Abstract
Background
Aging is a complex biological process characterized by obesity and immunosenescence throughout the organism. Immunosenescence involves a decline in immune function and the increase in chronic-low grade inflammation, called inflammaging. Adipose tissue expansion, particularly that of visceral adipose tissue (VAT), is associated with an increase in pro-inflammatory macrophages that play an important role in modulating immune responses and producing inflammatory cytokines. The leukotriene B4 receptor 1 (BLT1) is a regulator of obesity-induced inflammation. Its ligand, LTB4, acts as a chemoattractant for immune cells and induces inflammation. Studies have shown that BLT1 is crucial for cytokine production during lipopolysaccharide (LPS) endotoxemia challenge in younger organisms. However, the expression patterns and function of BLT1 in older organisms remains unknown.
Results
In this study, we investigated BLT1 expression in immune cell subsets within the VAT of aged male and female mice. Moreover, we examined how antagonizing BLT1 signaling could alter the inflammatory response to LPS in aged mice. Our results demonstrate that aged mice exhibit increased adiposity and inflammation, characterized by elevated frequencies of B and T cells, along with pro-inflammatory macrophages in VAT. BLT1 expression is the highest in VAT macrophages. LPS and LTB4 treatment result in increased BLT1 in young and aged bone marrow-derived macrophages (BMDMs). However, LTB4 treatment resulted in amplified Il6 from aged, but not young BMDMs. Treatment of aged mice with the BLT1 antagonist, U75302, followed by LPS-induced endotoxemia resulted in an increase in anti-inflammatory macrophages, reduced phosphorylated NFκB and reduced Il6.
Conclusions
This study provides valuable insights into the age- and sex- specific changes in BLT1 expression on immune cell subsets within VAT. This study offers support for the potential of BLT1 in modulating inflammation in aging.
Similar content being viewed by others
Background
Aging is associated with a severe decline in physiological functions and an increased susceptibility to various chronic diseases. The accumulation of cellular senescence, and immunosenescence in older individuals contribute to chronic inflammation, often referred to as inflammaging [1,2,3,4]. This low-grade, persistent inflammation plays a significant role in the aging process and age-related diseases. The immune system is altered with age, leading to an activation of immune cells and the release of pro-inflammatory cytokines [5]. Over time, this chronic inflammatory state within tissues contributes to the development of age-related diseases, including cardiovascular diseases, neurodegenerative diseases, metabolic dysfunction, and increased susceptibility to infections when compared to the young [6,7,8].
Aging is also accompanied by an increased visceral adiposity due to reduced total basal energy expenditure [9, 10] and decreased catecholamine-stimulated lipolysis [11,12,13,14]. The function of adipose tissue includes regulation of body temperature, storage and hydrolysis of energy, and modulation of immune responses [4, 15]. Adipose tissue consists of adipocytes, pre-adipocytes and stem cells, endothelial cells, and immune cells [16]. Immune cells within adipose tissue, particularly macrophages, play a crucial role in regulating tissue homeostasis and are a major source of inflammatory cytokines such as IL-6 and TNF-α [17, 18]. These cytokines normally support the inflammatory process but accumulate over time supporting the development of inflammaging [17, 18]. Macrophages can polarize into different types: M1-like pro-inflammatory macrophages and M2-like anti-inflammatory macrophages [19]. M1 macrophages overexpress markers including CD86, CD11c, CD38, and produces pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α [20, 21]. In contrast, M2 macrophages express markers like CD206, CD163, and produces anti-inflammatory cytokines such as IL-10 [20, 22]. The expansion of adipose tissue results in an accumulation of macrophages, which exhibit a pro-inflammatory phenotype, supporting a systemic increase in inflammation and immunosenescence [23, 24]. Senescent cell accumulation is also a contributor to immunosenescence [1]. Senescent cells express a senescence associated secretory phenotype that promotes the M1 pro-inflammatory polarization of macrophages that further exacerbates the immunosenescence microenvironment [25]. Taken together, the imbalance between pro and anti-inflammatory networks could lead to immunosenescence that is exhibited in adipose tissue in older organisms.
Leukotrienes are a part of the eicosanoid family that plays an important role in inflammation, asthma, and anaphylaxis. They are derived from arachidonic acids released from cellular phospholipids by cytosolic phospholipase A2. Arachidonic acids will then convert into leukotriene A4 through the action of 5-lipoxygenase and 5-lipoxygenase activator protein, and further hydrolyzed into leukotriene B4 (LTB4) by leukotriene A4 hydrolase; or synthesized into LTC4, LTD4 or LTE4 by leukotriene C4 synthase [26, 27]. LTB4 acts as a chemoattractant of leukocytes, including neutrophils, macrophages, and eosinophils [28,29,30]. LTB4 can trigger inflammation by stimulating the secretion of inflammatory cytokines through the interaction with its receptor. Leukotriene B4 receptor 1 (BLT1) and leukotriene B4 receptor 2 (BLT2), G-protein coupled receptors (GPCRs) which bind to LTB4, exhibit distinct characteristics. One such receptor is BLT1, a high-affinity receptor of LTB4 that is primarily expressed on myeloid cells [31,32,33]. SP1 is a transcriptional factor that controls ltb4r1 expression [34, 35]. Phosphorylation of ERK1/2, JNK1/2 and AKT is induced by the LTB4-BLT1 axis, leading to up-regulation of nuclear factor-κB (NF-κB) and cytokine production, including IL-17, IL-6 and IL-1β [36, 37]. BLT1 is also capable of inhibiting adenylyl cyclase and calcium entry through its interaction with the Gi- and Gq-classes of G proteins [38], which results in activation of NF-κB [39]. BLT1 is required for the inflammatory processes in acute and chronic diseases in younger organisms. Lipopolysaccharides (LPS) are the major component of the gram-negative bacteria outer membrane, which induces endotoxemia and increase BLT1 expression through toll-like receptor 4 (TLR4)-mediated cytokine production [37, 40, 41]. Moreover, obese BLT1-deficient mice exhibit improved insulin sensitivity, reduced inflammation and a decreased frequency of M1 macrophages, but an increased frequency of M2 macrophages [42].
