Abstract
Background
The purpose of this study is to report our clinical outcomes using intensity-modulated radiation therapy (IMRT) for adjuvant treatment of cervical cancer, compared with three-dimensional conformal radiation therapy (3DCRT), in terms of tumor control, complications and dose-volume histogram (DVH) parameters.
Methods
Between March 2008 and February 2014, 62 patients were treated with concurrent nedaplatin-based chemotherapy and whole-pelvic external beam radiation therapy (RT). Of these patients, 32 (52 %) received 3DCRT and 30 (48 %) received IMRT.
Results
The median follow-up periods were 40 months (range 2–74 months). The 3-year overall survival rate (OS), locoregional control rate (LRC) and progression-free survival rate (PFS) were 92, 95 and 92 % in the IMRT group, and 85, 82 and 70 % in the 3DCRT group, respectively. A comparison of OS, LRC and PFS showed no significant differences between IMRT and 3DCRT. The 3-year cumulative incidences of grade 2 or higher chronic gastrointestinal (GI) complications were significantly lower with IMRT compared to 3DCRT (3 % vs. 45 %, p < .02) and in patients with V40 of the small bowel loops of ≤340 mL compared to those with >340 mL (3 % vs. 45 %, p < .001). Patients treated with IMRT had a higher incidence of grade 3 acute hematologic complications (p < .05). V40 and V45 of the small bowel loops or bowel bag were predictive for development of both acute and chronic GI complications.
Conclusions
Our results suggest that IMRT for adjuvant treatment of cervical cancer is useful for decreasing GI complications without worsening outcomes.
Similar content being viewed by others
Background
Adjuvant whole-pelvic radiation therapy (RT) concurrent with platinum-based chemotherapy is standard treatment for patients after radical hysterectomy for uterine cervical cancer with high-risk clinicopathological factors [1]. For patients with intermediate-risk factors, whole-pelvic RT with or without chemotherapy can at least reduce locoregional recurrence [2, 3]. However, patients undergoing whole-pelvic RT with or without chemotherapy after radical hysterectomy may suffer acute and chronic gastrointestinal (GI) complications.
We previously reported that dose-volume histogram (DVH) parameters of the small bowel loops were predictive for development of chronic GI complications and that V40 of the small bowel loops >340 mL was an independent risk factor for chronic GI complications using conventional two-dimensional (2D) or three-dimensional (3D) conformal RT (CRT) concurrently with nedaplatin [4]. There is often a significant amount of small bowel in the pelvis that can be avoided to a greater degree with intensity-modulated RT (IMRT) than with 3DCRT. Therefore, since October 2010, we have used IMRT as adjuvant whole-pelvic RT concurrently with nedaplatin. The purpose of this study is to report our clinical outcomes using IMRT for adjuvant treatment of cervical cancer, compared with 3DCRT, in terms of tumor control and complications. We also evaluated whether DVH predictors for development of GI complications using 2D or 3DCRT were also useful parameters in IMRT.
Methods
Patients
The study was performed as a retrospective chart review and was approved by our institutional review board. A total of 102 patients with clinical stage IB1-IIB uterine cervical cancer underwent radical hysterectomy and postoperative RT at our institute between March 2008, when we changed from 2D to 3DCRT in postoperative concurrent nedaplatin-based chemoradiation therapy, and February 2014. Postoperative RT is indicated when a patient’s pathological report displays any one of the following high-risk prognostic factors: parametrial invasion, pelvic lymph node metastasis, a positive surgical margin, or one of the following intermediate-risk prognostic factors: deep stromal invasion, lymphovascular invasion, or a large tumor (>4 cm in diameter) [5, 6]. Forty patients were excluded from the study: 12 who received extended-field RT alone because of multiple lymph node metastases [7], 12 who underwent clinical trials of whole-pelvic RT with concurrent carboplatin and paclitaxel [8], 13 who refused concurrent chemotherapy, and 3 who received intracavitary brachytherapy because of a close surgical margin. Thus, data were retrospectively analyzed for 62 patients treated with concurrent nedaplatin-based chemotherapy and whole-pelvic external RT.
