Abstract
Aging is associated with systemic chronic, low-grade inflammation, termed ‘inflammaging’. This pattern of inflammation is multifactorial and is driven by numerous inflammatory pathways, including the inflammasome. However, most studies to date have examined changes in the transcriptomes that are associated with aging and inflammaging, despite the fact that inflammasome activation is driven by a series of post-translational activation steps, culminating in the cleavage and activation of caspase-1. Here, we utilized transgenic mice expressing a caspase-1 biosensor to examine age-associated inflammasome activation in various organs and tissues to define these post-translational manifestations of inflammaging. Consistent with other studies, we observe increased inflammation, including inflammasome activation, in aged mice and specific tissues. However, we note that the degree of inflammasome activation is not uniformly associated with transcriptional changes commonly used as a surrogate for inflammasome activation in tissues. Furthermore, we used a skull thinning technique to monitor central nervous system inflammasome activation in vivo in aged mice and found that neuroinflammation is significantly amplified in aged mice in response to endotoxin challenge. Together, these data reveal that inflammaging is associated with both transcriptional and post-translational inflammatory pathways that are not uniform between tissues and establish new methodologies for measuring age-associated inflammasome activation in vivo and ex vivo.
Similar content being viewed by others
Introduction
Biological aging is associated with a series of physiological and cellular changes that reduce organismal fitness. These changes include a number of hallmarks or pillars, that include genomic instability, altered intercellular communication, cellular senescence, mitochondrial dysfunction, deregulated nutrient-sensing, metabolic derangements and chronic inflammation [1, 2]. Age-associated chronic inflammation, or “inflammaging” is driven by the development of other hallmarks of aging and is thought to underly age-associated functional decline as well as the increased susceptibility to numerous diseases of inflammation, including diseases, cardiovascular disease, cancer, immune system disease, and musculoskeletal disorders [3,4,5,6,7,8,9,10,11,12,13]. Therefore, understanding the cellular and molecular mechanisms driving inflammaging may provide the opportunity to reduce age-associated functional decline and the incidence or severity of age-associated diseases.
Studies of age-associated changes in inflammation have identified multiple inflammatory programs and pathways that increase during aging. These include changes at the transcriptional level, including studies which have demonstrated changes in interferon signaling that occur during aging [14,15,16]. However, it is also clear that inflammaging cannot be completely explained by transcriptional changes that occur during aging. Specifically, genetic studies have clearly demonstrated that the inflammasome, which is a post-translational inflammatory response, contributes to age-associated functional decline [17]. Inflammasomes are induced by sensing of pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs). Numerous PRRs, including NOD-like receptors (NLRs) and AIM2-like receptors (ALRs), form inflammasome complexes in response to DAMP recognition, cellular injury and stress [18, 19]. Following assembly of these multi-protein complexes, inflammasome receptor oligomerization leads to the autoproteolytic cleavage and activation of caspase-1 [18]. Activated caspase-1 cleaves and thereby activates inflammatory cytokines, such as pro-IL-1β and pro-IL-18 which lead to propagation of the cellular inflammatory responses [20]. Caspase-1 also cleaves gasdermin D (GSDMD) into its functional form, promoting its membrane association and pore formation, which facilitate the release of inflammatory cytokines and can also promote an inflammatory cell death known as pyroptosis [20,21,22,23].
Genetic studies reveal that deletion of NLRP3 or other inflammasome genes ameliorate numerous aspects of age-related functional decline [17, 24,25,26]. However, monitoring chronic, low-grade inflammasome activation that occurs in this context can be technically challenging compared to disease models involving acute and robust levels of inflammasome activation. For this reason, many studies of inflammaging have focused on transcriptional changes of NLRP3 and other genes that participate in inflammasome activation. However, given that inflammasome activation is mediated through a series of post-translational protease cleavage events of caspase-1 and other proteins, monitoring inflammasome activation, rather than changes in inflammasome associated transcripts, is necessary to fully understand complete panorama of inflammatory sequelae associated with inflammaging.