Aging increases susceptibility and mortality to endotoxemia, due to expression of cytokines like IL-6 from the adipose tissue [8, 43]. While the function of BLT1 is well-established in the young, its expression patterns and role in natural mouse models of aging and during endotoxemia remains unknown. Here, we investigate the age-specific changes of BLT1 expression within immune cell subsets from multiple tissues. We identified an increase in BLT1 expression on adipose tissue macrophages in aged mice. Additionally, we observed that BLT1-inhibition can affect the ratio of pro-inflammatory to anti-inflammatory macrophages and Il6 levels during endotoxemia in the aged mice. These insights suggest that BLT1 may be a potential therapeutic target to treat age-related inflammation and diseases.
Methods
Animals
All mice were kept in specific pathogen-free facilities and provided free access to sterile water at the University of Minnesota. Sentinel mice in our animal rooms consistently tested negative for standard murine pathogens at various intervals during the studies. 3–8 months old male or female, 20–24 months old male or female C57BL6/J (wild-type) and 22–27 months old male or female Fabp4 knockout (AKO) mice were used for experiments. The aged WT mice were sourced from the National Institute of Aging, and the young WT mice were either bred in-house, acquired from The Jackson Laboratory or sourced from the National Institute of Aging. AKO mice were previously described [44]. All mice were treated according to National Institutes of Health guidelines for the care and use of experimental animals, approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Mice were maintained in a controlled environment with fixed temperature (21–23 °C), humidity (30–70%), lighting (14 h light/10 h dark) and free access of drinking water and standard chow (Teklad 2918).
Experimental design
Blinding of investigators was unfeasible during the experiments, given the apparent variations in phenotype resulting from age or LPS treatment. Aged mice displaying severe frailty, tumors, or other age-related pathologies were deliberately excluded from the study, constituting less than 5% of the total population of aged mice.
Mouse models
Lipopolysaccharide (LPS)-induced endotoxemia challenge
Young (3–8 months) and old (20–24 months) mice received intraperitoneal (IP) injections of either sterile phosphate-buffered solution (PBS; Corning) or 3 mg/kg lipopolysaccharide (LPS; E. coli O111-B4; Sigma, L3024) diluted in sterile PBS. Body temperature was measured using a rectal thermometer. Euthanasia was performed through exsanguination and cervical dislocation under isoflurane anesthesia, and tissues harvested after euthanasia.
BLT1 antagonist treatment
Mice were given IP injection with 1 mg/kg BLT1 antagonist-U75302 (Cayman Chemical) 1 h prior to 3 mg/kg LPS (E. coli O111-B4; Sigma, L3024) injection. Control mice were injected with DMSO and PBS.
Tissue digestion
Visceral adipose tissue (VAT) was harvested after euthanasia and weighed. Tissues underwent enzymatic digestion in 0.1% collagenase II (Worthington Biochemicals) in Hanks' Balanced Salt Solution (HBSS) (Life Technologies) for 30 min at 37 °C, 200 rpm agitation [45]. To ensure consistency and minimize procedural variations, both control and experimental groups were processed and stained on the same day. The stromal vascular fraction (SVF) was pelleted through centrifugation at 1500 rpm for 5 min. Cells were in RPMI with 10% FBS. ACK lysis buffer (Quality Biological Inc) was used to remove red blood cells. Cells were quenched with RPMI with 10% FBS after 2 min in ACK lysis buffer, filtered with a 40uM filter and centrifuge at 1500 rpm for 5 min to get single cell suspension. Splenocytes and peritoneal exudate cells were isolated into single cell suspension in a similar way, but without digestion in collagenase II.
Flow cytometry
Single cells were stained with Ghost Dye Red 780 Viability Dye (TONBO biosciences) for 30 min on ice, avoiding light. Then washed and incubated with FcBlock (BD Bioscience) and surface antibodies for another 30 min. Antibodies used are listed in Table S1. The BLT1 staining required a secondary antibody since it is unconjugated. AF647-conjugated anti-mouse IgG1 (Biolegend) was used as the secondary antibody for a 30-min stain. The control staining was incubated with Rabbit IgG Isotype Control (Invitrogen) instead of the BLT1 antibody. For intracellular staining, cells were fixed with the BD CytofixCytoperm kit (554715) for 20 min, followed by an intracellular antibody stain for 30 min. Flow cytometry analysis was performed on a BD FACSymphony A3 (R6609) cytometer and a FlowJo v10 software.
Bone Marrow-Derived Macrophages (BMDM) culture
Bone marrow was isolated from femur and tibia of young and aged mice. Cells were lysed in ACK lysis buffer, filtered and resuspended in RPMI + 10% FBS + 1% antibiotic–antimycotic, supplemented with 25 ng/ml of macrophage colony-stimulating factor (M-CSF) (R&D systems) for 5 days to differentiate the cells into macrophages. An extra 50 ng/ml of M-CSF was added to the cells after 5 days of culture, this supplementation allowed for an additional 2 days. For LPS stimulation, cells were stimulated with 1 μg/ml LPS for 4 h. For LTB4 stimulation, cells were stimulated with 100 nM LTB4 for 6 h. For macrophage polarization, cells were incubated with 1 μg/ml LPS and 20 ng/ml IFNg (M1 macrophages), or with 10 ng/ml IL-4 (M2 macrophages) for 24 h.
