell pathways involved in weight loss and tissue degradation in patients with cancer cachexia [5, 99, 100]. Tumor-derived factors interact with a variety of immune cells and endothelial cells, significantly influencing the tumor microenvironment [100, 101]. These factors are normally packaged within EVs [102,103,104] and modulate the function of immune cells such as T cells [105], macrophages [106], and natural killer (NK) cells [107]. This regulation results in immunosuppression and stimulates the release of cytokines (Fig. 3C) [104]. Furthermore, these EVs interplay with endothelial cells, including vascular endothelial cells [108], to promote angiogenesis and with lymphatic endothelial cells to facilitate cancer spread through the lymphatic system. This complex interaction creates a tumor microenvironment that is immunosuppressive, promotes angiogenesis, and enhances metastasis, thereby accelerating cachexia (Fig. 3D). Additionally, these EVs can transport tumor-derived nucleic acids, like microRNAs, influencing the inflammatory response in recipient cells and delivering pro-inflammatory factors that reinforce the overall inflammatory state [109, 110]. . This multifaceted role of EVs in promoting inflammation underscores their significance in the pathophysiology of cancer cachexia and highlights their potential as targets for therapeutic intervention.
In the cachectic state, tumor-derived EVs are also critical in activating inflammatory-related signaling pathways, notably NF-κB [111, 112], STAT3 [113, 114], and toll-like receptor (TLR) signaling (Fig. 3E) [115, 116]. A study has highlighted that EVs from LLC (Lewis Lung Carcinoma) and C26 tumor cells can induce adipocyte wasting, an effect attributed to the action of interleukin-8 (IL-8). IL-8, present outside the adipocytes, activates the NF-κB signaling pathway [59]. Furthermore, extracellular vesicles from LLC tumor cells affect muscle cells by inducing atrophy and stimulating the breakdown of fat in adipocytes. These effects are mediated by the extracellular presence of IL-6, which triggers the STAT3 pathway within the target cells [76]. The interplay between inflammation and signaling pathways is pivotal in the development and progression of cachexia. Managing this condition often involves targeting these inflammatory processes. This is typically done through interventions such as anti-inflammatory medications or treatments aimed at modulating the involved signaling pathways, underscoring the importance of understanding these mechanisms for effective therapeutic strategies.
EVs and metabolic alterations
Studies have shown that EVs from cancer cells or the tumor microenvironment frequently contain cargo molecules linked to metabolic disorders (Fig. 2D) [117, 118]. These molecules can be transferred to various recipient cells, including muscle cells, leading to alterations in their metabolic functions. One notable impact of cancer cell-derived EVs is the induction of insulin resistance in muscle cells, impairing their ability to uptake and utilize glucose [70]. This disruption in glucose metabolism is a key aspect of the metabolic reprogramming associated with cancer cachexia. In addition to affecting muscle cells, EVs can also influence lipid metabolism in adipocytes. They promote lipolysis, contributing to the systemic lipid imbalances commonly seen in CC [58, 59, 76, 81, 82, 85, 86, 119]. This alteration in lipid metabolism further exacerbates the wasting and systemic metabolic dysregulation characteristic of the condition. Moreover, EVs discharged by activated immune cells may transport inflammatory cytokines, contributing to the widespread systemic inflammation and metabolic dysregulation in CC [77].
Mitochondria, the cell’s energy-producing organelles, are critically linked to the development and progression of cancer cachexia [120]. EVs released by cancer cells can harbor specific cargo that directly affects mitochondrial function in recipient cells [57]. For instance, it has been demonstrated that tumor-derived EVs can induce mitochondrial dysfunction in skeletal muscle cells [69]. They also induce insulin resistance in muscle cells, leading to lipid accumulation and impairing glucose uptake and utilization [70]. A notable study uncovers the presence of mitochondria within EVs, suggesting a significant role in intercellular communication and tissue homeostasis [121]. The idea that EVs can transport entire mitochondria or mitochondrial components opens new avenues for understanding how cancer cells can influence the metabolism and function of distant tissues.
EVs and CNS homeostasis regulation
EVs can significantly impact CNS homeostasis in CC, primarily through the transfer of pro-inflammatory molecules. EVs originating from tumor cells often contain pro-inflammatory cytokines and chemokines, including TNF-α, IL-6, and IL-1β (Fig. 2E) [122]. These EVs, when they interact with CNS cells, can trigger an inflammatory response, leading to the activation of glial cells, the release of additional pro-inflammatory mediators, and a disruption of CNS homeostasis [123]. This inflammatory response in the CNS, instigated by EVs, affects several processes that are crucial in CC. For instance, the pro-inflammatory cytokines released by EVs can influence hypothalamic nuclei responsible for appetite regulation, potentially leading to reduced food intake and anorexia [122, 124]. Moreover, TNF-α from EVs can promote muscle protein breakdown by activating pathways that lead to the degradation of muscle proteins, especially myofibrillar proteins [125]. It is important to recognize that the regulation of muscle atrophy by the CNS is a multifaceted process, influenced by an array of factors. The involvement of EVs and their pro-inflammatory cargo provides a deeper understanding of the complex interactions at play in the CNS during the progression of cancer cachexia.
In feeding regulation, particularly in CC, the role of EVs is increasingly recognized. Elevated levels of growth differentiation factor 15 (GDF15) are often observed in CC, and the presence of GDF15 in exosomes may be a contributing factor to the appetite suppression and weight loss experienced by cancer patients (Fig. 2E) [126, 127]. GDF15 is known for its role in mediating anorexic responses, and its presence in EVs suggests a pathway by which tumors can systemically affect appetite and energy balance. Additionally, EVs can influence the sympathetic nervous system’s activity [128], leading to increased energy expenditure [129] and contributing to muscle wasting [130]. This interaction further exemplifies the complex ways in which EVs can modulate systemic metabolic responses in cancer cachexia. Beyond their effects on metabolism and appetite, EVs derived from tumor cells also carry molecules that can promote tumor growth and metastasis [45, 131]. These EVs can facilitate the spread of cancer cells to distant sites, including the CNS. Once in the CNS, cancer cells and their EVs can disrupt normal cellular functions and promote inflammation. This can exacerbate the progression of cachexia, highlighting the multifaceted impact of EVs in the pathophysiology of cancer and its systemic effects.
Additionally, research into small extracellular vesicles (sEVs) and their regulatory effects on hypothalamic AMPK (AMP-activated protein kinase) function is a case in point. Studies have shown that peripheral intravenous administration of specific sEVs can directly target neuronal cell populations in the hypothalamus [132, 133]. This targeted approach holds potential for treating cancer cachexia, particularly considering the interplay between hypothalamic AMPK activity, elevated brown adipose tissue (BAT) thermogenesis, and the browning of white fat [134,135,136]. Furthermore, characterizing EVs present in biofluids like blood or cerebrospinal fluid could provide valuable diagnostic and prognostic insights for cancer cachexia. By analyzing these biofluids for specific EVs and their cargo, clinicians may gain a better understanding of the disease’s progression and the effectiveness of treatments.
EVs as biomarkers of CC
Early detection and monitoring of CC are essential for effective management and treatment [137, 138]. Research on CC biomarkers has largely focused on mediators of skeletal muscle loss, produced by both tumor and host tissues. These include cachexia-inducible factors [139], pro-inflammatory cytokines [140, 141], lipids [142, 143], metabolic products of protein and fat [144], and non-coding RNAs [145,146,147]. However, none of these biomarkers have been extensively used in clinical practice to detect skeletal muscle wasting. Notable reviews by Loumaye et al. [148] and Cao et al. [149] discuss various CC biomarkers, emphasizing the identification of these markers and measuring their circulating levels in certain cancer types. In CC, the body undergoes significant metabolic and systemic changes, and both tumor and host tissues contribute to the altered profile of circulating EVs. It appears that the specific literature focusing on the relative contributions of tumor-derived versus host tissue-derived EVs in cancer cachexia, especially in the context of their presence in the circulation, is not readily accessible or may not be extensively covered in available research. This paper highlights recent developments in EVs as potential CC biomarkers, offering a foundation for future clinical research in this area.
EVs incorporating proteins as biomarkers
EVs can transport proteins that reflect cachexia-related processes, including systemic inflammation, muscle wasting, and metabolic changes. Analyzing the proteomic content of EVs can pinpoint specific proteins or patterns related to CC. This approach holds promise for creating diagnostic and prognostic tools for CC.
Inflammatory and metabolic markers
EVs carry numerous inflammatory cytokines from both host tissues and tumors, a process extensively studied in various cachexia models [138, 150]. IL-6 emerges as one of the most promising biomarkers for CC. IL-6 levels are directly associated with tumor stage, weight loss, and survival in lung and gastrointestinal cancer patients, as evidenced by studies [151, 152]. Additionally, potential biomarkers like TNF-alpha, β-dystroglycan, Monocyte Chemoattractant Protein-1 (MCP-1), IL-1β, and IL-8, originating from tumors and/or host tissues, show significant potential in CC diagnostics [140, 153, 154]. These bioactive molecules are integral to the inflammatory and metabolic alterations seen in cancer cachexia. Their levels in the body can provide insights into the severity of cachexia, and they offer potential targets for therapeutic interventions aimed at mitigating the devastating effects of this condition.
Non-inflammatory markers
Numerous studies have highlighted specific proteins carried by EVs that are associated with CC. These proteins impact target cells and contribute to cachexia development. Notably, EV-Growth and GDF15 from cancer cells emerge as key molecules in CC research [126, 155, 156]. Another significant protein, the Proteolysis-Inducing Factor (PIF), secreted by tumor cells, is known to cause muscle wasting [156, 157]. It can be transferred via exosomes, leading to muscle protein degradation. Additionally, exosomal Fatty Acid Binding Protein 4 (FABP4) has been implicated in muscle wasting and systemic inflammation in CC [158,159,160]. Each of these proteins contributes to the multifaceted nature of cachexia through different mechanisms. These proteins’ interactions and effects underscore the complexity of cachexia, making it a challenging condition to manage and treat effectively. Further exploration in this field could yield valuable markers for CC prevention and treatment.
Heat shock proteins (HSPs) cargo
Recent research has also highlighted a notable link between EVs and heat shock proteins (HSPs) in CC [55, 161]. It’s been discovered that certain HSPs are enclosed within EVs and released into the extracellular environment [162]. Furthermore, changes in HSP expression and activity have been noted in CC [163,164,165], indicating their role in cachexia’s development and progression. For example, increased levels of HSP70/90 are found in cachectic cancer patients compared to non-cachectic ones [166,167,168,169]. Additionally, higher EV concentrations in the serum of cancer patients have been associated with poorer prognosis [170, 171]. HSP27, another heat shock protein, has also been studied in CC contexts, with heightened levels detected in the skeletal muscles of cachectic cancer patients [172]. Another important study suggests that, in muscle-related diseases, upregulation of HSP70 and HSP90 may occur as a cellular response to alleviate protein folding stress and maintain protein homeostasis [164], although it’s essential to emphasize that this result is not specifically grounded in the context of CC. These insights underscore the potential of EV-associated HSPs as crucial molecular markers in CC management.
Skeletal muscle biomarkers
Research has pinpointed muscle-specific proteins as promising biomarkers linked to muscle wasting in CC [173, 174]. Myostatin, known for inhibiting muscle growth, is notably elevated in EVs from cachectic cancer patients [175]. Additionally, proteins crucial for muscle protein synthesis and degradation, such as Bone Morphogenetic Proteins (BMPs), irisin, TGFβ, activin A, atrogin-1, and Muscle RING-Finger Protein-1 (MuRF1) [176, 177], have been identified as potential EV-derived biomarkers. These protein changes are closely linked to the progression and severity of the disease, underscoring their value in diagnosis and prognosis. Regular tracking of these EV-related muscle-specific proteins could offer significant insights into the effectiveness of treatments and the progression of CC over time.