Radiotherapy and chemotherapy
Whole-pelvic RT was delivered with 3DCRT planning in 32 patients between April 2008 and September 2010, and with IMRT planning in 30 patients starting in October 2010. Whole-pelvic RT with 3DCRT or IMRT was performed as previously described [4, 8]. The differences between 3DCRT and IMRT planning are summarized in Table 1. The clinical target volume (CTV) was defined according to the consensus guidelines of the Radiation Therapy Oncology Group (RTOG) 0418 [9] and the atlas on the RTOG site, or using the Japanese Clinical Oncology Group (JCOG) guidelines [10]. The RTOG guidelines include a central vaginal CTV (proximal vagina and paravaginal tissues) and a CTV for the pelvic lymph nodes, whereas the JCOG guidelines include only a CTV for the pelvic nodes. We started using 3DCRT in 2008, and CTVs (central vaginal CTV and pelvic lymph nodes) were contoured using RTOG guidelines. In October 2010, we started to use IMRT, with pelvic lymph nodes contoured using JCOG guidelines and central vaginal CTV contoured using RTOG guidelines. Thus, in brief, CTVs in 3DCRT were contoured using RTOG guidelines and CTVs in IMRT were contoured using RTOG and JCOG guidelines.
During the 3DCRT era, no normal structures were contoured before treatment. In IMRT planning, the bladder, rectum, bowel bag and femoral head were contoured before treatment because of the use of normal tissue constraints. The bowel bag for 3DCRT and the small bowel loops, large bowel loop and pelvic bone for 3DCRT and IMRT were contoured retrospectively for analysis in this study. The contouring methods for the bowel bag, small bowel loops and large bowel loop have been previously described [4]. The pelvic bone was contoured as described by Mell et al. [11].
In IMRT, target criteria and normal tissue constraints have been previously described [8]. The pelvic bone was not included as a planning constraint.
Nedaplatin (40 mg/m2) was given intravenously on a weekly basis for 5–6 weeks during the course of whole-pelvic RT, as previously described [4, 5].
Evaluation of complications
GI, genitourinary (GU), and hematologic (HT) complications were assessed according to the Common Terminology Criteria for Adverse Events version 4.0. All patients received treatment with hospitalization. For acute complications, the patients were assessed for toxicity directly during treatment on a daily basis for GI and GU complications and on a weekly basis for HT complications. Thus, acute toxicity data including grade were collected prospectively. However, for chronic complications, toxicity data including the grade of each complication were collected retrospectively from follow-up records.
Statistical analysis
Differences in clinicopathological factors, DVH parameters and incidence of complications between 3DCRT and IMRT were analyzed by Mann–Whitney U test for quantitative variables and by Fisher exact test for categorical variables. The actuarial overall survival rate (OS), loco-regional control rate (LRC) and progression-free survival rate (PFS) or incidence of chronic GI complications were calculated using the Kaplan-Meier method and differences between groups were compared by log-rank test. Correlations between grades of complications and DVH parameters were analyzed by analysis of variance (ANOVA). All statistical tests were two-sided and p < .05 or a 95 % confidence interval (CI) not encompassing 1 was considered significant.
Results
The median follow-up periods from the start of RT were 40 months (range 2–74 months) for all patients, 57 months (5–74 months) for the 3DCRT group, and 28 months (2–44 months) for the IMRT group. Clinicopathological characteristics of the 3DCRT and IMRT groups are shown in Table 2. The characteristics were similar in the two groups, but the 3DCRT group had significantly more pathological T2 stage cases (44 % vs. 20 %, p = .04) and more pathological N1 stage cases (31 % vs. 20 %, not significant).
The mean V95% values for the planning target volume were 97 % (range 91–99 %) and 97 % (93-100 %) in the 3DCRT and IMRT groups, respectively, with no significant difference between the groups (p = .32). Similarly, the mean V93% did not differ significantly between the groups (99 % vs. 99 %, p = .57). A comparison of OS, LRC and PFS also showed no significant differences between IMRT and 3DCRT (Fig. 1). The 3-year OS, LRC and PFS were 92 %, 95 % and 92 % in the IMRT group, and 85 %, 82 % and 70 % in the 3DCRT group, respectively.