To characterize global and tissue-specific changes in inflammasome activation during the process of aging, we utilized a transgenic mouse model in which a luciferase-based caspase-1 biosensor is constitutively expressed in all tissues [27]. Proteolytic activation of caspase-1 thus induces luciferase activation in specific tissues, allowing inflammasome activation to be monitored in vivo and ex vivo [27,28,29,30,31,32,33]. These studies reveal a tissue-specific degree of overlap or discordance between age-associated inflammasome activation and upregulation of inflammatory transcripts commonly associated with inflammaging. We observe that inflammasome activation is not directly correlated with transcriptional upregulation of innate immune receptors or inflammatory cytokines. Additionally, using a model of endotoxemia, we establish a system to monitor age-associated changes in inflammasome activation in the central nervous system (CNS), which allows for more effective monitoring of post-translational inflammatory responses in the CNS.
Results
Aged Mice Exhibit Tissue-Specific Increases in Inflammasome Activation and Immunoglobulin Deposition
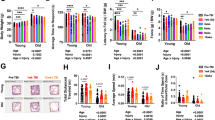
To characterize global changes in inflammasome activation during aging, we used caspase-1 reporter mice described previously to visualize caspase-1 activation in vivo and ex vivo [27, 30, 31, 33]. At ~ 18–24 months of age (18–24 mo), mice exhibited increased biosensor activation in vivo (Fig. 1A-B), consistent with age-dependent increase in low-grade systemic inflammation. To identify the source of this increased biosensor signal, we extracted tissues from young (6–12-week-old) and aged (18–24 mo) mice and biosensor activation was assessed ex vivo. We found elevated biosensor activation in some tissues from aged mice, including the pancreas, kidneys, heart, and brain, while biosensor signal remained unchanged in other tissues, such as the lungs and intestines (Fig. 1C, 1D). To better understand the development of age-associated changes in biosensor activation, we also examined biosensor activation in the same organs of mice at 15 months of age (15 mo). At 15 mo, significant biosensor activation was observed in the pancreas and kidney, while biosensor activation in the brain and the heart was not significantly different than young mice at this time point (Fig. 1D).
Caspase-1 activation increases in vivo during aging and in some tissues ex vivo. Representative images and quantification of in vivo caspase-1 biosensor activation in young (12 week old) and aging (18- 24 month old) mice (A-B). Tissues were extracted from young and aged mice and tissue bioluminescence was measured ex vivo (C-D). * = p
To confirm caspase-1 activation in these tissues, we attempted to corroborate biosensor signal by monitoring caspase-1 cleavage via western blot. However, the presence of murine immunoglobulins, particularly in aged tissue, complicated this assessment. Specifically, we used a commonly utilized mouse antibody to caspase-1 that can detect both the uncleaved p45 and cleaved p20 band of caspase-1. Consistent with our biosensor measurements, we observed apparent increases in the amount of cleaved caspase-1 in the brain, heart and kidneys (Fig S1). However, we also observed an increase in cleaved caspase-1 in the lungs, where we measured relatively little age-associated caspase-1 activation using our biosensor (Fig S1A). However, when lysates were analyzed using only the goat α-mouse secondary antibody, we noted bands of similar size and intensity to what was seen when the primary antibody was included, suggesting that much if not all of the observed signal by western blot was due to the presence of murine immunoglobulin accumulation in these aged tissues (Fig S1B). We therefore attempted to immunodeplete immunoglobulins from these tissues with protein A/G beads. However, immunodepletion did not effectively remove the non-specific 20 kDa band from these lysates (Fig S2), which prevented us from assessing the amount of cleaved caspase-1 in aged tissues. Assessment of age-associated immunoglobulins in various organs revealed that this phenomenon was not exclusive to IgG, as both IgM and IgA levels were also increased in many organs (Fig S3). Less deposition of all three immunoglobulins was observed in the brain, although an age-dependent deposition of IgG and IgM was still clearly observed (Fig S3).
As the NLRP3 inflammasome has been previously implicated in age-associated inflammation [17, 24,25,26], we treated aged mice with the NLRP3 inhibitor MCC950 to determine the degree to which increased biosensor activation observed in aged mice was derived from increased NLRP3 inflammasome activation [34]. Treatment of aged mice with MCC950 induced a significant reduction in biosensor signal emanating from the head, abdomen and paws of aged mice, reducing total biosensor signal by 50% or greater in these tissues (Fig. 2A, B). A similar trend was noted in the chest, but this difference was not statistically significant (Fig. 2B).