EVs incorporating miRNAs and RNAs as biomarkers
MicroRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) are crucial regulators of gene expression and cellular processes, each playing a distinct yet interconnected role [178]. These RNA types are intricately involved in the complex cellular dynamics of cancer cachexia, influencing muscle metabolism, inflammation, and metabolic changes associated with this condition. Their collective dysregulation in cancer cachexia underscores their importance in the pathophysiology of this syndrome, presenting potential targets for therapeutic intervention. RNA molecules transported by EVs offer critical insights into the biological mechanisms underlying CC. Notably, specific types of RNA, including miRNAs, lncRNAs, and circRNAs, are often dysregulated in EVs from CC patients [179]. These altered RNA profiles mirror the molecular shifts tied to muscle wasting and the metabolic changes characteristic of cachexia.
Cancer cell derived EVs -miRNAs and RNAs as biomarkers
Research has revealed specific miRNAs that are dysregulated in EVs derived from CC patients compared to non-cachectic individuals [180]. Various miRNAs, including miR-195a-5p, miR-125b-1-3p [42], miR-21, miR-29a [43, 66], miR-181a-3p [68], and miR-122-5p [69], are linked to muscle wasting and inflammation in CC. Additionally, miR-486 has been identified as a potential marker for cachexia severity and treatment response [181]. These miRNAs, often associated with cancer progression and muscle atrophy, are notably stable in the circulatory system when carried by EVs like exosomes. This stability positions them as effective biomarkers for cancer-related cachexia.
Beyond miRNAs, the potential of lncRNAs and circRNAs incorporated in EVs as biomarkers for CC is an area of active research, though their clinical applicability requires further validation. Initial studies have yielded promising results. For instance, lncRNAs like H19 [182] and LINC00355 [183] show different expression levels in EVs from cancer patients at risk of cachexia compared to healthy individuals, suggesting their utility in diagnosing or predicting CC. Other lncRNAs, such as Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) [184] and HOX transcript antisense intergenic RNA (HOTAIR) [185], have been linked to CC and are detectable in EVs [185, 186]. Regarding circRNAs, current research on cachexia is limited, but there are studies like one on CircPTK2 that illustrate its role in lipid metabolism regulation in CC [187]. The inherent stability of EV-incorporated circRNAs, due to their resistance to exonuclease degradation, makes them promising candidates for biomarker exploration.
Muscle specific EVs -miRNAs as biomarkers
The expression profiles of muscle-secreted miRNAs have been extensively studied, revealing their significant role in regulating muscle metabolism during CC [33, 145, 188]. Muscle-specific miRNAs, including miR-1, miR-133a, miR-133b, miR-206, miR-208a, miR-208b, and miR-499, along with muscle-enriched miR-486, are known to influence myogenesis, proliferation, differentiation, apoptosis of myotubes, and protein synthesis in skeletal muscle of CC patients [189, 190]. Recent research demonstrates that exosomes from skeletal muscle, containing myomiRs such as miR-1, miR-133a, miR-133b, and miR-206, play a dual role: they enter the circulatory system and facilitate inter-tissue communication between muscles, offering insights into new therapeutic approaches for muscle function [190]. However, detecting skeletal muscle-specific EVs remains challenging due to the complexity of EV types and a lack of specific markers for skeletal muscle-derived EVs. Nonetheless, these circulating EVs with muscle miRNAs, which contribute to muscle wasting, may serve as accessible and promising biomarkers in CC management.
EVs-based lipid composition as biomarkers
Lipidomic analysis of EVs could uncover lipid signatures serving as diagnostic markers for cachexia. For example, Fan et al. [191] demonstrates that lipid profiles of plasma exosomes can distinguish early-stage lung cancer from healthy individuals. Similar studies have utilized serum or plasma exosomal lipids in diagnosing pancreatic cancer [192], breast cancer [193], and colorectal cancer [194,195,196], suggesting the potential of lipid biomarkers in EVs for diagnosing various cancer stages. While cancer and its treatments can significantly impact the body’s metabolism and energy balance, the specific effects on adipose tissue are not well-documented [197]. Therefore, significant research is still required in exploring EV-encapsulated lipids as potential biomarkers for cancer-related cachexia.
EVs as therapeutic agents of CC
EVs indeed have gained attention in the field of medicine and therapeutics for their potential in treating various diseases, including cancer cachexia. These vesicles can originate from various cell types, such as mesenchymal stem cells, immune cells, and even tumor cells. Their ability to influence cellular processes and modulate immune responses makes them promising candidates for therapy. In this context, we focus specifically on the emerging role of EVs as therapeutic agents in CC, as detailed in Fig. 4.
Inhibiting production and release of cachectic EVs
The hypothesis is that EVs released by cancer cells or other cells contribute to the systemic effects of cachexia by transferring factors that promote muscle wasting, appetite suppression, and metabolic changes. Additionally, tumor-derived EVs can exacerbate cachexia by sending pro-cachectic signals to distant tissues. Therapeutically, targeting these tumor-derived EVs [198,199,200,201] or blocking their pathway activation [43, 56, 67] can mitigate cachexia-related complications. For example, amiloride, a commonly used diuretic, significantly improves metabolic disorders in cachectic gastrocnemius by effectively inhibiting tumor-derived exosome release, thus affecting muscle catabolism, protein synthesis, glycolysis, and ketone body oxidation [201]. Similarly, GW4869, an inhibitor of exosome production and release, shows potential in reducing lipolysis and adipose tissue browning in cachexia [198]. Moreover, IMO-8503 is found to suppress cancer cells’ release of EVs containing circulating miRNAs, thereby reversing cachexia with minimal side effects [66]. Another study indicates that omeprazole, a proton pump inhibitor (PPI), can ameliorate cancer-induced cachexia by limiting the release of EV surface proteins like Hsp70 and Hsp90 [202]. Additionally, blocking CD81 on EVs from senescent bone marrow-derived mesenchymal stem cells (BMSCs) can reduce muscle wasting [203], suggesting its potential in preventing muscle loss. Thus, suppressing EV release emerges as a promising cell-free therapeutic approach for CC treatment (Fig. 4A).
EVs as anti-inflammatory agents
Recent research indicates that EVs from certain cell types, like mesenchymal stem cells (MSCs) or immune cells, may have anti-inflammatory effects, suggesting their potential use as therapeutic agents in CC (Fig. 4B). These EVs can interact with and modulate immune cell functions. For instance, EVs from MSCs or immune cells [204,205,206,207] often carry anti-inflammatory proteins like IL-10 or TGF-β, which can suppress immune responses and reduce inflammation. Moreover, they can influence macrophage polarization, shifting them from a pro-inflammatory (M1) state to an anti-inflammatory (M2) state [208]. This shift towards M2 macrophages could lessen the inflammatory response, thereby potentially mitigating cachexia-related inflammation. Additionally, EVs transport specific miRNAs to target cells, impacting various signaling pathways involved in inflammation [209]. Certain miRNAs in EVs have demonstrated anti-inflammatory properties [210], offering a possible way to alleviate inflammation in CC.
Exercise related EVs for treating CC
Physical exercise is a key strategy to counteract muscle atrophy and dysfunction in CC [211, 212]. During physical activity, EV trafficking plays a crucial role in inter-tissue communication [213,214,215,216]. Originating from muscle, immune, or cancer cells, these EVs can mediate the systemic effects of exercise on diverse tissues and organs [181]. , potentially improving cachexia symptoms.
Exercise-induced EVs are gaining attention for their roles in oncology [181] and skeletal muscle [217]. They are particularly interesting for their possible correlation with muscle remodeling and homeostasis [218]. The molecules within these EVs can help modulate processes like reducing oxidative damage, influencing mitochondrial function [219] improving metabolism [220] and enhancing skeletal muscle insulin sensitivity (Fig. 4D) [221]. Physical training can alter the number of circulating EVs and their protein contents. For example, exercise can increase the release of EVs containing Hsp72 [216] and Hsp60 [222], with Hsp60 activating the Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha (PGC1α) pathway, crucial for modulating muscle wasting [72, 222]. However, it’s important to note that these findings are not specifically studied in the context of CC. The precise mechanisms and therapeutic uses of exercise-induced EVs in CC remain areas of active research.
In addition to combatting muscle dysfunction, EVs from physical exercise may help delay CC progression through their anti-inflammatory effects (Fig. 4D). Exercise-induced muscle-derived IL-6 has shown to inhibit other inflammatory factors, exerting anti-inflammatory impacts [223]. EVs released post-exercise carrying meteorin-like protein can increase delivery of anti-inflammatory cytokines [224, 225]. The ability of contracting skeletal muscle cells to communicate with other organs via EV-based humoral factors is key to how physical exercise induces systemic adaptations, enhancing overall health [226]. These insights suggest a significant role for exercise-induced EVs in cachexia treatment.
EVs as drug delivery system
Studies involving animal models and preclinical research have demonstrated the potential of EVs as a drug delivery system for muscle atrophy. For instance, EVs derived from MSCs have shown promise in promoting muscle regeneration, reducing inflammation, and improving muscle function in models of muscle atrophy (Fig. 4H) [227,228,229,230]. Additionally, research indicates that exosomes released from differentiating human skeletal myoblasts can stimulate myogenesis in human adipose-derived stem cells (HASCs), thereby accelerating muscle regeneration (Fig. 4H) [231]. Furthermore, the development of artificial nanovesicles, especially exosome-mimetic nanovesicles, is attracting interest in cancer research as potential therapeutic agents [232]. These nanovesicles are designed to replicate the properties of natural exosomes, potentially functioning as effectively or even more so [233]. Exosome-like systems, based on nanotechnology and surface engineering approaches, aim to overcome limitations of natural exosomes, showing potential as a competitive approach for innovative targeted anti-cancer therapies [234]. Nanoparticles, especially green nanoparticles with their environmental and biocompatibility benefits [235], are pivotal in cancer treatment as a drug delivery system [236,237,238]. Therefore, artificial nanovesicles and precision delivery systems may greatly improve treatment efficacy for cachexia in cancer (Fig. 4G).
Modulating EV cargo as therapeutic agents
Modifying the content of EVs presents a promising therapeutic strategy for CC. Engineering EVs or altering their cargo-loading process could enable the delivery of specific molecules to mitigate the effects of cachexia (Fig. 4E). For instance, EVs could be loaded with anti-inflammatory agents [239], anchor factors [240], or myostatin inhibitors [241] to reduce muscle wasting. Additionally, engineering EVs to display specific targeting ligands on their surface would allow them to selectively bind to receptors on CC-related cells. An example of this is Physiactisome, a nanovesicle incorporating Hsp60, which mimics the beneficial effects of exercise training in combating muscle atrophy and cachexia [242]. Further, by manipulating the cargo of EVs, the adverse impacts of cancer-derived EVs on cachexia might be lessened. Research efforts could focus on selectively removing or altering specific proteins or nucleic acids within EVs linked to cachexia progression. Another approach involves loading therapeutic agents into EVs, turning them into targeted delivery vehicles for specific cells or tissues implicated in cachexia. Recent perspectives suggest that artificial EVs could be more effective than natural vesicles for drug delivery [243].