RNA extraction and gene expression analysis
Tissues were homogenized in Trizol (Invitrogen, 15596026) using the Next Advantage Bullet Blender Storm 24. Chloroform was added to the Trizol mixture and incubated prior to centrifugation at 12,000 rpm for 15 min at 4 °C. RNA extraction on the aqueous phase of the centrifuged homogenate was performed using Invitrogen PureLink RNA Mini Kits according to the manufacturer's instructions. Reverse transcription and qPCR were performed as previously described [46]. 18 s was used as internal control for loading differences. Primer sequences—Il6 forward: 5’-CACAGAGGATACCACATCCCAACA-3’, Il6 reverse: 5’-TCCACGATTTCCCAGAGAACA-3’, Tnfα forward: 5’-GGTGCCTATGTCTCAGCCTCTT-3’, Tnfα reverse: 5’-CGATCACCCCGAAGTTCAGTA-3’, IL1β forward: 5’-GGTGCCTATGTCTCAGCCTCTT-3’, IL1β reverse: 5’-CGATCACCCCGAAGTTCAGTA-3’, 18 s forward: 5’-CCGCGGTTCTATTTTGTTGGT-3’, 18 s reverse: 5’-CTCTAGCGGCGCAATACGA-3’.
Western blotting
Whole VAT lysates were homogenized in RIPA with phosphatase and protease inhibitors (Sigma-Aldrich; P0044, P5726, P8340). Lysates were left on ice for 1 h and vortex every 15 min, pellet and lipids of lysates were discarded after spinning at 14,000 rpm for 10 min in 4 °C. A Bradford Assay (Fisher Sci, 23246) was used to measure total protein concentration and standardization (15 μg) for gel loading. LDS Sample Buffer (Thermo Fisher, B0008) and Sample Reducing Agent (Thermo Fisher, B0004) was added to samples. Protein was separated across a 4–12% Bis–Tris SDS Gel and then blotted onto a PVDF membrane (Invitrogen, PB5210) by semi-dry transfer. Blots were blocked with 5% non-fat milk in 0.5% TBST solution for 1 h on a benchtop rocking table. Blots were incubated in primary antibody (NF-κB: Cell Signaling, 8242S, p-NF-κB: Cell Signaling, 3033S, β-actin: Cell Signaling, 4967L) solution in 2.5% nonfat milk in 0.5% TBST, rocked overnight at 4 °C. Blots were then washed three times in a 0.5% TBST solution and incubated in goat anti-rabbit secondary antibody (Invitrogen, 31460) solution for 45 min at room temperature on a benchtop rocking table. Following three final washes in 0.5% TBST, blots were imaged with ECL substrate (Thermo Fisher, PI32106) on a chemiluminescent imaging camera. Quantification of bands were performed with Thermo Fisher’s iBright Analysis software.
Statistics
A confidence interval of 95% was used for all statistical tests. All data were assumed to be normally distributed, unless the standard deviation was identified as significantly different between groups. All statistical tests were performed using GraphPad Prism v9 for Windows (GraphPad Software). Data are expressed as mean ± s.e.m. Sample size of independent experiment are described in the figure legends. Statistical analysis was conducted using non-parametric T-test, 2-way ANOVA with a post-hoc test of Tucky or Sidak, or one-way ANOVA with a post-hoc test of Tukey. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Result
Aged male and female mice have increased visceral adiposity with altered immune cell composition
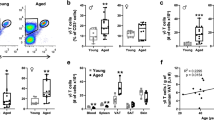
To examine the physiological difference between young and aged mice, we measured the body weight and VAT mass of young (3–8 months old) and aged (20–24 months old) mice. Aged female mice showed an increase in both body weight and VAT mass (Fig. 1a). Aged male mice showed comparable body weight with the young, but there was a significant increase in VAT mass (Fig. 1b). Due to the increase in body-fat percentage, both male and female mice exhibit increased visceral adiposity (Supplementary Fig. 1a).
Comparison of immune cell population in VAT of young (3–8 months) and aged (20–24 months) mice. a Quantification of young and aged female mice body weight (g) and VAT mass (g). b Quantification of young and aged male mice body weight (g) and VAT mass (g). c Flow cytometry gating strategy of immune cell subsets. d Frequencies of immune cell subsets in female mice. e Cells/g tissue of immune cell subset in female mice. f CD11c MFI of F4/80+ SiglecF− macrophages from female mice. g Frequencies of immune cell subsets in male mice. h Cells/g tissue of immune cell subsets in male mice. i CD11c MFI of F4/80+ SiglecF.− macrophages of male mice. Female (a, c-f) and male (b, g-i) mice were used in this experiment (n = 4–5/group). Chart error bars represent mean ± SEM. Statistical analysis was conducted using 2-way ANOVA with a post-hoc test of Sidak (d-e, g-h) or non-parametric T-tests (a-b, f, i). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
As immune cells are a critical component in regulating VAT homeostasis [15], we examined the subsets of immune cells in the young and aged mice. We investigated the frequency and cell counts of VAT immune cell subsets including B cells, T cells, eosinophils, macrophages, and neutrophils in female mice using multi-parameter flow cytometry. The gating strategy to identify each cell type is shown (Fig. 1c). B cells and CD3+ T cells were increased as a proportion of the CD45+ cells (Fig. 1d) and cells per gram of tissue with aging (Fig. 1e). In contrast, eosinophils showed a decrease as a proportion of CD45+ cells (Fig. 1d). We also examined the pro-inflammatory macrophage phenotype, using CD11c, in young and aged mice. We examined the expression level of CD11c on macrophages using mean fluorescence intensity (MFI) (Fig. 1f). The results revealed an increase in CD11c expression (MFI) on macrophages in VAT from aged female mice (Fig. 1f). We also quantified the frequency (Fig. 1g) and cells per gram of tissue (Fig. 1h) for adipose tissue immune cell subsets in young and aged male mice. Consistent with the results from female mice, the proportion and the cellularity of B cells and T cells were increased in VAT from aged male mice (Fig. 1g-h). We also detected a decrease in the frequency of eosinophils and macrophages (Fig. 1f). There was no difference in CD11c MFI on VAT macrophages from aged male mice (Fig. 1i). The frequencies and cells/g tissue of CD11c on macrophages were quantified but were not statistically different (Supplementary Fig. 1b-c). In conclusion, VAT from aged male and female mice exhibit similar changes in immune cell subsets, including increased B and T cells, but decreased frequency of macrophages and eosinophils. The female mice revealed a significant increase in CD11c MFI, a marker for pro-inflammatory macrophages.