MCC950 attenuated caspase-1 mediated inflammatory signal in aging mice. A Sample images show aged caspase-1 activation reporter mouse (22–24 months old) bioluminescence signals before and after MCC950 treatment. MCC950 was treated within 24 h prior to the second imaging session. The analyzed ROIs (red circles) were quantified across both time points. B Significantly elevated head, abdominal, and paw caspase-1 activation associated signals in aged mice and significantly decrease post MCC950 treatment. n = 5 mice per group; *p
We similarly monitored biosensor activation in the brain ex vivo using a slice culture model. We imaged brain slice cultures from 6 aged animals, applied MCC950 to these cultures and measured biosensor activation again 24 h after addition of MCC950 (Fig. 2C). Similar to what we observed following MCC950 administration in vivo, we noted a significant decrease in biosensor activation in brain slice cultures following addition of MCC950 (Fig. 2C, D). Collectively, these data demonstrate that the increased biosensor activation observed in aged mice is due to NLRP3 inflammasome activation.
Aging associated changes in inflammatory transcripts are not predictive of changes in inflammasome activation.
Next, we assessed changes in commonly measured inflammatory transcripts in tissues of young and aged mice. Notably, although we observed a statistically significant increase of caspase-1 activity in the brain, kidneys, and heart, these changes were not associated with similar differences in other inflammasome related transcripts. Specifically, we did not observe statistically significant changes in NLRP3 or IL-18 in these tissues, although a trend towards an increase in NLRP3 was observed in the kidneys and heart, as well as in the lungs, despite the lungs not exhibiting increased caspase-1 biosensor activation in aged animals (Fig. 3). We observed statistically significant increases in pro-IL-1β and caspase-1 transcripts in some organs, with the largest and most significant increases observed in the lungs (Fig. 3). Smaller but significant increases in pro-IL-1β and caspase-1 were observed in the heart and a statistically significant increase in caspase-1 transcripts was observed in the brain. No significant increase in pro-IL-1β transcripts was observed in the brain and no inflammasome associated transcripts were increased in the kidney, despite the increase in caspase-1 biosensor observed in aged kidneys. Analysis of other inflammatory genes, including IL-6 and interferon stimulated genes, revealed relatively modest changes in transcription in most organs, with significant increases in interferon stimulated genes ISG15 and IFI16 noted in the brain and ISG15 in the kidneys. Together, these data demonstrate that tissue-specific changes in inflammasome-specific transcripts are not predictive of increases in caspase-1 activation in aged mice.
Aging aggravates endotoxemia-induced inflammasome activation in the CNS
We next asked if age influenced inflammasome activation in response to endotoxemia, a common model of sepsis. It is known that peripheral administration of lipopolysaccharide (LPS) induces systemic and neuroinflammation through activation of immune cells in the periphery and the release of cytokines and chemokines [35, 36]. Age is known to increase the inflammatory and cognitive sequelae induced during sepsis in both humans and mouse models [37, 38]. However, most studies of CNS responses to LPS injection have been unable to directly monitor inflammasome activation, instead relying on changes in transcription that may or may not be indicative of inflammasome activation in the CNS. We therefore injected young and aged mice with LPS, and caspase-1 activation and transcriptional changes were measured in tissues 24 h later. We detected a robust increase in biosensor activation and upregulation of inflammatory transcripts, most notably IL-6, in all tissues (Fig S4). We observed a significant increase in biosensor activation following LPS injection in both young and aged mice, specifically in the kidney, brain, heart and lungs, despite a relatively small number of aged biosensor mice included in this experiment (n = 3) (Fig. 4).
To further characterize age-dependent changes in the brain, we utilized caspase-1 reporter mice with cranial windows to visualize inflammasome activation in the CNS in vivo. We have recently used this approach to monitor inflammasome activation in the CNS following mild traumatic brain injury [28]. Skulls of young and aged mice were thinned completely, and a layer of transparent glue was applied. Windows remain optically clear for up to a year, allowing the continued monitoring of luciferase activity in the brain of living mice. We injected young and aged mice with LPS and monitored CNS inflammasome activation in vivo. As expected, we measured increased biosensor activation in young and aged mice treated with LPS, relative to the untreated controls (Fig. 5A). However, since both the cranial window surgery and LPS injection increase mortality of aged mice, we also monitored biosensor activation in organotypic brain slices ex vivo. We observed increased biosensor activation in slices derived from aged mice that was exacerbated following LPS exposure (Fig. 5 C-D). Surprisingly, we did not observe a significant increase in biosensor activation in CNS slices from young mice following LPS addition, suggesting that peripheral chemokines or other inflammatory mediators drive this response following LPS injection in vivo. Together, these data indicate that endotoxin challenge in aged mice results in amplified inflammasome activation in the brain.