EVs in ameliorating nutrient uptake and metabolism
EVs are known to transport a variety of bioactive factors capable of influencing the expression and activity of nutrient transporters. EVs from healthy cells, for example, may contain molecules that boost the uptake of essential nutrients like amino acids, glucose, and fatty acids by recipient cells. This enhancement in nutrient absorption can promote anabolism, helping to counteract the metabolic imbalances caused by CC (Fig. 4C) [244, 245]. On the other hand, CC is often accompanied by changes in gut microbiota composition, which can impact nutrient absorption and metabolism. EVs originating from specific bacterial strains, or those engineered to carry beneficial microbiota-derived molecules, might be capable of modifying the gut microbiota. Such alterations could potentially improve nutrient absorption and overall metabolism in CC patients [246].
EVs in regulating appetite
Recent studies highlight the role of EVs from the hypothalamus and adipose tissue in regulating feeding behavior and energy balance [247,248,249]. Adipose tissue-derived EVs (EVs-AT) have been found to influence appetite-regulating pathways by altering the expression of appetite-related genes in target cells (Fig. 4F) [248]. Furthermore, EVs released by gut cells, including enteroendocrine cells, may contain bioactive molecules that facilitate signaling between the gut and the brain [250], thereby modulating appetite pathways and affecting satiety and hunger cues [251, 252]. Additionally, microbiota-derived extracellular vesicles (MEVs), released by gut bacteria, interact with host cells involved in appetite regulation. These MEVs can transport various bioactive molecules that influence appetite-related pathways (Fig. 4F) [253, 254]. However, it is important to note that more research is needed to fully understand the role of EVs in appetite regulation, particularly in the context of CC, and to investigate their potential as therapeutic targets.
EVs as immunotherapeutic tactics
The use of EVs for immunomodulation in CC is a burgeoning area of research. EVs have the capability to influence the activity and function of various immune cells implicated in CC, such as MSCs, macrophages, B cells and T cells (Fig. 4B). Notably, they can shift macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype [208]. This polarization shift can reduce tissue damage and facilitate tissue repair. Moreover, EVs might also boost the cytotoxicity of T cells against tumor cells [255, 256], aiding in tumor suppression and potentially easing the effects of cachexia. Therefore, manipulating the immune response via immune cell-derived EVs could significantly impact the progression of CC.
Current perspectives and future challenges
EVs have emerged as significant players in the field of CC due to their vital role in intercellular communication and their potential in diagnostic and therapeutic applications. These vesicles, containing a diverse array of molecules such as proteins, nucleic acids, and lipids, mirror the phenotype of their originating cells, making them promising candidates for diagnostic and prognostic biomarkers in CC. Identifying specific EV biomarkers could enhance early detection and provide insights into cachexia’s progression. Furthermore, the ability to engineer EVs to deliver targeted treatments, such as small interfering RNAs (siRNAs) or drugs, directly to affected cells presents a novel avenue for CC therapy. However, the development of effective methods to load EVs with therapeutic agents and ensure their precise delivery to target tissues, such as muscle or adipose tissue, remains a challenge.
The potential of EVs in CC treatment is substantial, yet it is hindered by several technical obstacles. Standardizing methods for EV isolation and characterization, developing sensitive assays for cargo analysis, and scaling up therapeutic EV production are critical steps that need to be addressed [257,258,259]. Moreover, ensuring robust and reproducible methodologies is essential for the reliability and comparability of EV-based studies [260]. As EV-based approaches transition from preclinical to clinical settings, overcoming regulatory hurdles, establishing scalable manufacturing processes, and conducting comprehensive clinical trials to assess safety and efficacy become paramount [261]. Given the complexity of cancer cachexia, which varies among individuals and cancer types, and the diversity in EV cargo and composition, personalized approaches targeting specific cachexia mechanisms are necessary.
In total, while EVs offer significant promise in the management of cancer cachexia, overcoming the challenges associated with their development is crucial for realizing their full potential as diagnostic and therapeutic tools. A deeper understanding of EVs’ role in muscle wasting and metabolic dysfunction, coupled with ongoing research, collaboration, and technological innovation, is key to advancing the field. Integrating EV-based therapies with existing interventions, such as nutritional support, exercise, or pharmacological treatments, could provide synergistic effects, improving patient outcomes. Therefore, exploring multimodal approaches that combine EVs with other therapeutic modalities is essential for advancing the management of cancer cachexia.
Conclusions
EVs are vital in cancer biology, mainly because they facilitate intercellular communication by transporting bioactive molecules. This function is critical for various cancer-related processes, including the development and spread of tumors, evasion of the immune response, and development of resistance to drugs. The composition of EVs, which mirrors the condition of their originating cells, offers significant insights for diagnosing, prognosticating, and tracking cancer progression. Moreover, their capacity to traverse biological barriers makes them promising for creating targeted drug delivery systems, potentially transforming cancer treatment strategies. While EVs hold great potential for cancer therapy, challenges such as achieving specificity to cancer cells, scaling up production, efficiently loading and securing the stability of therapeutic agents, and overcoming regulatory and safety obstacles, remain to be addressed.
EVs also play a crucial role in cancer-associated cachexia, a condition prevalent in advanced cancer stages that significantly affects patient quality of life and survival. By mediating interactions between tumor cells and the tumor microenvironment, EVs contribute to cachexia through inflammatory responses, metabolic reprogramming, and direct effects on muscle and adipose tissue. Understanding the role of EVs in cachexia is vital for early detection, monitoring, and identifying therapeutic targets to alleviate the condition’s impact on patient outcomes. Research has shown the diagnostic and prognostic potential of EVs in cancer cachexia, with ongoing studies exploring their therapeutic possibilities. The integration of EV-based biomarkers and therapies into clinical practice promises to improve patient outcomes by enabling earlier diagnosis, more accurate prognosis, and personalized treatment strategies. As knowledge of EV-mediated molecular mechanisms expands, targeted interventions can be developed, highlighting the importance of continued research and investment in this area. Overall, EVs represent a significant advancement in managing and treating cancer cachexia, offering a new frontier in cancer care.
The role of inflammatory factors from tumor and immune cells in cancer cachexia. (A) EVs from tumor cells carry various pro-inflammatory molecules. (B) The molecules contained within EVs, such as cytokines, chemokines, and growth factors, contribute to cancer progression and accelerate the onset of cancer-associated cachexia. (C) Regulating inflammatory molecules leads to immunosuppression and stimulates cytokine release, which in turn boosts metastasis and speeds up cachexia. (D) EVs interact with endothelial cells to encourage angiogenesis and with lymphatic endothelial cells to aid in cancer dissemination. (E) Tumor-derived EVs are pivotal in triggering inflammation-related signaling pathways, specifically NF-κB, STAT3, and TLR pathways. Abbreviations NF-κB, Nuclear Factor-kappa B; STAT3, signal transducer and activator of transcription 3; TLR, toll-like receptor
Therapeutic potential of EVs in cancer-induced cachexia. EVs have demonstrated promising therapeutic effects in managing cancer-induced cachexia, through various mechanisms: (A) Inhibition of EV Production: Reducing the production and release of cancer cell-secreted EVs can alleviate symptoms of cancer cachexia. (B) Immune Cell-Derived EVs: EVs from immune cells or anti-inflammatory sources carry factors that can suppress inflammation and modulate the immune response, thus helping to manage cancer cachexia. These EVs can regulate immune responses, diminish systemic inflammation, and restore immune homeostasis, potentially slowing the progression of cachexia. (C) Nutrient-Derived EVs: EVs carrying nutrients or those derived from nutrient sources are being explored for their therapeutic effects in cancer cachexia. (D) Exercise-Induced EVs: EVs generated through exercise may improve body weight, muscle mass, and physical performance in cachectic patients. (E) Cargo Modulation: Altering the cargo of EVs presents a novel approach for treating cancer cachexia. (F) Gut and Adipose Tissue-Derived EVs: These EVs could regulate appetite by affecting signaling pathways in the brain’s appetite-control centers. (G) Loaded EVs: EVs can be engineered to carry anti-inflammatory agents, specific anti-cancer drugs, and other therapeutic agents, making them effective for therapeutic cargo delivery. (H) MSC and HASC-Derived EVs: Mesenchymal stem cell (MSC) and human adipose-derived stem cell (HASC)-derived EVs have shown promise in enhancing muscle tissue regeneration in preclinical studies. Abbreviations MSC, mesenchymal stem cell; HASCs, human adipose-derived stem cells; EVs-AT, adipose tissue-derived EVs; MEVs, microbiota-derived extracellular vesicles
Data availability
Not applicable.
Abbreviations
- CC:
-
Cancer cachexia
- EVs:
-
Extracellular vesicles
- MVBs:
-
Endosomal multivesicular bodies
- ILVs:
-
Intra-luminal vesicles
- CNS:
-
Central nervous system
- IGF-1:
-
Lnsulin-like growth factor-1
- mTOR:
-
mechanistic target of rapamycin
- IL-1β:
-
L Interleukin-1 beta
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IFN-γ:
-
Interferon-γ
- TNF-α:
-
Tumor necrosis factor-alpha
- VEGF:
-
Vascular endothelial growth factor
- TGF-β:
-
Transforming growth factor-beta
- NK:
-
Natural killer
- NF-κB:
-
Nuclear factor-kappa B
- STAT3:
-
Signal transducer and activator of transcription 3
- TLR:
-
Toll-like receptor
- LLC:
-
Lewis lung carcinoma
- GDF15:
-
Growth differentiation factor 15
- AMPK:
-
AMP-activated protein kinase
- BAT:
-
Brown adipose tissue
- MCP-1:
-
Monocyte chemoattractant protein-1
- PIF:
-
Proteolysis-inducing factor
- FABP4:
-
Fatty acid binding protein 4
- HSPs:
-
Heat shock proteins
- BMPs:
-
Morphogenetic proteins
- MuRF1:
-
Muscle RING-finger protein-1
- MALAT1:
-
Metastasis ssociated lung adenocarcinoma transcript 1
- HOTAIR:
-
HOX transcript antisense intergenic RNA
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- MSCs:
-
Mesenchymal stem cells
- PGC1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- HASCs:
-
Human adipose-derived stem cells
- EVs-AT:
-
Adipose tissue-derived EVs
- MEVs:
-
Microbiota-derived extracellular vesicles
References
Brown JS, Amend SR, Austin RH, Gatenby RA, Hammarlund EU, Pienta KJ. Updating the definition of Cancer. Mol Cancer Res. 2023;21:1142–7. https://doi.org/10.1158/1541-7786.Mcr-23-0411.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29. https://doi.org/10.1038/nrclinonc.2017.44.
Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. 2021;219:107709. https://doi.org/10.1016/j.pharmthera.2020.107709.
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–62. https://doi.org/10.1038/nrc3829.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. https://doi.org/10.1038/nrdp.2017.105.
Brianna LSH. Chemotherapy: how to reduce its adverse effects while maintaining the potency? Med Oncol. 2023;40:88. https://doi.org/10.1007/s12032-023-01954-6.
Bukowski K, Kciuk M, Kontek R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int J Mol Sci. 2020;21:3233. https://doi.org/10.3390/ijms21093233.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218. https://doi.org/10.1038/s41392-021-00641-0.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21. https://doi.org/10.1038/s41423-020-0488-6.
Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11:6370–92. https://doi.org/10.7150/thno.57828.
Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–60. https://doi.org/10.1210/en.2007-0828.
Huang KC, Chiang YF, Huang TC, Chen HY, Lin PH, Ali M, Hsia SM. Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo. J Cachexia Sarcopenia Muscle 2023, 14:182–197. 2023;14:182 – 97. https://doi.org/10.1002/jcsm.13120.