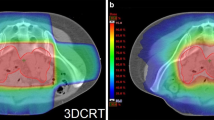
Comparisons of DVH parameters for small bowel loops, bowel bag, large bowel loop and pelvic bone between 3DCRT and IMRT are shown in Fig. 2, Tables 3 and 4. Patients who received IMRT had significantly reduced V40 and V45 volumes of the small bowel loops, bowel bag and large bowel loop, compared to patients who received 3DCRT (Fig. 2 and Table 3). Patients who received IMRT also had a reduced V30 of the small bowel loops and bowel bag, but a significantly increased V40 of the pelvic bone, compared to those treated with 3DCRT (Fig. 2 and Table 4).
The grades of acute or chronic complications and numbers of patients with these complications are summarized in Table 5. IMRT patients had fewer acute and chronic GI complications than those treated with 3DCRT, with the IMRT group having significantly fewer grade 2 or higher acute GI complications (63 % vs. 94 %, p < .01), grade 3 acute GI complications (20 % vs. 56 %, p < .01), and grade 2 or higher chronic GI complications (3 % vs. 28 %, p < .01); and fewer grade 3 chronic GI complications (3 % vs. 19 %, not significant). The 3-year cumulative incidences of grade 2 or higher chronic GI complications were significantly lower with IMRT compared to 3DCRT (3 % vs. 45 %, HR = 7.5, 95 % CI = 1.2-15.0, p < .02) and in patients with V40 of the small bowel loops of ≤340 mL compared to those with >340 mL (3 % vs. 45 %, HR = 7.7, 95 % CI = 3.2-61.0, p < .001) (Fig. 3). Patients treated with IMRT had a higher incidence of grade 3 acute HT complications (38 % vs. 63 %, p < .05).
Kaplan-Meier estimates of cumulative incidence curves for a grade 2 or higher chronic GI complications between IMRT and 3DCRT, and b stratified by V40 of the small bowel loops. a Patients who received IMRT had fewer chronic GI toxicities than those treated with 3DCRT. b, c Patients with V40 > 340 mL had higher rates of grade 2 or higher GI complications compared to those with V40 ≤ 340 mL
Correlations between grades of complications and DVH parameters (V15-45) for all patients are shown in Fig. 4. Patients with grade 2 or higher chronic GI complications had significantly greater V15-45 volumes in the small bowel loops and bowel bag (Fig. 4a, b). Patients with grade 3 acute GI complications had significantly greater V40 and V45 volumes in the small bowel loops and bowel bag, compared to patients with grade 0–1 complications (Fig. 4c, d). The grades of acute GI complications increased in a volume-dependent manner based on V40 and V45 of the small bowel loops or bowel bag, although without significance. These data indicate that V40 and V45 of small bowel loops or bowel bag were predictive for development of both acute and chronic GI complications. There was no correlation between the grades of acute HT complications and DVH values in pelvic bone (Fig. 4e).
Correlations of grades of complications with DVH parameters for a small bowel loops for chronic GI complications, b bowel bag for chronic complications, c small bowel loops for acute GI complications, d bowel bag for acute GI complications, and e pelvic bone for acute HT complications in IMRT and 3DCRT
Discussion
This study provides a comparison of the outcomes of patients with uterine cervical cancer treated with postoperative IMRT versus postoperative 3DCRT concurrent with weekly nedaplatin. There were no significant differences in OS, LRC and PFS between the cohorts, but IMRT reduced acute and chronic GI complications compared with 3DCRT. Previous reports have suggested a potential role for IMRT in adjuvant treatment of cervical cancer with adverse risk factors [12, 13]. Our retrospective data support the benefit of IMRT in reducing GI complications in postoperative chemoradiation for cervical cancer. A randomized phase III trial of postoperative treatment of endometrial and cervical cancer (RTOG1203) is ongoing for comparison of outcomes and complications between IMRT and 3DCRT, with a focus on acute GI complications.