BLT1 is highly expressed in the macrophages found in VAT
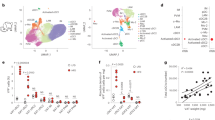
BLT1 is reported to be highly expressed in neutrophils, macrophages, and eosinophils [31,32,33]. BLT1 is also reported to be expressed on B2 B cells in the adipose tissue [47]. To examine which immune cell subset in the young and aged mice expresses BLT1, we compared the MFI of BLT1 on B cells, eosinophils, macrophages, and neutrophils of VAT. The antibody used for BLT1 detection (anti-BLT1, clone 7A8) was validated by utilizing BLT1-overexpressing cells and mouse peripheral blood leukocytes that express BLT1. Importantly, this antibody does not cross-react with BLT2 or other GPCRs [48]. The gating strategy is shown using an isotype control to gate on BLT1+ cells in designated immune cell subsets (Fig. 2a). All experiments examining BLT1+ cells used the same gating strategy. We also examined peritoneal exudate cells (PECs), and spleen, tissues that exhibits well-characterized changes with age [49]. The results show that BLT1 MFI is the highest in VAT macrophages in young mice, as compared to other tissues (Fig. 2b-c). In VAT, BLT1 MFI on B cells and T cells are comparable to the isotype control, indicating that BLT1 is not expressed on those cell types (Fig. 2c). Next, we quantified BLT1+ cells in young and aged female mice. However, we did not observe any differences in frequencies and cells/g tissue of BLT1+ macrophages, eosinophils, and neutrophils in VAT from young and aged mice (Fig. 2d). We performed a similar analysis in VAT from male mice. Aged mice exhibited an increase in BLT1+ macrophages/g tissue (Fig. 2e). Overall, this experiment indicates that BLT1 is highly expressed in VAT macrophages compared to other tissues and immune cells.
Comparison of BLT1 in immune cell subsets of young (3–8 months) and aged (20–24 months) mice. a Histogram to identify BLT1+ cells. b BLT1 MFI of immune cell subset in VAT, peritoneal exudate cells (PEC), and spleen. c Histogram of BLT1 in VAT immune cell subset. d Frequencies and count of BLT1+ cells in immune cell subset in female young vs. aged mice. e Frequencies and count of BLT1+ cells in immune cell subset in male young vs. aged mice. Female (a-d) and male (e) mice were used in this experiment (n = 4–5/group). Error bars represent mean ± SEM. Statistical analysis was conducted using paired one-way ANOVA with a post-hoc test of Tukey (b) or 2-way ANOVA with a post-hoc test of Sidak (d-e). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
LTB4 amplifies Il6 from aged, but not young BMDMs
The differentiation of bone marrow-derived macrophages (BMDMs) is a well-established in vitro system for studying macrophage biology [50]. To address whether inflammation would affect BLT1 expression in young and aged macrophages to a comparable extent, we generated BMDMs from marrow of young and aged mice by differentiating them for 7 days in M-CSF (Fig. 3a). We left them untreated or treated with LPS (1 mg/ml; 4 h) and used flow cytometry to measure BLT1 MFI and CD86 as a marker of the inflammatory phenotype. CD11c along with CD86, are both reported to be markers highly express on M1 type macrophages, which can both be used to identify pro-inflammatory macrophages [51]. BLT1 MFI and frequency of BLT1+ cells were increased with LPS treatment in young and aged BMDMs (Fig. 3b-c). To more closely examine the pro-inflammatory phenotypes, we assessed CD86 expression on the whole macrophage population and on the BLT1+ macrophage subset. When examining the total macrophage population, CD86 MFI was increased in young BMDMs when treated with LPS, but not in aged BMDMs (Fig. 3d). However, when gating through the BLT1+ macrophages, we found that CD86 MFI was significantly increased in the aged BLT1+ BMDMs, but not in the young (Fig. 3e). Since BLT1 expression showed comparable values during LPS stimulation in young and aged BMDMs, we asked whether increased receptor activity could support the inflammatory phenotype of the aged BMDMs. We treated BMDMs with LTB4 and examined the BLT1+ population of cells. LTB4 stimulation (100 nM; 6 h) increased the frequency of BLT1+ BMDMs from both young and aged mice (Fig. 3f). Further examination of gene expression of downstream cytokines showed that ll6 is significantly increased in LTB4 treated-aged BMDMs compared to the young. This is consistent with the pro-inflammatory phenotype of aged macrophages, where the aged macrophage is more sensitive to stimuli, resulting in increased cytokines like Il6.