Aged mice exhibit increased inflammasome activation in the brain in response to endotoxin challenge. Representative image of a mouse 6 months following skull-thinning cranial window surgery (A). Young (6–12 week old) and aged (18 month – 2 year old) mice were challenged with LPS (100 μg, i.p). Representative in vivo IVIS images prior to (0 h) and 24 h-post LPS administration (B). C. Brains were excised from young (3–5 month old) and aged (22 – 23.5 month old) caspase-1 biosensor mice and 400-450 μm coronal sections were generated (~ 25–45 slices per group). Slices were treated with and without LPS and imaged using the IVIS (D). (C-D) N = 6–10 animals per group, * = p
Discussion
While most work to date has focused on systemic changes in inflammasome signaling during aging, altered inflammatory pathways in specific organs relevant to an age-dependent disease, or aberrant inflammatory pathways activated in specific immune cell populations, the objective of this work was to broadly assess age-associated changes in inflammasome activation in major organ systems and compare this to changes in inflammatory transcripts occurring in these same tissues. To do this, we utilized transgenic mice expressing a bioluminescent reporter of caspase-1, which allowed us to determine which organs and tissues exhibit the strongest degree of age-associated inflammasome activation. We observed elevated caspase-1 activation in vivo and in extracted organs ex vivo, including the pancreas, heart, kidneys, and brain (Fig. 1–2, Supplement 1).
Once we identified tissues with age-dependent increased caspase-1 activity, we further interrogated alterations in inflammatory transcriptional signatures in these tissues. A number of prior studies have performed single-cell or bulk RNA sequencing on aging tissues and demonstrated upregulation of innate immune and inflammatory signaling pathways in aged tissues [14, 16, 39, 40]. However, when we compared changes in inflammatory transcripts, including transcripts commonly measured to monitor inflammasome upregulation, we observed that these changes were not predictive of the degree of biosensor activation observed in these organs. For example, we observed the most consistent and statistically significant increase in inflammasome associated transcripts in the lungs (Fig. 3), despite not observing increased biosensor activation in the lungs (Fig. 1D). However, we did measure a strong increase in biosensor activation in the lungs of both old and young mice following LPS injection (Fig. 4), demonstrating that substantial inflammasome activation can occur in the lungs and that this response can be reliably measured using this model. We measured an increased trend in the upregulation of NLRP3 and pro-IL-1β transcripts that was not significant for the number of mice used in these studies, whereas procaspase-1 transcripts were significantly upregulated in most aged tissues (Fig. 3). Our results demonstrate that age-dependent increases in caspase-1 activation in the heart, brain, and kidneys occurred in the absence of large/significant transcriptional changes in inflammatory pathways in these tissues. An important caveat to our approach is that it relied on bulk RNA isolation, which could obscure cell-type specific changes in inflammatory transcript expression.
We wanted to further assess age-associated changes in inflammasome/caspase-1 activation in response to an inflammatory stimulus. We hypothesized that caspase-1 activation would be exacerbated in response to endotoxemia in aged mice. Despite a relatively small number of animals available for these experiments, we observed statistically significant increases in biosensor activation in the heart, kidney, lungs and brain (Fig. 4). We also attempted to monitor CNS inflammasome activation directly using cranial windows. These data corroborated our ex vivo findings (Fig. 5B), although a small number of aged animals surviving the window surgery and LPS injection precluded assessment of statistical significance. We also observed a significantly increased response to LPS in brain slice cultures from aged mice (Fig. 5C-D). Notably, we did not observe an increase in biosensor activation in LPS-treated brain slices from young animals, compared to pretreatment levels (Fig. 5 C-D). Since we observed a significant increase in young brains imaged ex vivo following LPS injections (Fig. 4) and observed a similar pattern in young mice with cranial windows (Fig. 5B), this suggests that peripheral inflammation is required to drive CNS inflammation in young mice. In this context, future studies are required to understand the differential responsiveness of aged CNS tissue to LPS, which could be due to an increased number of peripheral immune cells present in the CNS of aged animals or differential responsiveness of CNS resident cells.