Vagnildhaug OM, Blum D, Wilcock A, Fayers P, Strasser F, Baracos VE, Hjermstad MJ, Kaasa S, Laird B, Solheim TS. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J Cachexia Sarcopenia Muscle. 2017;8:789–97. https://doi.org/10.1002/jcsm.12220.
Mason MC, Garcia JM, Sansgiry S, Walder A, Berger DH, Anaya DA. Preoperative cancer cachexia and short-term outcomes following surgery. J Surg Res. 2016;205:398–406. https://doi.org/10.1016/j.jss.2016.06.076.
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37:1101–13. https://doi.org/10.1016/j.clnu.2017.07.010.
Coletti D. Chemotherapy-induced muscle wasting: an update. Eur J Transl Myol. 2018;28:7587. https://doi.org/10.4081/ejtm.2018.7587.
Takayama K, Atagi S, Imamura F, Tanaka H, Minato K, Harada T, Katakami N, Yokoyama T, Yoshimori K, Takiguchi Y, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer. 2016;24:3473–80. https://doi.org/10.1007/s00520-016-3156-8.
Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4:95–109. https://doi.org/10.1007/s13539-012-0087-1.
Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer Cachexia: its mechanism and clinical significance. Int J Mol Sci. 2021;22:8491. https://doi.org/10.3390/ijms22168491.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. https://doi.org/10.1146/annurev-cellbio-101512-122326.
Ståhl AL, Johansson K, Mossberg M, Kahn R, Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34:11–30. https://doi.org/10.1007/s00467-017-3816-z.
Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, Shlomi T, Gil Z. Transfer of miRNA in macrophage-derived Exosomes induces Drug Resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78:5287–99. https://doi.org/10.1158/0008-5472.Can-18-0124.
Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, Zhang S, Wang J, Komarova S, Wang J, et al. Apoptotic cell-derived extracellular vesicles promote malignancy of Glioblastoma Via Intercellular transfer of splicing factors. Cancer Cell. 2018;34:119–e135110. https://doi.org/10.1016/j.ccell.2018.05.012.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. https://doi.org/10.1038/s41586-018-0392-8.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. https://doi.org/10.1038/s41556-018-0250-9.
Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–81. https://doi.org/10.1016/j.semcancer.2017.02.006.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a Distance. Dev Cell. 2019;49:347–60. https://doi.org/10.1016/j.devcel.2019.04.011.
Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466–77. https://doi.org/10.1016/j.annonc.2021.01.074.
Shu S, Yang Y, Allen CL, Maguire O, Minderman H, Sen A, Ciesielski MJ, Collins KA, Bush PJ, Singh P, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2018;8:12905. https://doi.org/10.1038/s41598-018-31323-7.
Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18:59. https://doi.org/10.1186/s12943-019-0980-8.
Fu X, Song J, Yan W, Downs BM, Wang W, Li J. The biological function of tumor-derived extracellular vesicles on metabolism. Cell Commun Signal. 2023;21:150. https://doi.org/10.1186/s12964-023-01111-6.
Gerwing M, Kocman V, Stölting M, Helfen A, Masthoff M, Roth J, Barczyk-Kahlert K, Greune L, Schmidt MA, Heindel W, et al. Tracking of Tumor Cell-Derived Extracellular vesicles in vivo reveals a specific distribution pattern with consecutive Biological effects on Target sites of Metastasis. Mol Imaging Biol. 2020;22:1501–10. https://doi.org/10.1007/s11307-020-01521-9.
Narasimhan A, Ghosh S, Stretch C, Greiner R, Bathe OF, Baracos V, Damaraju S. Small RNAome profiling from human skeletal muscle: novel miRNAs and their targets associated with cancer cachexia. J Cachexia Sarcopenia Muscle. 2017;8:405–16. https://doi.org/10.1002/jcsm.12168.
Aoi W, Tanimura Y. Roles of skeletal muscle-derived exosomes in Organ metabolic and immunological communication. Front Endocrinol (Lausanne). 2021;12:697204. https://doi.org/10.3389/fendo.2021.697204.
Tai YL, Chu PY, Lee BH, Chen KC, Yang CY, Kuo WH, Shen TL. Basics and applications of tumor-derived extracellular vesicles. J Biomed Sci. 2019;26:35. https://doi.org/10.1186/s12929-019-0533-x.
Rome S. Muscle and adipose tissue communicate with Extracellular vesicles. Int J Mol Sci. 2022;23:7052. https://doi.org/10.3390/ijms23137052.
Yamaguchi A, Maeshige N, Yan J, Ma X, Uemura M, Matsuda M, Nishimura Y, Hasunuma T, Kondo H, Fujino H, Yuan ZM. Skeletal myotube-derived extracellular vesicles enhance itaconate production and attenuate inflammatory responses of macrophages. Front Immunol. 2023;14:1099799. https://doi.org/10.3389/fimmu.2023.1099799.
Huang T, Song C, Zheng L, Xia L, Li Y, Zhou Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18:62. https://doi.org/10.1186/s12943-019-0967-5.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. https://doi.org/10.1126/science.aau6977.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617–38. https://doi.org/10.1038/s41571-018-0036-9.
Turiák L, Misják P, Szabó TG, Aradi B, Pálóczi K, Ozohanics O, Drahos L, Kittel A, Falus A, Buzás EI, Vékey K. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteom. 2011;74:2025–33. https://doi.org/10.1016/j.jprot.2011.05.023.
Miao C, Zhang W, Feng L, Gu X, Shen Q, Lu S, Fan M, Li Y, Guo X, Ma Y, et al. Cancer-derived exosome miRNAs induce skeletal muscle wasting by bcl-2-mediated apoptosis in colon cancer cachexia. Mol Ther Nucleic Acids. 2021;24:923–38. https://doi.org/10.1016/j.omtn.2021.04.015.
He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci U S A. 2014;111:4525–9. https://doi.org/10.1073/pnas.1402714111.
Mohan A, Agarwal S, Clauss M, Britt NS, Dhillon NK. Extracellular vesicles: novel communicators in lung diseases. Respir Res. 2020;21:175. https://doi.org/10.1186/s12931-020-01423-y.
Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell. 2023;186:1610–26. https://doi.org/10.1016/j.cell.2023.03.010.
Venturella M, Criscuoli M, Carraro F, Naldini A, Zocco D. Interplay between Hypoxia and Extracellular vesicles in Cancer and inflammation. Biology (Basel). 2021;10:606. https://doi.org/10.3390/biology10070606.
Ho J, Chaiswing L, St Clair DK. Extracellular vesicles and Cancer Therapy: insights into the role of oxidative stress. Antioxid (Basel). 2022;11:1194. https://doi.org/10.3390/antiox11061194.
Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, Endzeliņš E, Llorente A, Linē A, Riekstiņa U. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17. https://doi.org/10.1186/s12964-018-0229-y.
Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, Zhao X, Lu C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via mir-21-5p delivery. J Exp Clin Cancer Res. 2019;38:62. https://doi.org/10.1186/s13046-019-1027-0.
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, Grivel JC. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55. https://doi.org/10.1186/s12943-019-0965-7.
Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64. https://doi.org/10.1038/nrc2501.
Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157. https://doi.org/10.1186/s12943-019-1089-9.
Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–8. https://doi.org/10.1016/j.jconrel.2017.09.019.
Prada I, Meldolesi J. Binding and Fusion of Extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17:1296. https://doi.org/10.3390/ijms17081296.
Yang J, Zhang Z, Zhang Y, Ni X, Zhang G, Cui X, Liu M, Xu C, Zhang Q, Zhu H, et al. ZIP4 promotes muscle wasting and Cachexia in mice with Orthotopic pancreatic tumors by stimulating RAB27B-Regulated release of Extracellular vesicles from Cancer cells. Gastroenterology. 2019;156:722–e734726. https://doi.org/10.1053/j.gastro.2018.10.026.
Gao X, Wang Y, Lu F, Chen X, Yang D, Cao Y, Zhang W, Chen J, Zheng L, Wang G, et al. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J Extracell Vesicles. 2021;10:e12060. https://doi.org/10.1002/jev2.12060.
Pin F, Beltrà M, Garcia-Castillo L, Pardini B, Birolo G, Matullo G, Penna F, Guttridge D, Costelli P. Extracellular vesicles derived from tumour cells as a trigger of energy crisis in the skeletal muscle. J Cachexia Sarcopenia Muscle. 2022;13:481–94. https://doi.org/10.1002/jcsm.12844.
Hu W, Xiong H, Ru Z, Zhao Y, Zhou Y, Xie K, Xiao W, Xiong Z, Wang C, Yuan C, et al. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 2021;12:134. https://doi.org/10.1038/s41419-020-03382-0.
Xiong H, Ye J, Xie K, Hu W, Xu N, Yang H. Exosomal IL-8 derived from lung Cancer and Colon Cancer cells induced adipocyte atrophy via NF-κB signaling pathway. Lipids Health Dis. 2022;21:147. https://doi.org/10.1186/s12944-022-01755-2.
Wang G, Li J, Bojmar L, Chen H, Li Z, Tobias GC, Hu M, Homan EA, Lucotti S, Zhao F, et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature. 2023;618:374–82. https://doi.org/10.1038/s41586-023-06114-4.
Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, Xu F, Wang L, Shen Y, Wang T, et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368–79. https://doi.org/10.1093/carcin/bgy115.
Pitzer CR, Paez HG, Alway SE. The contribution of Tumor Derived exosomes to Cancer Cachexia. Cells. 2023;12:292. https://doi.org/10.3390/cells12020292.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. https://doi.org/10.1016/s1470-2045(10)70218-7.
Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer Cachexia: more than skeletal muscle wasting. Trends Cancer. 2018;4:849–60. https://doi.org/10.1016/j.trecan.2018.10.001.
Zhang X, Zhao Y, Yan W. The role of extracellular vesicles in skeletal muscle wasting. J Cachexia Sarcopenia Muscle. 2023;14:2462–72. https://doi.org/10.1002/jcsm.13364.
Calore F, Londhe P, Fadda P, Nigita G, Casadei L, Marceca GP, Fassan M, Lovat F, Gasparini P, Rizzotto L, et al. The TLR7/8/9 antagonist IMO-8503 inhibits Cancer-Induced Cachexia. Cancer Res. 2018;78:6680–90. https://doi.org/10.1158/0008-5472.Can-17-3878.
Kuang JX, Shen Q, Zhang RQ, Fang QY, Deng X, Fan M, Cheng CR, Zhang XW, Liu X. Carnosol attenuated atrophy of C2C12 myotubes induced by tumour-derived exosomal mir-183-5p through inhibiting Smad3 pathway activation and keeping mitochondrial respiration. Basic Clin Pharmacol Toxicol. 2022;131:500–13. https://doi.org/10.1111/bcpt.13795.
Qiu L, Chen W, Wu C, Yuan Y, Li Y. Exosomes of oral squamous cell carcinoma cells containing miR-181a-3p induce muscle cell atrophy and apoptosis by transmissible endoplasmic reticulum stress signaling. Biochem Biophys Res Commun. 2020;533:831–7. https://doi.org/10.1016/j.bbrc.2020.09.066.
Ruan X, Cao M, Yan W, Jones YZ, Gustafsson ÅB, Patel HH, Schenk S, Wang SE. Cancer-cell-secreted extracellular vesicles target p53 to impair mitochondrial function in muscle. EMBO Rep. 2023;24:e56464. https://doi.org/10.15252/embr.202256464.