Pro-inflammatory (CD86+) phenotype in LPS-treated BLT1+ BMDMs. a Schematic represents the method used to differentiate BMDMs and treat with 1 µg/ml of LPS. b Representative histogram of BLT1 of macrophages in untreated vs. LPS treated BMDMs. c Frequency of BLT1+ cells and BLT1 MFI on macrophages with or without LPS treatment. d CD86 MFI on macrophages with or without LPS treatment. e CD86 MFI on BLT1.+ macrophages with or without LPS treatment. f BLT1 frequencies of macrophages with or without LTB4 treatment. g II6 expression of BMDMs treated with or without LTB4. mRNA expression is normalized to 18 s and the young untreated group. Male mice were used in this experiment (n = 5–6/group). Error bars represent mean ± SEM. Statistical analysis was conducted using 2-way ANOVA with a post-hoc test of Sidak. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
M2 polarized BLT1+ BMDMs from aged mice exhibit reduced CD206, a marker for M2 macrophages
Next, we examined if BLT1 was preferentially expressed on M1 or M2 polarized macrophages generated from young or aged BMDMs. BMDMs were polarized to M1 macrophages with LPS and IFNγ and M2 macrophages with IL-4 for 24 h (Fig. 4a). Upon M1 polarization, young and aged BMDMs showed no difference in CD11c expression (Fig. 4b.) M2 polarized aged BMDMs expressed lower levels of CD206 MFI compared to young counterparts (Fig. 4c). Aged BLT1+ M2-polarized macrophages showed decreased CD206 expression as compared to young BLT1+ M2 macrophages (Fig. 4d). These data indicate that aged BLT1+ M2 macrophages have reduced CD206 expression, which may be consistent with a reduced M2-phenotype.
Pro-inflammatory (CD11c+) and anti-inflammatory (CD206+) phenotype macrophages in M1 and M2 polarized BMDMs of young and aged mice. a Schematic represents the method used to differentiate and polarize BMDMs. CD11c MFI was examined in M1 polarized BMDMs, and CD206 was examined in M2 polarized BMDMs b CD11c MFI of macrophages in M1 polarized BMDMs. c CD206 MFI of macrophages in M2 polarized BMDMs. d CD206 MFI in BLT1.+ macrophages in M2 polarized BMDMs. Male mice were used in this experiment (n = 4/group). Error bars represent mean ± SEM. Statistical analysis was conducted using non-parametric T-tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
The BLT1 antagonist alters the ratio of pro-inflammatory and anti-inflammatory VAT macrophages, reduces pNFκB and reduces Il6
The BLT1 antagonist, U75302, binds BLT1 to block downstream signaling and prevent LPS-induced inflammation in young mice [37]. As older individuals have increased susceptibility to endotoxemia [8], we asked whether inhibition of LTB4-BLT1 axis with U75302 treatment would reduce severity of endotoxemia in aged mice. To test this, aged male mice (24 months old) were IP injected with 1 mg/kg U75302 1 h prior to 3 mg/kg LPS treatment for 6 h (Fig. 5a). Injection of BLT1 antagonist did not affect hypothermia induced by LPS treatment (Fig. 5b), VAT B cells were decreased (Supplementary Fig. 2a). Lymphocyte activation (CD69) level was comparable between vehicle and U75302 group (Supplementary Fig. 2b, 2c). We also examined CD11c+ and CD206+ macrophage subsets to identify whether macrophages exhibited a pro- or anti-inflammatory phenotype. We measured the ratio of CD11c to CD206 using both the frequency and MFI. This analysis revealed a significant decrease in the ratio of CD11c/CD206 macrophages in the antagonist group (Fig. 5c, d). There’s also a significant increase in the frequency of CD206+ macrophages, with a trend towards a decrease in the CD11c+ macrophages from the antagonist group compared to the vehicle group (Supplementary Fig. 2d). The activation of NF-κB, a major transcription factors downstream from BLT1, leads to cytokine production [36, 37]. We identified an increase in phosphorylated NF-κB in VAT from aged mice following LPS that was decreased by treatment with the BLT1 antagonist (Fig. 5e). We also quantified cytokines that would be regulated by NF-κB activity. The gene expression of Il6 was significantly increased in LPS treated aged mice compare to control. We also identified a significant reduction in Il6 from VAT of aged mice treated with LPS plus the BLT1 antagonist (Fig. 5f). While there were expected trends towards increases in Il1β and Tnfα with LPS treatment, there were no differences with the BLT1 antagonist (Fig. 5f). These results reveals that the BLT1 antagonist reduces inflammation by altering the ratio of pro-inflammatory CD11c+ macrophages and CD206+ anti-inflammatory macrophages and reducing the phosphorylation of NF-κB, resulting in reduced Il6 expression.
BLT1 antagonist (U75302) in LPS-exposed aged (24 months) male mice. a Schematic represents the experimental design. Mice were injected with 3 mg/kg LPS and 1 mg/kg U75302 (BLT1 antagonist) through IP. b Body temperature of vehicle, LPS treated and LPS with BLT1 antagonist treated mice. c Ratio of CD11c+ and CD206+ to frequency and MFI of F4/80+ SiglecF.− macrophages in VAT. d Dot plot of LPS vs. LPS with BLT1 antagonist treated mouse in VAT. e Protein expression of NF-κB and phospho-NF-κB in VAT of vehicle, LPS treated and LPS with BLT1 antagonist treated mice. Phospho-NF-κB is nomalized to NF-κB and β-actin. f Relative gene expression in VAT of vehicle, LPS treated and LPS with BLT1 antagonist treated mice. mRNA expression is normalized to 18 s and the vehicle group. Male mice were performed in this experiment (n = 4 in vehicle, n = 8 in LPS, n = 7 in LPS with BLT1 antagonist). Error bars represent mean ± SEM. Statistical analysis was conducted using one-way ANOVA with a post-hoc test of Tukey. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
Lifelong Fabp4-deficiency in aged male mouse reduces BLT1 expression in VAT macrophages
FABP4 (fatty acid binding protein 4) is a protein secreted by adipocytes to regulate metabolic and inflammatory pathways [52]. Lifelong Fabp4-deficiency protects against deterioration of glucose homeostasis and metabolic pathologies in VAT during aging [52, 53]. We examined BLT1 expression on the VAT macrophages from aged wild-type or Fabp4-knockout male mice (AKO) at 23 months of age (Supplementary Fig. 3a). BLT1 MFI was reduced when comparing wild-type to AKO (Supplementary Fig. 3b, 3c). These results are consistent with improved metabolic homeostasis and reduced inflammation of aged AKO mice.