In these studies, we also tried to validate caspase-1 activation in tissues using western blot to detect cleaved caspase-1. However, the presence of age-associated immunoglobulins precluded the detection of cleaved caspase-1. Broadly speaking, the reliable detection of cleaved caspase-1 has been limited to two commercially available antibodies to caspase-1. The first, a rabbit polyclonal antibody, is no longer commercially available, leaving most studies reliant on a commercially available mouse monoclonal antibody to caspase-1. However, as noted in another study [41], the similar size of murine IgG immunoglobulin components to immature and mature caspase-1 confounds detection of caspase-1 in some experimental model systems. We find that aging studies are particularly impacted by this technical limitation due to the comparatively high amount of immunoglobulin deposition observed in aged tissue (Fig S1). Although we were able to validate the biosensor signal observed in aged animals using the NLRP3 inhibitor MCC950 (Fig. 2), the prevalence of age-associated immunoglobulins in the organs examined demonstrates that detection of caspase-1 cleavage using this antibody should be avoided and that secondary antibody controls are required in studies of inflammasome activation in aged animals.
Although our studies and other studies clearly demonstrate a role for the NLRP3 inflammasome in age-associated inflammasome activation, these studies to not exclude the role of other inflammasomes in this response. Most inflammasomes promote the cleavage of gasdermin D or gasdermin E, which leads to the formation of pores on the plasma membrane that are able to drive changes in cytoplasmic ion concentrations, including K + efflux, which is known to promote NLRP3 activation [42]. Thus, although we observe that a substantial amount of inflammasome activation occurring in aged animals is sensitive to NLRP3 inhibition, it is possible that other inflammasomes initiate inflammasome activation in some organs or tissues, and that these responses induce subsequent activation of the NLRP3 inflammasome.
Conclusion
In this study, we demonstrate that transgenic mice expressing a caspase-1 biosensor are an attractive model to study age-associated inflammasome activation in mice. In a relatively small cohort of animals, we were able to detect statistically significant increases in inflammasome activation in many organs. Moreover, we observe that inflammasome activation in many organs is not correlated with transcriptional changes of inflammasome associated transcripts, demonstrating the importance of measuring activation of this post-translational inflammatory pathway directly or indirectly, as we have done here.
Materials and Methods
Mice
Caspase-1 biosensor mice and C57Bl/6 J (strain #000664, Jackson Laboratory) were used for these studies. Mice were bred in house and maintained in pathogen-free conditions at Loyola University Chicago. All experiments were performed in accordance with protocols approved by Loyola University Chicago’s Institutional Animal Care and Use Committee.
IVIS measurements
For IVIS imaging, caspase-1 biosensor mice were weighed and injected intraperitoneally with 150 mg/kg VivoGlo Luciferin (Promega). Mice were anesthetized with 2% isoflurane/air mixture and imaged 10 min following administration of the luciferase substrate using the IVIS 100 Imaging system (Xenogen). For ex vivo imaging, tissues were extracted from sacrificed mice, placed in a solution of diluted luciferase substrate (300 μg/mL) and imaged. Bioluminescent images were acquired and analyzed using Living Image software (PerkinElmer).
Western blot
Tissues isolated from young and aged caspase-1 biosensor mice or C57Bl/6 J mice were flash frozen. Tissues were homogenized using an electric homogenizer in lysis buffer (1% NP-40, 100 mM Tris, pH 8.0, and 150 mM NaCl) containing a protease inhibitor mixture (Sigma Aldrich) and shaken on ice for 30 min. Lysates were collected following centrifugation and protein content was quantified by BCA (Pierce; Thermo Fisher Scientific). Samples were mixed with 2 × Laemmli sample buffer and boiled for 5 min at 95˚C. Equal amounts of protein were loaded into a 4–15% gradient gel (Bio-Rad) and transferred onto a nitrocellulose membrane (Bio-Rad), which were blocked with 5% milk for 1 h and subsequently incubated with anti-mouse caspase-1 (Adipogen) or anti-β-Actin (Santa-Cruz Biotechnology) antibodies. Chemiluminescence was measured using SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific) and a FluorChem E machine (Protein Simple).