Wang L, Zhang B, Zheng W, Kang M, Chen Q, Qin W, Li C, Zhang Y, Shao Y, Wu Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7:5384. https://doi.org/10.1038/s41598-017-05541-4.
Rong S, Wang L, Peng Z, Liao Y, Li D, Yang X, Nuessler AK, Liu L, Bao W, Yang W. The mechanisms and treatments for Sarcopenia: could exosomes be a perspective research strategy in the future? J Cachexia Sarcopenia Muscle. 2020;11:348–65. https://doi.org/10.1002/jcsm.12536.
Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6:197–207. https://doi.org/10.1002/jcsm.12043.
Shi X, Yang J, Liu M, Zhang Y, Zhou Z, Luo W, Fung KM, Xu C, Bronze MS, Houchen CW, Li M. Circular RNA ANAPC7 inhibits Tumor Growth and muscle wasting via PHLPP2-AKT-TGF-β Signaling Axis in Pancreatic Cancer. Gastroenterology. 2022;162:2004–17. https://doi.org/10.1053/j.gastro.2022.02.017. e2002.
Webster JM, Kempen L, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during Cachexia. Front Physiol. 2020;11:597675. https://doi.org/10.3389/fphys.2020.597675.
Pucci M, Raimondo S, Urzì O, Moschetti M, Di Bella MA, Conigliaro A, Caccamo N, La Manna MP, Fontana S, Alessandro R. Tumor-derived small Extracellular vesicles induce pro-inflammatory cytokine expression and PD-L1 regulation in M0 macrophages via IL-6/STAT3 and TLR4 signaling pathways. Int J Mol Sci. 2021;22:12118. https://doi.org/10.3390/ijms222212118.
Hu W, Ru Z, Zhou Y, Xiao W, Sun R, Zhang S, Gao Y, Li X, Zhang X, Yang H. Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1091–102. https://doi.org/10.1016/j.bbalip.2019.04.006.
Kasprzak A. The role of Tumor Microenvironment cells in Colorectal Cancer (CRC) Cachexia. Int J Mol Sci. 2021;22:1565. https://doi.org/10.3390/ijms22041565.
Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, Chen X, Yao X, Gu X, Qi L, et al. Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol. 2023;214:115664. https://doi.org/10.1016/j.bcp.2023.115664.
Hardee JP, Carson JA. Muscular contraction’s therapeutic potential for cancer-induced wasting. Am J Physiol Cell Physiol. 2022;323:C378–84. https://doi.org/10.1152/ajpcell.00021.2022.
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. https://doi.org/10.1038/nature13528.
Liu A, Pan W, Zhuang S, Tang Y, Zhang H. Cancer cell-derived exosomal mir-425-3p induces white adipocyte atrophy. Adipocyte. 2022;11:487–500. https://doi.org/10.1080/21623945.2022.2108558.
Di W, Zhang W, Zhu B, Li X, Tang Q, Zhou Y. Colorectal cancer prompted adipose tissue browning and cancer cachexia through transferring exosomal miR-146b-5p. J Cell Physiol. 2021;236:5399–410. https://doi.org/10.1002/jcp.30245.
Wan Z, Chen X, Gao X, Dong Y, Zhao Y, Wei M, Fan W, Yang G, Liu L. Chronic myeloid leukemia-derived exosomes attenuate adipogenesis of adipose derived mesenchymal stem cells via transporting miR-92a-3p. J Cell Physiol. 2019;234:21274–83. https://doi.org/10.1002/jcp.28732.
Sun S, Wang Z, Yao F, Sun K, Li Z, Sun S, Li C. Breast cancer cell-derived exosome-delivered microRNA-155 targets UBQLN1 in adipocytes and facilitates cancer cachexia-related fat loss. Hum Mol Genet. 2023;32:2219–28. https://doi.org/10.1093/hmg/ddad055.
Liu Y, Wang M, Deng T, Liu R, Ning T, Bai M, Ying G, Zhang H, Ba Y. Exosomal miR-155 from gastric cancer induces cancer-associated cachexia by suppressing adipogenesis and promoting brown adipose differentiation via C/EPBβ. Cancer Biol Med. 2022;19:1301–14. https://doi.org/10.20892/j.issn.2095-3941.2021.0220.
Sun D, Ding Z, Shen L, Yang F, Han J, Wu G. Mir-410-3P inhibits adipocyte differentiation by targeting IRS-1 in cancer-associated cachexia patients. Lipids Health Dis. 2021;20:115. https://doi.org/10.1186/s12944-021-01530-9.
Hu Y, Liu L, Chen Y, Zhang X, Zhou H, Hu S, Li X, Li M, Li J, Cheng S, et al. Cancer-cell-secreted mir-204-5p induces leptin signalling pathway in white adipose tissue to promote cancer-associated cachexia. Nat Commun. 2023;14:5179. https://doi.org/10.1038/s41467-023-40571-9.
Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST, Mukhopadhyay D. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65:1165–74. https://doi.org/10.1136/gutjnl-2014-308350.
Kong F, Li L, Du Y, Zhu H, Li Z, Kong X. Exosomal adrenomedullin derived from cancer-associated fibroblasts promotes lipolysis in adipose tissue. Gut. 2018;67:2226–7. https://doi.org/10.1136/gutjnl-2017-315778.
Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, Sabet S, Khoshbakht MA, Hashemi M, Hushmandi K, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15:83. https://doi.org/10.1186/s13045-022-01305-4.
Hayasaka R, Tabata S, Hasebe M, Ikeda S, Ohnuma S, Mori M, Soga T, Tomita M, Hirayama A. Metabolomic analysis of small extracellular vesicles derived from pancreatic Cancer cells cultured under Normoxia and Hypoxia. Metabolites. 2021;11:215. https://doi.org/10.3390/metabo11040215.
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96. https://doi.org/10.1038/s41577-021-00547-6.
Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. https://doi.org/10.1146/annurev-immunol-032713-120145.
Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiol (Bethesda). 2010;25:85–101. https://doi.org/10.1152/physiol.00045.2009.
Paval DR, Patton R, McDonald J, Skipworth RJE, Gallagher IJ, Laird BJ. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. 2022;13:824–38. https://doi.org/10.1002/jcsm.12912.
Singh SK, Singh R. Cytokines and chemokines in Cancer Cachexia and its long-term impact on COVID-19. Cells. 2022;11:579. https://doi.org/10.3390/cells11030579.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Terlecki G, Gamian A. Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med. 2008;46:359–64. https://doi.org/10.1515/cclm.2008.089.
Balsano R, Kruize Z, Lunardi M, Comandatore A, Barone M, Cavazzoni A, Re Cecconi AD, Morelli L, Wilmink H, Tiseo M, et al. Transforming growth factor-Beta signaling in Cancer-Induced Cachexia: from Molecular pathways to the clinics. Cells. 2022;11:2671. https://doi.org/10.3390/cells11172671.
Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol. 2022;22:309–21. https://doi.org/10.1038/s41577-021-00624-w.
VanderVeen BN, Murphy EA, Carson JA. The impact of Immune cells on the skeletal muscle Microenvironment during Cancer Cachexia. Front Physiol. 2020;11:1037. https://doi.org/10.3389/fphys.2020.01037.
Preuss SF, Grieshober D, Augustin HG. Systemic reprogramming of endothelial cell signaling in Metastasis and Cachexia. Physiol (Bethesda). 2023;38:0. https://doi.org/10.1152/physiol.00001.2023.
Dalla PV, Santos J, Milthorpe BK, Padula MP. Selectively-packaged proteins in breast Cancer Extracellular vesicles involved in Metastasis. Int J Mol Sci. 2020;21:4990. https://doi.org/10.3390/ijms21144990.
Chitti SV, Fonseka P, Mathivanan S. Emerging role of extracellular vesicles in mediating cancer cachexia. Biochem Soc Trans. 2018;46:1129–36. https://doi.org/10.1042/bst20180213.
Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22:560–70. https://doi.org/10.1038/s41590-021-00899-0.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18. https://doi.org/10.1038/cr.2016.151.
Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–39. https://doi.org/10.1016/j.it.2015.02.004.
Cózar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11:34–44. https://doi.org/10.1158/2159-8290.Cd-20-0655.
Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20:154. https://doi.org/10.1186/s12943-021-01463-y.
Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, Wen SW, Wiegmans AP, Möller A. Breast Cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front Immunol. 2018;9:871. https://doi.org/10.3389/fimmu.2018.00871.
Jorquera-Cordero C, Lara P, Cruz LJ, Schomann T, van Hofslot A, de Carvalho TG, Guedes P, Creemers L, Koning RI, Chan AB, de Araujo Junior RF. Extracellular vesicles from M1-Polarized macrophages combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated macrophages in breast Cancer. Pharmaceutics. 2022;14:1068. https://doi.org/10.3390/pharmaceutics14051068.
Zhang J, Zheng J, Chen H, Li X, Ye C, Zhang F, Zhang Z, Yao Q, Guo Y. Curcumin Targeting NF-κB/Ubiquitin-Proteasome-System Axis Ameliorates Muscle Atrophy in Triple-Negative Breast Cancer Cachexia Mice. Mediators Inflamm 2022, 2022:2567150. https://doi.org/10.1155/2022/2567150.
Vaes RDW, van Dijk DPJ, Farshadi EA, Olde Damink SWM, Rensen SS, Langen RC. Human pancreatic tumour organoid-derived factors enhance myogenic differentiation. J Cachexia Sarcopenia Muscle. 2022;13:1302–13. https://doi.org/10.1002/jcsm.12917.
Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41. https://doi.org/10.1016/j.semcdb.2016.02.009.
Ying L, Yao Y, Lv H, Lu G, Zhang Q, Yang Y, Zhou J. IL-17A contributes to skeletal muscle atrophy in lung cancer-induced cachexia via JAK2/STAT3 pathway. Am J Physiol Cell Physiol. 2022;322:C814–24. https://doi.org/10.1152/ajpcell.00463.2021.
Henriques F, Lopes MA, Franco FO, Knobl P, Santos KB, Bueno LL, Correa VA, Bedard AH, Guilherme A, Birbrair A, et al. Toll-like Receptor-4 disruption suppresses adipose tissue remodeling and increases survival in Cancer Cachexia Syndrome. Sci Rep. 2018;8:18024. https://doi.org/10.1038/s41598-018-36626-3.
McCarty MF, Iloki-Assanga S, Lujany LML. Nutraceutical targeting of TLR4 signaling has potential for prevention of cancer cachexia. Med Hypotheses. 2019;132:109326. https://doi.org/10.1016/j.mehy.2019.109326.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. https://doi.org/10.7554/eLife.10250.
Jiang C, Jiang Z, Sha G, Wang D, Tang D. Small extracellular vesicle-mediated metabolic reprogramming: from tumors to pre-metastatic niche formation. Cell Commun Signal. 2023;21:116. https://doi.org/10.1186/s12964-023-01136-x.
Shibata C, Otsuka M, Seimiya T, Kishikawa T, Ishigaki K, Fujishiro M. Lipolysis by pancreatic cancer-derived extracellular vesicles in cancer-associated cachexia via specific integrins. Clin Transl Med. 2022;12:e1089. https://doi.org/10.1002/ctm2.1089.
Beltrà M, Pin F, Ballarò R, Costelli P, Penna F. Mitochondrial dysfunction in Cancer Cachexia: impact on muscle health and regeneration. Cells. 2021;10:3150. https://doi.org/10.3390/cells10113150.