Discussion
VAT is an organ that shows early signs of immune activation and inflammation during aging [4, 12, 15]. In this study, we examined the immune cell composition from VAT and the expression of the BLT1 on immune cells from VAT, PEC and spleen of young and aged, male and female mice. Our findings reveal that BLT1 expression is limited to innate immune cells of the VAT and PEC, and that macrophages from aged mice have the highest BLT1 expression. In BMDM experiments, we examined BLT1 in LPS-, LTB4- stimulated or M1 and M2 polarized macrophages. In LPS stimulated conditions, we identified that BLT1+ BMDMs from aged mice express higher levels of CD86, as compared to the BLT1+ cells from young mice (Fig. 3e). This suggests that the LTB4-BLT1 pathway may be more active in aged pro-inflammatory BMDMs. In agreement, we identified increased Il6 after LTB4 treatment in the aged BMDMs. We also found that M2-polarized BLT1+ macrophages from aged mice have reduced CD206 that suggests reduced anti-inflammatory functions. Additionally, BLT1 antagonism reverses the LPS-induced M1/M2 ratio, reduces pNF-κB and reduces Il6 in a mouse model of endotoxemia. Finally, we show that macrophages from VAT of aged AKO mice trended towards reduced BLT1 expression. Taken together, these findings highlight key changes in the VAT from aged mice. We provide additional insight into the changes in BLT1 expression with age, the consequence of the pro-inflammatory BLT1+ macrophages, and the impact of acute BLT1 inhibition during endotoxemia.
Previous works have implicated the LTB4-BLT1 pathway in obesity related inflammation and metabolic dysfunction [42, 54,55,56]. Spite et al. described the ability of BLT1 to regulate the accumulation of macrophages in the adipose tissue leading to inflammation and insulin resistance. The obese BLT1-null mice exhibit reduced M1 macrophages and pro-inflammatory cytokines; and increase of M2 macrophages in the adipose tissue [42]. These results are consistent with our data in which BLT1 antagonist alters the ratio of pro-inflammatory macrophages and anti-inflammatory macrophages (Fig. 5c, d, Supplementary Fig. 2d-e). Ying et al. described the accumulation of B2 B cells in the VAT of obese male mice that was dependent on B cell-specific expression of BLT1 [56]. B cells are increased in the VAT of aged, obese mice, but we did not identify any expression of BLT1 on these cells (Fig. 2c). This suggests obesity-induced and age-induced B cell accumulation may occur through separate mechanisms. Ramalho et al. describe the BLT1-dependent accumulation of fatty acid, and altered metabolism in macrophages from diabetic mice [55]. Furthermore, the metabolites of arachidonic acid: LTB4 and PGE2 may be increased in aging, which could support the development of inflammation and immunosenescence [57, 58]. Our findings add to this body of literature by focusing on macrophages during aging.
Aging causes an increase in pro-inflammatory M1 macrophages and a decrease in M2 macrophages, which supports the increased inflammatory cytokines [59]. Recent work identified that BLT1 expression is significantly higher in the M1-polarized macrophages, as compared to M2-polarized macrophages in BMDMs [41]. Obese BLT1 knockout mice display an increase in M2 macrophages and decrease in M1 macrophages within VAT [29]. FABP4 has a high binding affinity to fatty acids, LTA4 and arachidonic acids, the precursors of LTB4. The binding of FABP4 with LTA4 stabilizes LTA4 and promotes its conversion into LTB4 [60]. In young mice, Fabp4-deletion reduces the expression of BLT1, the abundance of M1 macrophages, and increases the M2 macrophages [41]. Lifelong Fabp4-deficiency reduces leukocytes in aged VAT [52, 53], which would be consistent with our findings that VAT macrophages from old AKO male mice have reduced BLT1 expression. These data point towards the importance of BLT1 in regulating the frequency of pro and anti-inflammatory macrophages that is linked to Fabp4-mediated inflammation during aging.
Conclusions
The LTB4-BLT1 pathway is a strong inducer of producing pro-inflammatory cytokines, indicating that BLT1 is a potential target for treating infection and chronic inflammatory diseases. This study provides valuable insights into the age-specific changes in BLT1 expression and its impact on macrophage subsets within VAT. The use of a BLT1 antagonist in mitigating macrophage inflammation opens avenues for further exploration of BLT1 as a potential therapeutic target for treating age-related diseases. Taken together, BLT1 promotes inflammation within the VAT of aged mice by modulating pro-inflammatory and anti-inflammatory macrophages.
Limitation of study
We used a single moderate LPS dose (3 mg/kg) that leads to significant mortality in aged mice, so we are limited to the conclusions from this acute antagonist study. Previous research used a dose of 6 mg/kg LPS or larger to examine the action of the BLT1 antagonist in young mice [37, 61, 62]. It would be expected that life-long BLT1 deficiency would have a greater impact, but that would require breeding and aging Blt1-/- mice. We identified an improved LPS-induced macrophage phenotype with BLT1 antagonism; however, whether CD206+ macrophages have increased function (eg. secretion of IL-10 or phagocytic capabilities) would need to be directly tested.