RNA isolation and qRT-PCR
Tissues were homogenized by electric homogenization in TRIzol™ (Invitrogen) and RNA was extracted following the manufacturer’s instructions. cDNA was synthesized using the GoScript Reverse Transcription System (Promega) and quantitative real-time PCR was conducted as described previously [30].
Cranial window skull thinning surgery for in vivo CNS imaging
Mice were anesthesized with Avertin and fixed in a small animal stereotaxic system under a dissecting microscope. The skin and periosteum layers were excised to expose the skull, and the skull was thoroughly cleaned. Skulls were thinned using a Microtorque Foredom K. 1070 drill (Foredom Inc.). The drill bit was angled parallel to the skull surface and the microdrill was moved uniformly from anterior to posterior. Every few minutes, the skull was irrigated with saline and dried with compressed air and cotton swabs and the drilling motion was repeated until the skull was optimally thin and the microvasculature was clearly visible. A thin layer of cyanoacrylate glue (C1000, Ted Pella Science) was applied to provide protection, followed immediately by a drop of insta-set accelerator (Bob Smith Industries Incorporated) so that the glue would dry transparent. Animals were allowed to recover for two weeks prior to baseline IVIS imaging and LPS exposure.
Statistical analysis
Statistical significance was determined using a student’s t test when comparing two groups or one-way ANOVA followed by a Bonferroni multiple comparisons test when comparing multiple groups using GraphPad Prism software (GraphPad Software, Inc.).
Brain slice preparation and imaging
Mice were anesthetized with 125–250 mg/kg tribromoethanol (intraperitoneally) and decapitated with scissors. Whole brain was then removed and immediately placed in an ice-cold 4 °C oxygenated sucrose artificial cerebral spinal fluid (s-ACSF) solution (206 mM/L sucrose, 2 mM/L KCl, 1 mM/L MgCl2, 2 mM/L MgSO4, 1.25 mM/L NaH2PO4, 26 mM/L NaHCO3, 10 mM/L D-glucose, 1 mM/L CaCl2). Brains were placed in 4 °C s-ACSF and sectioned with a vibrotome at 350–400 μm thick (Leica VT1200S; Leica, Nusslock, Germany). Collected slices were then incubated at 32 °C for 1 h in i-ACSF (124 mM/L NaCl, 3 mM/L KCl, 2 mM/L MgSO4, 1.25 mM/L NaH2PO4, 26 mM/L NaHCO3, 10 mM/L d-glucose, 1 mM/L CaCl2) before imaging sessions. Each experimental group contained 3–4 animals. Brain slices were imaged at a 2-min interval for a total of 8 min for each series. 100 μL of d-luciferin (20 mg/mL) was added to each well one minute prior to the start of the first imaging series to a final concentration of 2 μg/mL. 100 μL of MCC950 (10 mg/mL) was added to the same chamber up to a final concentration of 1 μg/mL 5 min prior to the second imaging series.
Data Availability
Primary data is available upon request to EC.
References
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–78.
Liu RM. 2022. Aging, Cellular Senescence, and Alzheimer's Disease. Int J Mol Sci 23.
Chou YH, Chen YM. Aging and Renal Disease: Old Questions for New Challenges. Aging Dis. 2021;12:515–28.
Machiela E, Southwell AL. Biological Aging and the Cellular Pathogenesis of Huntington’s Disease. J Huntingtons Dis. 2020;9:115–28.
Easter M, Bollenbecker S, Barnes JW, Krick S. 2020. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int J Mol Sci 21.
Brahadeeswaran S, Sivagurunathan N, Calivarathan L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol Neurobiol. 2022;59:2288–304.
Ostolaza A, Corroza J, Ayuso T. Multiple sclerosis and aging: comorbidity and treatment challenges. Mult Scler Relat Disord. 2021;50:102815.
Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–85.
Lansdorp PM. Telomeres, aging, and cancer: the big picture. Blood. 2022;139:813–21.
Perez RF, Tejedor JR, Fernandez AF, Fraga MF. Aging and cancer epigenetics: Where do the paths fork? Aging Cell. 2022;21:e13709.
Santos AL, Sinha S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res Rev. 2021;67:101268.