Crewe C, Funcke JB, Li S, Joffin N, Gliniak CM, Ghaben AL, An YA, Sadek HA, Gordillo R, Akgul Y, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33:1853–e18681811. https://doi.org/10.1016/j.cmet.2021.08.002.
Guo J, Duan Z, Zhang C, Wang W, He H, Liu Y, Wu P, Wang S, Song M, Chen H, et al. Mouse 4T1 breast Cancer cell-derived exosomes induce Proinflammatory Cytokine production in Macrophages via miR-183. J Immunol. 2020;205:2916–25. https://doi.org/10.4049/jimmunol.1901104.
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. https://doi.org/10.1038/nrclinonc.2012.209.
Dalton B, Leppanen J, Campbell IC, Chung R, Breen G, Schmidt U, Himmerich H. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav Immun. 2020;85:88–95. https://doi.org/10.1016/j.bbi.2019.05.012.
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. Faseb j. 1998;12:871–80. https://doi.org/10.1096/fasebj.12.10.971.
Zhang W, Sun W, Gu X, Miao C, Feng L, Shen Q, Liu X, Zhang X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022;8:162. https://doi.org/10.1038/s41420-022-00972-z.
Talbert EE, Guttridge DC. Emerging signaling mediators in the anorexia-cachexia syndrome of cancer. Trends Cancer. 2022;8:397–403. https://doi.org/10.1016/j.trecan.2022.01.004.
Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. Cancer-Associated neurogenesis and nerve-Cancer cross-talk. Cancer Res. 2021;81:1431–40. https://doi.org/10.1158/0008-5472.Can-20-2793.
Zhang XH, Tang LY, Wang XY, Shen CL, Xiong WF, Shen Y, Wan YH, Wu YB, Wang YC, Zhang HX, et al. ADGRA1 negatively regulates energy expenditure and thermogenesis through both sympathetic nervous system and hypothalamus-pituitary-thyroid axis in male mice. Cell Death Dis. 2021;12:362. https://doi.org/10.1038/s41419-021-03634-7.
Delbono O, Rodrigues ACZ, Bonilla HJ, Messi ML. The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia. Ageing Res Rev. 2021;67:101305. https://doi.org/10.1016/j.arr.2021.101305.
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in Cancer: cell-to-cell mediators of Metastasis. Cancer Cell. 2016;30:836–48. https://doi.org/10.1016/j.ccell.2016.10.009.
López M. Hypothalamic AMPK as a possible target for energy balance-related diseases. Trends Pharmacol Sci. 2022;43:546–56. https://doi.org/10.1016/j.tips.2022.04.007.
Milbank E, Dragano NRV, González-García I, Garcia MR, Rivas-Limeres V, Perdomo L, Hilairet G, Ruiz-Pino F, Mallegol P, Morgan DA, et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKα1 corrects obesity through BAT activation. Nat Metab. 2021;3:1415–31. https://doi.org/10.1038/s42255-021-00467-8.
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–47. https://doi.org/10.1016/j.cmet.2014.06.011.
Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15:9–20. https://doi.org/10.1038/s41574-018-0123-0.
Kir S, Spiegelman BM. CACHEXIA & BROWN FAT: A BURNING ISSUE IN CANCER. Trends Cancer. 2016;2:461–3. https://doi.org/10.1016/j.trecan.2016.07.005.
Chiappalupi S, Sorci G, Vukasinovic A, Salvadori L, Sagheddu R, Coletti D, Renga G, Romani L, Donato R, Riuzzi F. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J Cachexia Sarcopenia Muscle. 2020;11:929–46. https://doi.org/10.1002/jcsm.12561.
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. https://doi.org/10.1016/j.cell.2010.07.011.
Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. https://doi.org/10.1016/j.cmet.2012.06.011.
Talbert EE, Lewis HL, Farren MR, Ramsey ML, Chakedis JM, Rajasekera P, Haverick E, Sarna A, Bloomston M, Pawlik TM, et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2018;9:358–68. https://doi.org/10.1002/jcsm.12251.
Han J, Meng Q, Shen L, Wu G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17:14. https://doi.org/10.1186/s12944-018-0657-0.
Morigny P, Zuber J, Haid M, Kaltenecker D, Riols F, Lima JDC, Simoes E, Otoch JP, Schmidt SF, Herzig S, et al. High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J Cachexia Sarcopenia Muscle. 2020;11:1459–75. https://doi.org/10.1002/jcsm.12626.
Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. 2018;234:13–22. https://doi.org/10.1002/jcp.26811.
Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J, van Helvoort A, Smidt ML, Kruitwagen R, Baade-Corpelijn L, et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle. 2021;12:2007–21. https://doi.org/10.1002/jcsm.12804.
van de Worp W, Schols A, Dingemans AC, Op den Kamp CMH, Degens J, Kelders M, Coort S, Woodruff HC, Kratassiouk G, Harel-Bellan A, et al. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2020;11:452–63. https://doi.org/10.1002/jcsm.12512.
Donzelli S, Farneti A, Marucci L, Ganci F, Sacconi A, Strano S, Sanguineti G, Blandino G. Non-coding RNAs as putative biomarkers of Cancer-Associated Cachexia. Front Cell Dev Biol. 2020;8:257. https://doi.org/10.3389/fcell.2020.00257.
Santos JMO, Peixoto da Silva S, Gil da Costa RM, Medeiros R. The emerging role of MicroRNAs and other non-coding RNAs in Cancer Cachexia. Cancers (Basel). 2020;12:1004. https://doi.org/10.3390/cancers12041004.
Loumaye A, Thissen JP. Biomarkers of cancer cachexia. Clin Biochem. 2017;50:1281–8. https://doi.org/10.1016/j.clinbiochem.2017.07.011.
Cao Z, Zhao K, Jose I, Hoogenraad NJ, Osellame LD. Biomarkers for Cancer Cachexia: a Mini Review. Int J Mol Sci. 2021;22:4501. https://doi.org/10.3390/ijms22094501.
Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, Harrison CA, Gregorevic P. Differential effects of IL6 and activin A in the development of Cancer-Associated Cachexia. Cancer Res. 2016;76:5372–82. https://doi.org/10.1158/0008-5472.Can-15-3152.
Suh SY, Choi YS, Yeom CH, Kwak SM, Yoon HM, Kim DG, Koh SJ, Park J, Lee MA, Lee YJ, et al. Interleukin-6 but not tumour necrosis factor-alpha predicts survival in patients with advanced cancer. Support Care Cancer. 2013;21:3071–7. https://doi.org/10.1007/s00520-013-1878-4.
Batista ML Jr., Olivan M, Alcantara PS, Sandoval R, Peres SB, Neves RX, Silverio R, Maximiano LF, Otoch JP, Seelaender M. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine. 2013;61:532–9. https://doi.org/10.1016/j.cyto.2012.10.023.
Stephens NA, Skipworth RJ, Gallagher IJ, Greig CA, Guttridge DC, Ross JA, Fearon KC. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:53–61. https://doi.org/10.1002/jcsm.12005.
Hou YC, Wang CJ, Chao YJ, Chen HY, Wang HC, Tung HL, Lin JT, Shan YS. Elevated serum Interleukin-8 level correlates with Cancer-Related Cachexia and Sarcopenia: an Indicator for Pancreatic Cancer outcomes. J Clin Med. 2018;7:502. https://doi.org/10.3390/jcm7120502.
Kim H, Kim KM, Kang MJ, Lim S. Growth differentiation factor-15 as a biomarker for Sarcopenia in aging humans and mice. Exp Gerontol. 2020;142:111115. https://doi.org/10.1016/j.exger.2020.111115.
Monitto CL, Dong SM, Jen J, Sidransky D. Characterization of a human homologue of proteolysis-inducing factor and its role in cancer cachexia. Clin Cancer Res. 2004;10:5862–9. https://doi.org/10.1158/1078-0432.Ccr-04-0435.
Gonçalves EM, Salomão EM, Gomes-Marcondes MC. Leucine modulates the effect of Walker factor, a proteolysis-inducing factor-like protein from Walker tumours, on gene expression and cellular activity in C2C12 myotubes. Cytokine. 2013;64:343–50. https://doi.org/10.1016/j.cyto.2013.05.018.
Wang B, Xu J, Ren Q, Cheng L, Guo F, Liang Y, Yang L, Tan Z, Fu P, Ma L. Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis. Cell Death Dis. 2022;13:333. https://doi.org/10.1038/s41419-022-04794-w.
de Castro GS, Correia-Lima J, Simoes E, Orsso CE, Xiao J, Gama LR, Gomes SP, Gonçalves DC, Costa RGF, Radloff K, et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin Nutr. 2021;40:2443–55. https://doi.org/10.1016/j.clnu.2020.10.050.
Fischer H, Gustafsson T, Sundberg CJ, Norrbom J, Ekman M, Johansson O, Jansson E. Fatty acid binding protein 4 in human skeletal muscle. Biochem Biophys Res Commun. 2006;346:125–30. https://doi.org/10.1016/j.bbrc.2006.05.083.
Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, Zhu ZJ, Flores R, Wen Y, Gong X, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8:589. https://doi.org/10.1038/s41467-017-00726-x.
Caruso Bavisotto C, Marino Gammazza A, Campanella C, Bucchieri F, Cappello F. Extracellular heat shock proteins in cancer: from early diagnosis to new therapeutic approach. Semin Cancer Biol. 2022;86:36–45. https://doi.org/10.1016/j.semcancer.2021.09.010.
Niu M, Song S, Su Z, Wei L, Li L, Pu W, Zhao C, Ding Y, Wang J, Cao W, et al. Inhibition of heat shock protein (HSP) 90 reverses signal transducer and activator of transcription (STAT) 3-mediated muscle wasting in cancer cachexia mice. Br J Pharmacol. 2021;178:4485–500. https://doi.org/10.1111/bph.15625.
Thakur SS, Swiderski K, Ryall JG, Lynch GS. Therapeutic potential of heat shock protein induction for muscular dystrophy and other muscle wasting conditions. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160528. https://doi.org/10.1098/rstb.2016.0528.
Ihnatko R, Post C, Blomqvist A. Proteomic profiling of the hypothalamus in a mouse model of cancer-induced anorexia-cachexia. Br J Cancer. 2013;109:1867–75. https://doi.org/10.1038/bjc.2013.525.
Ren B, Luo S, Xu F, Zou G, Xu G, He J, Huang Y, Zhu H, Li Y. The expression of DAMP proteins HSP70 and cancer-testis antigen SPAG9 in peripheral blood of patients with HCC and lung cancer. Cell Stress Chaperones. 2017;22:237–44. https://doi.org/10.1007/s12192-016-0758-5.
Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, et al. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1733–7. https://doi.org/10.1158/1055-9965.Epi-06-0005.
Dutta SK, Girotra M, Singla M, Dutta A, Otis Stephen F, Nair PP, Merchant NB. Serum HSP70: a novel biomarker for early detection of pancreatic cancer. Pancreas. 2012;41:530–4. https://doi.org/10.1097/MPA.0b013e3182374ace.
Kocsis J, Mészáros T, Madaras B, Tóth EK, Kamondi S, Gál P, Varga L, Prohászka Z, Füst G. High levels of acute phase proteins and soluble 70 kDa heat shock proteins are independent and additive risk factors for mortality in colorectal cancer. Cell Stress Chaperones. 2011;16:49–55. https://doi.org/10.1007/s12192-010-0220-z.
Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409–18. https://doi.org/10.1002/gcc.21926.
Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–6. https://doi.org/10.3816/CLC.2009.n.006.