We used nedaplatin as concurrent chemotherapy with RT. Nedaplatin (cis-diammine-glycoplatinum), a derivative of cisplatin, was developed by Shionogi Pharmaceutical Company in Japan, with the aim of reduced renal and gastrointestinal toxicity, but similar effectiveness, compared to cisplatin [14]. Nedaplatin has a particularly favorable efficacy postoperatively [5, 15] and in locally advanced cervical cancer [16]. Therefore, we considered that substitution of nedaplatin for cisplatin in concurrent chemotherapy may be beneficial for patients with cervical cancer.
We previously reported that V15-V45 of the small bowel loops has high accuracy for prediction of chronic GI complications and that V40 of the small bowel loops >340 mL is an independent risk factor for chronic GI complications in patients treated with adjuvant whole-pelvic RT using conventional 2D or 3DCRT [4]. However, dose patterns differ considerably between conventional 2D or 3DCRT and IMRT, and this raises the question of whether our previous findings for predictors apply in IMRT. In the current study, patients with grade 2 or higher chronic GI complications had significantly greater V15-V45 of the small bowel loops and the 3-year cumulative incidences of these complications were 3 and 45 % in patients with V40 values of ≤340 mL and >340 mL, respectively (p < .001). Therefore, our previous findings for predictors of chronic GI complications after 2D or 3DCRT are also useful in IMRT.
Chopra et al. found that V15 of the small bowel loops and large bowel loop are independent predictors of chronic grade 3 or higher complications [17], and recommended risk cut-off values of <275 mL and <250 mL, respectively. The difference in cut-off values in Chopra et al. and the current study may be due to treatment with or without brachytherapy, different endpoints (grade 2 or 3), different chemotherapy regimens (nedaplatin or cisplatin), and the higher DVH parameters of the small bowel loops and large bowel loop in our study (Table 3). In fact, only 9 of our patients (15 %) had V15 of the small bowel loops <275 mL. Therefore, the difference in DVH parameters might be due to differences in the physical characteristics of the patients in the two studies. There were many thin patients in our study (41/62 patients had BMI <22). However, a further study is required to determine the correlation between physical characteristics and bowel volume, and to seek better predictors of chronic GI complications.
A predictive model of acute GI complications is described in the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) guidelines [18]. QUANTEC indicated that V15 of the small bowel loops should be <125 mL or V45 of the bowel bag should be <195 mL to reduce the grade 3 complication rate to <10 %. However, in the current study the mean volumes of V15 of the small bowel loops for 3DCRT and IMRT were 428 and 411 mL, respectively, and the mean volumes of V45 of the bowel bag for 3DCRT and IMRT were 891 and 489 mL, respectively (Table 3). Therefore, the volumes of the small bowel loops or bowel bag were in excess of the QUANTEC volumes to reduce grade 3 complications to <10 % in both IMRT and 3DCRT. Consequently, a high rate of grade 3 acute GI complications of 20 % occurred in the IMRT group, but this was still less than the rate of acute GI complications after 3DCRT.
We previously reported that the small bowel loops may be better predictors of chronic GI complications compared to the bowel bag in 2D and 3DCRT [4]. However, in this study using 3DCRT and IMRT, patients with grade 2 or higher chronic GI complications had significantly greater V15-V45 volumes in the small bowel loops and bowel bag (Fig. 4a, b); and patients with grade 3 acute GI complications had significantly greater V40 and V45 volumes in the small bowel loops and bowel bag (Fig. 4c, d). The grades of acute GI complications also increased in a volume-dependent manner using V40 and V45 of the small bowel loops or bowel bag, although the relationship was not significant. Wedlake et al. found that cumulative acute GI symptoms measured by questionnaire are associated with consequential late symptoms [19]. Additionally, QUANTEC predicted that chronic GI complications are likely to be related to maximum dose or volume threshold parameters that are qualitatively similar to those related to the risk of acute GI complications [18]. Collectively, these findings indicate that bowel bag parameters are useful predictors of chronic and acute GI complications in 3DCRT and IMRT.