North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108.
Allen WE, Blosser TR, Sullivan ZA, Dulac C, Zhuang X. Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell. 2023;186(194–208):e18.
Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Gluck S, Thacker VV, Favre L, Mangeat B, Kroese LJ, Krimpenfort P, Prinz M, Ablasser A. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620:374–80.
Rasa SMM, Annunziata F, Krepelova A, Nunna S, Omrani O, Gebert N, Adam L, Kappel S, Hohn S, Donati G, Jurkowski TP, Rudolph KL, Ori A, Neri F. Inflammaging is driven by upregulation of innate immune receptors and systemic interferon signaling and is ameliorated by dietary restriction. Cell Rep. 2022;39:111017.
Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–32.
Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–14.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71.
Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73.
Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68.
Bauernfeind F, Niepmann S, Knolle PA, Hornung V. Aging-Associated TNF Production Primes Inflammasome Activation and NLRP3-Related Metabolic Disturbances. J Immunol. 2016;197:2900–8.
Talley S, Kalinina O, Winek M, Paik W, Cannon AR, Alonzo F 3rd, Choudhry MA, Knight KL, Campbell EM. A Caspase-1 Biosensor to Monitor the Progression of Inflammation In Vivo. J Immunol. 2019;203:2497–507.
Nguyen T, Nguyen N, Cochran AG, Smith JA, Al-Juboori M, Brumett A, Saxena S, Talley S, Campbell EM, Obukhov AG, White FA. Repeated closed-head mild traumatic brain injury-induced inflammation is associated with nociceptive sensitization. J Neuroinflammation. 2023;20:196.
Talley S, Rademacher DJ, Campbell EM. Inflammasome activation occurs in CD4(+) and CD8(+) T cells during graft-versus-host disease. Cell Death Dis. 2023;14:632.
Talley S, Bonomo R, Gavini C, Hatahet J, Gornick E, Cook T, Chun BJ, Kekenes-Huskey P, Aubert G, Campbell E, Mansuy-Aubert V. 2022. Monitoring of inflammation using novel biosensor mouse model reveals tissue- and sex-specific responses to Western diet. Dis Model Mech 15.
Talley S, Valiauga R, Anderson L, Cannon AR, Choudhry MA, Campbell EM. DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J Neuroinflammation. 2021;18:263.
Bonomo R, Talley S, Hatahet J, C G, Cook T, B C, P K-H, G A, Campbell EM, Mansuy-Aubert V. 2021. Live Monitoring of Inflammation Reveals Tissue and Sex-specific Responses to Western Diet and Butyrate treatment BioRXIV 461384:461384.
Kalinina O, Talley S, Zamora-Pineda J, Paik W, Campbell EM, Knight KL. Amelioration of Graft-versus-Host Disease by Exopolysaccharide from a Commensal Bacterium. J Immunol. 2021;206:2101–8.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62.
Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP. 2019. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int J Mol Sci 20.
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–11.
Manabe T, Heneka MT. Cerebral dysfunctions caused by sepsis during ageing. Nat Rev Immunol. 2022;22:444–58.
Takemon Y, Chick JM, Gerdes Gyuricza I, Skelly DA, Devuyst O, Gygi SP, Churchill GA, Korstanje R. 2021. Proteomic and transcriptomic profiling reveal different aspects of aging in the kidney. Elife 10.
Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115:E1896–905.
Wang Y, Weber M, Foreman O, Remy H, Mracsko EZ, Hanson JE. 2023. Comment on "Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice". Sci Transl Med 15:eade8728.
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53.
Acknowledgements
EC was supported by R03 AI156507 and R21 AI171879. ST was supported the Parkinson’s Foundation PF-PRF-1045269. FAW was supported by 1R01AR083130, R01 NS102415 TL1 TR002531from NIH and VA grant I01RX004297.
Author information
Authors and Affiliations
Contributions
ST, RV, JD, TN, LVN, JM performed experiments, ST, LVN and EC wrote the main manuscript text, EC, ST and TN prepared the figures, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Talley, S., Nguyen, T., Van Ye, L. et al. Characterization of age-associated inflammasome activation reveals tissue specific differences in transcriptional and post-translational inflammatory responses. Immun Ageing 21, 60 (2024). https://doi.org/10.1186/s12979-024-00462-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-024-00462-z