Barone R, Caruso Bavisotto C, Rappa F, Gargano ML, Macaluso F, Paladino L, Vitale AM, Alfano S, Campanella C, Gorska M, et al. JNK pathway and heat shock response mediate the survival of C26 colon carcinoma bearing mice fed with the mushroom Pleurotus Eryngii var. Eryngii without affecting tumor growth or cachexia. Food Funct. 2021;12:3083–95. https://doi.org/10.1039/d0fo03171b.
Crawford Parks TE, Ravel-Chapuis A, Bondy-Chorney E, Renaud JM, Côté J, Jasmin BJ. Muscle-specific expression of the RNA-binding protein Staufen1 induces progressive skeletal muscle atrophy via regulation of phosphatase tensin homolog. Hum Mol Genet. 2017;26:1821–38. https://doi.org/10.1093/hmg/ddx085.
Choi S, Jeong HJ, Kim H, Choi D, Cho SC, Seong JK, Koo SH, Kang JS. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy. 2019;15:1069–81. https://doi.org/10.1080/15548627.2019.1569931.
Loumaye A, de Barsy M, Nachit M, Lause P, Frateur L, van Maanen A, Trefois P, Gruson D, Thissen JP. Role of activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100:2030–8. https://doi.org/10.1210/jc.2014-4318.
Luan Y, Zhang Y, Yu SY, You M, Xu PC, Chung S, Kurita T, Zhu J, Kim SY. Development of ovarian tumour causes significant loss of muscle and adipose tissue: a novel mouse model for cancer cachexia study. J Cachexia Sarcopenia Muscle. 2022;13:1289–301. https://doi.org/10.1002/jcsm.12864.
Kalinkovich A, Livshits G. Sarcopenia–The search for emerging biomarkers. Ageing Res Rev. 2015;22:58–71. https://doi.org/10.1016/j.arr.2015.05.001.
Toden S, Zumwalt TJ, Goel A. Non-coding RNAs and potential therapeutic targeting in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188491. https://doi.org/10.1016/j.bbcan.2020.188491.
Du G, Zhang Y, Hu S, Zhou X, Li Y. Non-coding RNAs in exosomes and adipocytes cause fat loss during cancer cachexia. Noncoding RNA Res. 2021;6:80–5. https://doi.org/10.1016/j.ncrna.2021.04.001.
Krauss T, Heisz S, Honecker J, Prokopchuk O, Martignoni M, Janssen KP, Claussnitzer M, Hauner H, Seeliger C. Specific miRNAs are associated with human cancer cachexia in an organ-specific manner. J Cachexia Sarcopenia Muscle. 2023;14:1381–94. https://doi.org/10.1002/jcsm.13224.
Siqueira IR, Batabyal RA, Freishtat R, Cechinel LR. Potential involvement of circulating extracellular vesicles and particles on exercise effects in malignancies. Front Endocrinol (Lausanne). 2023;14:1121390. https://doi.org/10.3389/fendo.2023.1121390.
Hashemi M, Moosavi MS, Abed HM, Dehghani M, Aalipour M, Heydari EA, Behroozaghdam M, Entezari M, Salimimoghadam S, Gunduz ES, et al. Long non-coding RNA (lncRNA) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol Res. 2022;184:106418. https://doi.org/10.1016/j.phrs.2022.106418.
Zhao W, Zhang Y, Zhang W, Sun Y, Zheng B, Wang J, Gu Y, Qi J, Li J, Wang XJ, et al. Exosomal LINC00355 promotes the malignant progression of gastric cancer through histone deacetylase HDAC3-mediated TP53INP1 transcriptional inhibition. Life Sci. 2023;315:121387. https://doi.org/10.1016/j.lfs.2023.121387.
Han J, Shen L, Zhan Z, Liu Y, Zhang C, Guo R, Luo Y, Xie Z, Feng Y, Wu G. The long noncoding RNA MALAT1 modulates adipose loss in cancer-associated cachexia by suppressing adipogenesis through PPAR-γ. Nutr Metab (Lond). 2021;18:27. https://doi.org/10.1186/s12986-021-00557-0.
Zhang C, Xu L, Deng G, Ding Y, Bi K, Jin H, Shu J, Yang J, Deng H, Wang Z, Wang Y. Exosomal HOTAIR promotes proliferation, migration and invasion of lung cancer by sponging miR-203. Sci China Life Sci. 2020;63:1265–8. https://doi.org/10.1007/s11427-019-1579-x.
Xu J, Xiao Y, Liu B, Pan S, Liu Q, Shan Y, Li S, Qi Y, Huang Y, Jia L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer Res. 2020;39:54. https://doi.org/10.1186/s13046-020-01562-6.
Ding Z, Sun D, Han J, Shen L, Yang F, Sah S, Sui X, Wu G. Novel noncoding RNA CircPTK2 regulates lipolysis and adipogenesis in cachexia. Mol Metab. 2021;53:101310. https://doi.org/10.1016/j.molmet.2021.101310.
Camargo RG, Quintas Teixeira Ribeiro H, Geraldo MV, Matos-Neto E, Neves RX, Carnevali LC Jr., Donatto FF, Alcântara PS, Ottoch JP, Seelaender M. Cancer Cachexia and MicroRNAs. Mediators Inflamm 2015, 2015:367561. https://doi.org/10.1155/2015/367561.
Belli R, Ferraro E, Molfino A, Carletti R, Tambaro F, Costelli P, Muscaritoli M. Liquid Biopsy for Cancer Cachexia: focus on muscle-derived microRNAs. Int J Mol Sci. 2021;22:9007. https://doi.org/10.3390/ijms22169007.
Mytidou C, Koutsoulidou A, Katsioloudi A, Prokopi M, Kapnisis K, Michailidou K, Anayiotos A, Phylactou LA. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. Faseb j. 2021;35:e21279. https://doi.org/10.1096/fj.201902468RR.
Fan TWM, Zhang X, Wang C, Yang Y, Kang WY, Arnold S, Higashi RM, Liu J, Lane AN. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Anal Chim Acta. 2018;1037:256–64. https://doi.org/10.1016/j.aca.2018.02.051.
Tao L, Zhou J, Yuan C, Zhang L, Li D, Si D, Xiu D, Zhong L. Metabolomics identifies serum and exosomes metabolite markers of pancreatic cancer. Metabolomics. 2019;15:86. https://doi.org/10.1007/s11306-019-1550-1.
Nishida-Aoki N, Izumi Y, Takeda H, Takahashi M, Ochiya T, Bamba T. Lipidomic Analysis of Cells and extracellular vesicles from high- and low-metastatic triple-negative breast Cancer. Metabolites. 2020;10:67. https://doi.org/10.3390/metabo10020067.
Eylem CC, Yilmaz M, Derkus B, Nemutlu E, Camci CB, Yilmaz E, Turkoglu MA, Aytac B, Ozyurt N, Emregul E. Untargeted multi-omic analysis of colorectal cancer-specific exosomes reveals joint pathways of colorectal cancer in both clinical samples and cell culture. Cancer Lett. 2020;469:186–94. https://doi.org/10.1016/j.canlet.2019.10.038.
Elmallah MIY, Ortega-Deballon P, Hermite L, Pais-De-Barros JP, Gobbo J, Garrido C. Lipidomic profiling of exosomes from colorectal cancer cells and patients reveals potential biomarkers. Mol Oncol. 2022;16:2710–8. https://doi.org/10.1002/1878-0261.13223.
Bestard-Escalas J, Reigada R, Reyes J, de la Torre P, Liebisch G, Barceló-Coblijn G. Fatty acid unsaturation degree of plasma exosomes in Colorectal Cancer patients: a promising biomarker. Int J Mol Sci. 2021;22:5060. https://doi.org/10.3390/ijms22105060.
Van Soom T, Tjalma W, Papadimitriou K, Gebruers N, van Breda E. The effects of chemotherapy on resting energy expenditure, body composition, and cancer-related fatigue in women with breast cancer: a prospective cohort study. Cancer Metab. 2023;11:21. https://doi.org/10.1186/s40170-023-00322-2.
Hu W, Ru Z, Xiao W, Xiong Z, Wang C, Yuan C, Zhang X, Yang H. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem Biophys Res Commun. 2018;506:122–9. https://doi.org/10.1016/j.bbrc.2018.09.139.
Fan M, Sun W, Gu X, Lu S, Shen Q, Liu X, Zhang X. The critical role of STAT3 in biogenesis of tumor-derived exosomes with potency of inducing cancer cachexia in vitro and in vivo. Oncogene. 2022;41:1050–62. https://doi.org/10.1038/s41388-021-02151-3.
Fan M, Gu X, Zhang W, Shen Q, Zhang R, Fang Q, Wang Y, Guo X, Zhang X, Liu X. Atractylenolide I ameliorates cancer cachexia through inhibiting biogenesis of IL-6 and tumour-derived extracellular vesicles. J Cachexia Sarcopenia Muscle. 2022;13:2724–39. https://doi.org/10.1002/jcsm.13079.
Zhou L, Zhang T, Shao W, Lu R, Wang L, Liu H, Jiang B, Li S, Zhuo H, Wang S, et al. Amiloride ameliorates muscle wasting in cancer cachexia through inhibiting tumor-derived exosome release. Skelet Muscle. 2021;11:17. https://doi.org/10.1186/s13395-021-00274-5.
Liu Z, Xiong J, Gao S, Zhu MX, Sun K, Li M, Zhang G, Li YP. Ameliorating cancer cachexia by inhibiting cancer cell release of Hsp70 and Hsp90 with omeprazole. J Cachexia Sarcopenia Muscle. 2022;13:636–47. https://doi.org/10.1002/jcsm.12851.
Dai H, Zheng W, Luo J, Yu G, Song C, Wu Y, Xu J. Inhibiting uptake of extracellular vesicles derived from senescent bone marrow mesenchymal stem cells by muscle satellite cells attenuates Sarcopenia. J Orthop Translat. 2022;35:23–36. https://doi.org/10.1016/j.jot.2022.06.002.
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other Extracellular vesicles as new remedies in the Therapy of Inflammatory diseases. Cells. 2019;8:1605. https://doi.org/10.3390/cells8121605.
Wang S, Lei B, Zhang E, Gong P, Gu J, He L, Han L, Yuan Z. Targeted therapy for inflammatory diseases with mesenchymal stem cells and their derived exosomes: from Basic to Clinics. Int J Nanomed. 2022;17:1757–81. https://doi.org/10.2147/ijn.S355366.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. https://doi.org/10.1038/nri3622.
Cargnoni A, Papait A, Masserdotti A, Pasotti A, Stefani FR, Silini AR, Parolini O. Extracellular vesicles from perinatal cells for anti-inflammatory therapy. Front Bioeng Biotechnol. 2021;9:637737. https://doi.org/10.3389/fbioe.2021.637737.
Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. https://doi.org/10.1016/j.intimp.2021.107823.
Marinho R, Alcântara PSM, Ottoch JP, Seelaender M. Role of exosomal MicroRNAs and myomiRs in the development of Cancer Cachexia-Associated muscle wasting. Front Nutr. 2017;4:69. https://doi.org/10.3389/fnut.2017.00069.
Ragni E, Papait A, Perucca Orfei C, Silini AR, Colombini A, Viganò M, Libonati F, Parolini O, de Girolamo L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10:1044–62. https://doi.org/10.1002/sctm.20-0390.
Tsitkanou S, Murach KA, Washington TA, Greene NP. Exercise counteracts the deleterious effects of Cancer Cachexia. Cancers (Basel). 2022;14:2512. https://doi.org/10.3390/cancers14102512.