Availability of data and materials
No datasets were generated or analyzed during the current study. All raw data is available upon request.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- VAT:
-
Visceral adipose tissue
- BLT1:
-
Leukotriene B4 receptor 1
- BLT2:
-
Leukotriene B4 receptor 2
- LTB4:
-
Leukotriene B4
- LPS:
-
Lipopolysaccharide
- BMDM:
-
Bone marrow-derived macrophage
- IP:
-
Intraperitoneal
- AKO:
-
Fabp4 Knockout mouse
- PEC:
-
Peritoneal exudate cells
References
Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18(10):611–27.
Frasca D, Romero M, Diaz A, Garcia D, Thaller S, Blomberg BB. B Cells with a Senescent-Associated Secretory Phenotype Accumulate in the Adipose Tissue of Individuals with Obesity. Int J Mol Sci. 2021;22(4).
Pawelec G. Age and immunity: What is “immunosenescence”? Exp Gerontol. 2018;105:4–9.
Dahlquist KJV, Camell CD. Aging Leukocytes and the Inflammatory Microenvironment of the Adipose Tissue. Diabetes. 2022;71(1):23–30.
Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17(1):7–19.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22.
Camell CD, Yousefzadeh MJ, Zhu Y, Prata L, Huggins MA, Pierson M, et al. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021;373(6552).
Carey A, Nguyen K, Kandikonda P, Kruglov V, Bradley C, Dahlquist KJV, et al. Age-associated accumulation of B cells promotes macrophage inflammation and inhibits lipolysis in adipose tissue during sepsis. Cell Rep. 2024;43(3): 113967.
Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr). 2016;38(1):23.
Speakman JR, de Jong JMA, Sinha S, Westerterp KR, Yamada Y, Sagayama H, et al. Total daily energy expenditure has declined over the past three decades due to declining basal expenditure, not reduced activity expenditure. Nat Metab. 2023;5(4):579–88.
Tchernof MJTaA. Lipid metabolism in the elderly.pdf. Eur J Clin Nutr. 2000;54(Suppl 3):121-S125.
Camell CD. Adipose tissue microenvironments during aging: Effects on stimulated lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(5): 159118.
Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790(10):1117–23.
Nguyen HP, Lin F, Yi D, Xie Y, Dinh J, Xue P, et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev Cell. 2021;56(10):1437-51.e3.
Zhang YX, Ou MY, Yang ZH, Sun Y, Li QF, Zhou SB. Adipose tissue aging is regulated by an altered immune system. Front Immunol. 2023;14:1125395.
Man K, Kallies A, Vasanthakumar A. Resident and migratory adipose immune cells control systemic metabolism and thermogenesis. Cell Mol Immunol. 2022;19(3):421–31.
Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology. 2009;150(9):4124–34.
Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. 2002;8:75–9.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877: 173090.
Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm Sin B. 2021;11(6):1493–512.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614.
Qi LYH, Zhang Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget. 2016;7(44):71673–85.
Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187(12):6208–16.
Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550(7674):119–23.
Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, et al. Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat Metab. 2020;2(11):1265–83.
He R, Chen Y, Cai Q. The role of the LTB4-BLT1 axis in health and disease. Pharmacol Res. 2020;158: 104857.
W SE. Leukotriene receptor antagonists and related compounds. Can Respir J. 1999;6(2):189–93.
Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev Cell. 2012;22(5):1079–91.
Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21(3):239–47.
Patnode ML, Bando JK, Krummel MF, Locksley RM, Rosen SD. Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J Exp Med. 2014;211(7):1281–8.
Tager AM DJ, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. Bltr Mediates Leukotriene B4–Induced Chemotaxis and Adhesion and Plays a Dominant Role in Eosinophil Accumulation in a Murine Model of Peritonitis. J Exp Med. 2000;192(3):439–46.
Serezani CH, Lewis C, Jancar S, Peters-Golden M. Leukotriene B4 amplifies NF-kappaB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest. 2011;121(2):671–82.
Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203(4):829–35.
Kato K YT, Izumi T, Shimizu T. Cell-Specific Transcriptional Regulation of Human Leukotriene B4 Receptor Gene. J Exp Med. 2000;7;192(3):413–20.
Koncz G, Jenei V, Toth M, Varadi E, Kardos B, Bacsi A, et al. Damage-mediated macrophage polarization in sterile inflammation. Front Immunol. 2023;14:1169560.
Sanchez-Galan E, Gomez-Hernandez A, Vidal C, Martin-Ventura JL, Blanco-Colio LM, Munoz-Garcia B, et al. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81(1):216–25.
Kwon SY, Ro M, Kim JH. Mediatory roles of leukotriene B(4) receptors in LPS-induced endotoxic shock. Sci Rep. 2019;9(1):5936.
Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: metabolism and signal transduction. Arch Biochem Biophys. 2001;385(2):231–41.
Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem. 2002;269(18):4559–65.
Kawasaki T, Kawai T. Toll-like receptor signaling pathways Front Immunol. 2014;5:461.
Hertzel AV, Xu H, Downey M, Kvalheim N, Bernlohr DA. Fatty acid binding protein 4/aP2-dependent BLT1R expression and signaling. J Lipid Res. 2017;58(7):1354–61.
Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187(4):1942–9.
Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64(7):723–30.
Hertzel AV, Smith LA, Berg AH, Cline GW, Shulman GI, Scherer PE, et al. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am J Physiol Endocrinol Metab. 2006;290(5):E814–23.