Patients treated with IMRT, for which the pelvic bone was not used as a planning constraint, showed a greater incidence of grade 3 or higher acute HT complications (p < .05). Klopp et al. found that V40 of the pelvic bone predicted development of HT complications in the RTOG 0418 prospective trial [20]. Therefore, in our study, the cause of the significant increase in HT complications in IMRT may have been a significantly greater V40 of the pelvic bone, compared to patients who received 3DCRT (Fig. 2 and Table 4). These data indicate that bone marrow sparing IMRT is useful because IMRT is particularly effective at reducing the volume receiving a relatively high dose. Conversely, Mell et al. and Albuquerque et al. found that V10 and V20 of the pelvic bone more accurately predicted HT complications, compared to V30 or V40 [11, 21]. However, patients in our study who received IMRT had a greater incidence of HT complications and a significantly reduced V20 of the pelvic bone compared to patients who received 3DCRT (Table 4). These data indicate that the relationship between HT complications and DVH parameters of the pelvic bone is complicated. Therefore, future studies are required to examine the clinical benefit of IMRT in reducing HT complications and to validate the critical DVH predictors of these complications.
The findings in this study should be interpreted with an understanding of the following limitations. First, the heterogeneity in the treatment planning approach over the periods of the study (3DCRT and IMRT); the low number of events, especially in IMRT; and the lack of a pre-specified model or protocol are important limitations of the data and analysis. Second, we used weekly nedaplatin as concurrent chemotherapy, whereas chemoradiation therapy with 40 mg/m2 weekly cisplatin is now accepted as the standard first-line treatment. Therefore, we cannot exclude the possibility that the DVH parameter predictors found in this study may be chemotherapy-type specific, particularly as Bazan et al. showed that DVH predictors for acute HT complications in patients receiving IMRT are dependent on the type of chemotherapy [22].
Conclusions
We conclude that IMRT is useful for decreasing GI complications without worsening outcomes. Further studies are required to identify critical DVH parameters for avoidance of acute HT complications.
Abbreviations
- IMRT:
-
Intensity-modulated radiation therapy
- 3DCRT:
-
Three-dimensional conformal radiation therapy
- DVH:
-
Dose-volume histogram
- RT:
-
Radiation therapy
- OS:
-
Overall survival rate
- LRC:
-
Loco-regional control rate
- PFS:
-
Progression-free survival rate
- GI:
-
Gastrointestinal
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- CRT:
-
Conformal radiation therapy
- CTV:
-
Clinical target volume
- RTOG:
-
the Radiation Therapy Oncology Group
- GU:
-
Genitourinary
- HT:
-
Hematologic
- CI:
-
Confidence interval
- QUANTEC:
-
the Quantitative Analyses of Normal Tissue Effects in the Clinic
References
Peters WA 3rd, Liu PY, Barret RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage caner of the cervix. J Clin Oncol. 2000;18:1606–13.
Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–83.
Ryu SY, Park SI, Nam BH, Cho CK, Kim K, Kim BJ, et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int J Radiat Oncol Biol Phys. 2011;79:794–9.
Isohashi F, Yoshioka Y, Mabuchi S, Konishi K, Koizumi M, Takahashi Y, et al. Dose-volume histogram predictors of chronic gastrointestinal complications after radical hysterectomy and postoperative concurrent nedaplatin-based chemoradiation therapy for early-stage cervical cancer. Int J Radiat Oncol Biol Phys. 2013;85:728–34.
Mabuchi S, Morishige K, Isohashi F, Yoshioka Y, Takeda T, Yamamoto T, et al. Postoperative concurrent nedaplatin-based chemoradiotherapy improves survival in early-stage cervical cancer patients with adverse risk factors. Gynecol Oncol. 2009;115:482–7.
Okazawa M, Mabuchi S, Isohashi F, Suzuki O, Yoshioka Y, Sasano T, et al. Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage IB1-IIB cervical cancer patients with intermediate-risk or high-risk factors: a 13-year experience. Int J Gynecol Cancer. 2013;23:567–75.