Leal LG, Lopes MA, Peres SB, Batista ML Jr. Exercise Training as Therapeutic Approach in Cancer Cachexia: a review of potential anti-inflammatory effect on muscle wasting. Front Physiol. 2020;11:570170. https://doi.org/10.3389/fphys.2020.570170.
Cortiula F, Hendriks LEL, van de Worp W, Schols A, Vaes RDW, Langen RCJ, De Ruysscher D. Physical exercise at the crossroad between muscle wasting and the immune system: implications for lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2022;13:55–67. https://doi.org/10.1002/jcsm.12900.
Denham J, Spencer SJ. Emerging roles of extracellular vesicles in the intercellular communication for exercise-induced adaptations. Am J Physiol Endocrinol Metab. 2020;319:E320–9. https://doi.org/10.1152/ajpendo.00215.2020.
Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: insights from Exercise Science. Front Physiol. 2020;11:604274. https://doi.org/10.3389/fphys.2020.604274.
Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. https://doi.org/10.3402/jev.v4.28239.
Vechetti IJ Jr., Valentino T, Mobley CB, McCarthy JJ. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J Physiol. 2021;599:845–61. https://doi.org/10.1113/jp278929.
Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, Luchetti F, Papa S, Stocchi V. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs in the Bloodstream. PLoS ONE. 2015;10:e0125094. https://doi.org/10.1371/journal.pone.0125094.
Chu-Tan JA, Kirkby M, Natoli R. Running to save sight: the effects of exercise on retinal health and function. Clin Exp Ophthalmol. 2022;50:74–90. https://doi.org/10.1111/ceo.14023.
Wang Y, Liu Y, Zhang S, Li N, Xing C, Wang C, Wang J, Wei M, Yang G, Yuan L. Exercise improves metabolism and alleviates atherosclerosis via muscle-derived extracellular vesicles. Aging Dis. 2023;14:952–65. https://doi.org/10.14336/ad.2022.1131.
Wang D, Zhang X, Li Y, Jia L, Zhai L, Wei W, Zhang L, Jiang H, Bai Y. Exercise-Induced Browning of White Adipose tissue and improving skeletal muscle insulin sensitivity in Obese/Non-obese growing mice: do not neglect exosomal miR-27a. Front Nutr. 2022;9:940673. https://doi.org/10.3389/fnut.2022.940673.
Barone R, Macaluso F, Sangiorgi C, Campanella C, Marino Gammazza A, Moresi V, Coletti D, Conway de Macario E, Macario AJ, Cappello F, et al. Skeletal muscle heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Sci Rep. 2016;6:19781. https://doi.org/10.1038/srep19781.
Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–6. https://doi.org/10.1016/j.tips.2007.02.002.
Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12:504–17. https://doi.org/10.1038/nrendo.2016.76.
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–91. https://doi.org/10.1016/j.cell.2014.03.065.
Trovato E, Di Felice V, Barone R. Extracellular vesicles: Delivery vehicles of Myokines. Front Physiol. 2019;10:522. https://doi.org/10.3389/fphys.2019.00522.
Ye Q, Qiu X, Wang J, Xu B, Su Y, Zheng C, Gui L, Yu L, Kuang H, Liu H, et al. MSCs-derived apoptotic extracellular vesicles promote muscle regeneration by inducing Pannexin 1 channel-dependent creatine release by myoblasts. Int J Oral Sci. 2023;15:7. https://doi.org/10.1038/s41368-022-00205-0.
Song J, Liu J, Cui C, Hu H, Zang N, Yang M, Yang J, Zou Y, Li J, Wang L, et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia Muscle. 2023;14:915–29. https://doi.org/10.1002/jcsm.13177.
Cho KA, Choi DW, Kim YH, Kim J, Ryu KH, Woo SY. Mesenchymal stem cell-derived exosomes protect muscle loss by mir-145-5p activity targeting activin A receptors. Cells. 2021;10:2169. https://doi.org/10.3390/cells10082169.
Magarotto F, Sgrò A, Dorigo Hochuli AH, Andreetta M, Grassi M, Saggioro M, Nogara L, Tolomeo AM, Francescato R, Collino F, et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials. 2021;269:120653. https://doi.org/10.1016/j.biomaterials.2021.120653.
Choi JS, Yoon HI, Lee KS, Choi YC, Yang SH, Kim IS, Cho YW. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J Control Release. 2016;222:107–15. https://doi.org/10.1016/j.jconrel.2015.12.018.
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lötvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–710. https://doi.org/10.1021/nn402232g.
Kim YS, Kim JY, Cho R, Shin DM, Lee SW, Oh YM. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med. 2017;49:e284. https://doi.org/10.1038/emm.2016.127.
García-Fernández J, Fuente Freire M. Exosome-like systems: nanotechnology to overcome challenges for targeted cancer therapies. Cancer Lett. 2023;561:216151. https://doi.org/10.1016/j.canlet.2023.216151.
Gupta D, Boora A, Thakur A, Gupta TK. Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ Res. 2023;231:116316. https://doi.org/10.1016/j.envres.2023.116316.
Khalilov R, Bakishzade A, Nasibova AJAB, Sciences E. FUTURE PROSPECTS OF BIOMATERIALS IN NANOMEDICINE. Adv Biology Earth Sci. 2024;9:5–10. https://doi.org/10.62476/abes.9s5.
Rosic G. Cancer signaling, cell/gene therapy, diagnosis and role of nanobiomaterials. Adv Biology Earth Sci. 2024;9:11–34. https://doi.org/10.62476/abes9s11.
Huseynov E, NOVEL NANOMATERIALS FOR HEPATOBILIARY DISEASES TREATMENT AND FUTURE PERSPECTIVES. Adv Biology Earth Sci. 2024;9:81–91. https://doi.org/10.62476/abes9s81.
Tang TT, Wang B, Lv LL, Liu BC. Extracellular vesicle-based Nanotherapeutics: emerging frontiers in anti-inflammatory therapy. Theranostics. 2020;10:8111–29. https://doi.org/10.7150/thno.47865.
Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q, Moulton HM, Seow Y, Yin H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:eaat0195. https://doi.org/10.1126/scitranslmed.aat0195.
Ran N, Gao X, Dong X, Li J, Lin C, Geng M, Yin H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials. 2020;236:119826. https://doi.org/10.1016/j.biomaterials.2020.119826.
Di Felice V, Barone R, Trovato E, D’Amico D, Macaluso F, Campanella C, Marino Gammazza A, Muccilli V, Cunsolo V, Cancemi P, et al. Physiactisome: a New Nanovesicle Drug Containing Heat shock protein 60 for treating muscle wasting and Cachexia. Cells. 2022;11:1406. https://doi.org/10.3390/cells11091406.
Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, Hu XB, Xiang DX. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19:242. https://doi.org/10.1186/s12951-021-00986-2.
Mendivil-Alvarado H, Sosa-León LA, Carvajal-Millan E, Astiazaran-Garcia H. Malnutrition and biomarkers: a journey through Extracellular vesicles. Nutrients. 2022;14:1002. https://doi.org/10.3390/nu14051002.
Galley JD, Besner GE. The therapeutic potential of breast milk-derived extracellular vesicles. Nutrients. 2020;12:745. https://doi.org/10.3390/nu12030745.
Feng T, Zhang W, Li Z. Potential mechanisms of gut-derived extracellular vesicle participation in glucose and lipid homeostasis. Genes (Basel). 2022;13:1964. https://doi.org/10.3390/genes13111964.
Tan CF, Teo HS, Park JE, Dutta B, Tse SW, Leow MK, Wahli W, Sze SK. Exploring Extracellular vesicles Biogenesis in Hypothalamic cells through a heavy isotope Pulse/Trace Proteomic Approach. Cells. 2020;9:1320. https://doi.org/10.3390/cells9051320.
Gao J, Li X, Wang Y, Cao Y, Yao D, Sun L, Qin L, Qiu H, Zhan X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). 2020;228:e13339. https://doi.org/10.1111/apha.13339.
Mutt SJ, Herzig KH. Adipocyte-derived extracellular vesicles as new communication signals in the regulation of food intake. Acta Physiol (Oxf). 2020;228:e13411. https://doi.org/10.1111/apha.13411.
Kim MH, van Noort D, Sung JH, Park S. Organ-on-a-Chip for studying gut-brain Interaction mediated by Extracellular vesicles in the Gut Microenvironment. Int J Mol Sci. 2021;22:13513. https://doi.org/10.3390/ijms222413513.
van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: modulator of host metabolism and appetite. J Nutr. 2017;147:727–45. https://doi.org/10.3945/jn.116.240481.
Zanchi D, Depoorter A, Egloff L, Haller S, Mählmann L, Lang UE, Drewe J, Beglinger C, Schmidt A, Borgwardt S. The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev. 2017;80:457–75. https://doi.org/10.1016/j.neubiorev.2017.06.013.
Cuesta CM, Guerri C, Ureña J, Pascual M. Role of Microbiota-Derived Extracellular vesicles in Gut-Brain communication. Int J Mol Sci. 2021;22:4235. https://doi.org/10.3390/ijms22084235.
Leeuwendaal NK, Cryan JF, Schellekens H. Gut peptides and the microbiome: focus on ghrelin. Curr Opin Endocrinol Diabetes Obes. 2021;28:243–52. https://doi.org/10.1097/med.0000000000000616.
Oh DY, Fong L. Cytotoxic CD4(+) T cells in cancer: expanding the immune effector toolbox. Immunity. 2021;54:2701–11. https://doi.org/10.1016/j.immuni.2021.11.015.
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10:4355. https://doi.org/10.1038/s41467-019-12321-3.
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, et al. Exosome Processing and characterization approaches for Research and Technology Development. Adv Sci (Weinh). 2022;9:e2103222. https://doi.org/10.1002/advs.202103222.
Min L, Wang B, Bao H, Li X, Zhao L, Meng J, Wang S. Advanced nanotechnologies for Extracellular Vesicle-based Liquid Biopsy. Adv Sci (Weinh). 2021;8:e2102789. https://doi.org/10.1002/advs.202102789.
Grangier A, Branchu J, Volatron J, Piffoux M, Gazeau F, Wilhelm C, Silva AKA. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021;176:113843. https://doi.org/10.1016/j.addr.2021.113843.
Zhang Q, Jeppesen DK, Higginbotham JN, Franklin JL, Coffey RJ. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 2023;18:1462–87. https://doi.org/10.1038/s41596-023-00811-0.
Gupta D, Zickler AM, El Andaloussi S. Dosing extracellular vesicles. Adv Drug Deliv Rev. 2021;178:113961. https://doi.org/10.1016/j.addr.2021.113961.
Acknowledgements
We extend our gratitude to BioRender (BioRender.com) and FIGdraw (www.figdraw.com) for their support in creating the figures.
Funding
This work is funded by the grant from National Natural Science Foundation of China (82103021 to S Ding), China Postdoctoral Science Foundation (2022M721725 to S Ding). “The 14th-Five Year Plan” Nantong Clinical Medical Center (Innovation Platform), and the Youth Medical Expert Training Program, which are sponsored by the Nantong Municipal Health Commission.
Author information
Authors and Affiliations
Contributions
WY conceived the idea and drafted the manuscript, while DS critically revised it. Both authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The two authors agree to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Ding, S. Extracellular vesicles in cancer cachexia: deciphering pathogenic roles and exploring therapeutic horizons. J Transl Med 22, 506 (2024). https://doi.org/10.1186/s12967-024-05266-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05266-9