Carey A, Camell CD. Protocol to examine murine visceral adipose tissue immune cells using fluorescence based flow cytometry. STAR Protoc. 2024;5(3):103227. epub ahead of print.
Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1(3):1559–82.
Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J Clin Invest. 2017;127(3):1019–30.
Sasaki F, Koga T, Saeki K, Okuno T, Kazuno S, Fujimura T, et al. Biochemical and immunological characterization of a novel monoclonal antibody against mouse leukotriene B4 receptor 1. PLoS ONE. 2017;12(9): e0185133.
Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK(+) CD8(+) T Cells as Conserved Hallmark of Inflammaging. Immunity. 2021;54(1):99-115.e12.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20.
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang W, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115(2):22.
Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015;11(10):592–605.
Charles KN, Li MD, Engin F, Arruda AP, Inouye K, Hotamisligil GS. Uncoupling of Metabolic Health from Longevity through Genetic Alteration of Adipose Tissue Lipid-Binding Proteins. Cell Rep. 2017;21(2):393–402.
Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, Lopez-Parra M, Ferre N, Titos E, et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184(7):3978–87.
Ramalho T, Ramalingam L, Filgueiras L, Festuccia W, Jancar S, Moustaid-Moussa N. Leukotriene-B4 modulates macrophage metabolism and fat loss in type 1 diabetic mice. J Leukoc Biol. 2019;106(3):665–75.
Khan S, Tsai S, Winer DA. Adipose Tissue B Cells Come of Age: The AABs of Fat Inflammation. Cell Metab. 2019;30(6):997–9.
Das UN. "Cell Membrane Theory of Senescence" and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules. 2021;11(2).
Tesicky M, Krajzingrova T, Swiderska Z, Syslova K, Bilkova B, Elias J, et al. Longitudinal evidence for immunosenescence and inflammaging in free-living great tits. Exp Gerontol. 2021;154: 111527.
Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67(5):827–36.
Zimmer JS, Dyckes DF, Bernlohr DA, Murphy RC. Fatty acid binding proteins stabilize leukotriene A4: competition with arachidonic acid but not other lipoxygenase products. J Lipid Res. 2004;45(11):2138–44.
Sun M, Wang R, Han Q. Inhibition of leukotriene B4 receptor 1 attenuates lipopolysaccharide-induced cardiac dysfunction: role of AMPK-regulated mitochondrial function. Sci Rep. 2017;7:44352.
Zhang J, Wang M, Ye J, Liu J, Xu Y, Wang Z, et al. The Anti-inflammatory Mediator Resolvin E1 Protects Mice Against Lipopolysaccharide-Induced Heart Injury. Front Pharmacol. 2020;11:203.
Acknowledgements
Biorender Agreement #’s: KF26JYXJR4, ZK26JYXO5I, TU26JYY4IG. We thank Dr. David A. Bernlohr for providing the FABP4 knockout mice. We acknowledge and thank the University of Minnesota Flow Cytometry Resource for their expertise.
Funding
This work was supported by National Institute of Health grants R01 AG079913 (CDC), R01 AG069819 (PI Bernlohr) and the McKnight Land-Grant Professorship (CDC).
Author information
Authors and Affiliations
Contributions
WS carried out all experiments. VK, SC and CDC assisted with experiments. IJ provided insight into experimental design and interpretation of data. DD and DAB provided scientific insight, mice, experimental design, and edited the manuscript. WS and CDC conceived the project, analyzed the data, and wrote the manuscript. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12979_2024_461_MOESM1_ESM.pdf
Supplementary Material 1:Supplementary Fig. 1: Comparison of CD11c frequencies and cellularity of young (3-8 months) and aged (20-24 months) mice. a Ratio of VAT mass and body weight of female and male, young and aged mice. b Frequency of CD11c+ F4/80+ SiglecF- macrophages and cells/g tissue in VAT from female mice. c Frequency of CD11c+ F4/80+ SiglecF- macrophages and cells/g tissue of male mice. (n=4-5/group). Error bars represent mean ± SEM. Statistical analysis was conducted using non-parametric T-tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Supplementary Fig. 2: CD11c and CD206 frequencies in BLT1 antagonist (U75302) treated aged (24 months) mice. a Immune cell frequency of CD45+ live cells in VAT. b CD69 population in CD3e+ T cells and CD19+ B cells of spleen. c Histogram of CD69 population in CD3e+ T cells and CD19+ B cells of spleen d Frequency of CD11c+ F4/80+ SiglecF- macrophages and CD206+ F4/80+ SiglecF- macrophages. Male mice were used in this experiment (n=4 in vehicle, n=8 in LPS, n=7 in LPS with BLT1 antagonist). Error bars represent mean ± SEM. Statistical analysis was conducted using one-way ANOVA with a post-hoc test of Tucky. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Supplementary Fig. 3: BLT1 expression on VAT macrophages from aged WT and AKO male mice. a Flow cytometry gating strategy of BLT1+ subsets. b BLT1 frequency and MFI of F4/80+ SiglecF- macrophages in VAT from male mice. c Histogram of BLT1 population in F4/80+ SiglecF- VAT macrophages of aged male mice. Male mice were used in this experiment (n=4 in WT, n=2 in AKO). Error bars represent mean ± SEM. Statistical analysis was conducted using non-parametric T-tests. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Supplementary Table 1: Antibodies used for flow cytometry
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shih, WC., Jang, I.H., Kruglov, V. et al. Role for BLT1 in regulating inflammation within adipose tissue immune cells of aged mice. Immun Ageing 21, 57 (2024). https://doi.org/10.1186/s12979-024-00461-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-024-00461-0