Mabuchi S, Okazawa M, Isohashi F, Ohta Y, Maruoka S, Yoshioka Y, et al. Postoperative whole pelvic radiotherapy plus concurrent chemotherapy versus extended-field irradiation for early-stage cervical cancer patients with multiple pelvic lymph node metastases. Gynecol Oncol. 2011;120:94–100.
Mabuchi S, Takahashi R, Isohashi F, Yokoi T, Ito K, Tsutui T, et al. A phase I study of concurrent weekly carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy as an adjuvant treatment for early-stage cervical cancer patients with positive pelvic lymph nodes. Int J Gynecol Cancer. 2013;23:1279–86.
Small W Jr, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428–34.
Japan Clinical Oncology Group, Toita T, Ohno T, Kaneyasu Y, Uno T, Yoshimura R, et al. A consensus-based guideline defining the clinical target volume for pelvic lymph nodes in external beam radiotherapy for uterine cervical cancer. Jpn J Clin Oncol. 2010;40:456–63.
Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–65.
Chen MF, Tseng CJ, Tseng CC, Kuo YC, Yu CY, Chen WC. Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent Cisplatin and intensity-modulated pelvic radiotherapy: comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1438–44.
Folkert MR, Shih KK, Abu-Rustum NR, Jewell E, Kollmeier MA, Makker V, et al. Postoperative pelvic intensity-modulated radiotherapy and concurrent chemotherapy in intermediate- and high-risk cervical cancer. Gynecol Oncol. 2013;128:288–93.
Mabuchi S, Kimura T. Nedaplatin: a radiosensitizing agent for patients with cervical cancer. Chemother Res Pract. 2011;2011:963159.
Kobayashi Y, Ohara T, Wada Y, Okuda Y, Kondo H, Okuma Y, et al. Concurrent chemoradiotherapy with nedaplatin after radical hysterectomy in patients with stage IB and II cervical cancer. J Obstet Gynaecol Res. 2009;35:490–4.
Niibe S, Tsunoda T, Jobo T, Imai M, Matsuo K, Matsunaga K, et al. Phase II study of radiation therapy combined with weekly nedaplatin in locally advanced uterine cervical carcinoma (LAUCC): Kitasato Gynecologic Radiation Oncology Group (KGROG 0501): initial analysis. Eur J Gynaecol Oncol. 2008;29:222–4.
Chopra S, Dora T, Chinnachamy AN, Thomas B, Kannan S, Engineer R, et al. Predictors of grade 3 or higher late bowel toxicity in patients undergoing pelvic radiation for cervical cancer: results from a prospective study. Int J Radiat Oncol Biol Phys. 2014;88:630–5.
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76 Suppl 3:S101–7.
Wedlake LJ, Thomas K, Lalji A, Blake P, Khoo VS, Tait D, et al. Predicting late effects of pelvic radiotherapy: is there a better approach? Int J Radiat Oncol Biol Phys. 2010;78:1163–70.
Klopp AH, Moughan J, Portelance L, Miller BE, Salehpour MR, Hildebrandt E, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90.
Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79:1043–7.
Bazan JG, Luxton G, Kozak MM, Anderson EM, Hancock SL, Kapp DS, et al. Impact of chemotherapy on normal tissue complication probability models of acute hematologic toxicity in patients receiving pelvic intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:983–91.
Acknowledgement
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 25861097.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FI coordinated the entire study. Data acquisition was performed by FI, SM, YY, YS, OS, KT, MY, HU, YK, KK, IS, YO and TK. Data analysis was performed by FI, SM, YY, TK, and KO. The manuscript was prepared by FI. Corrections and improvements were suggested by SM, YY and KO. Revisions were done by SM, YY, YS, OS, KT, MY, HU, YK, KK, IS, YO, TK and KO. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Isohashi, F., Mabuchi, S., Yoshioka, Y. et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose-volume histogram parameters. Radiat Oncol 10, 180 (2015). https://doi.org/10.1186/s13014-015-0486-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-015-0486-5