Abstract
Background
To analysis the clinical outcomes of concurrent chemoradiotherapy (CCRT) alone based on 10-year results for loco-regionally advanced nasopharyngeal carcinoma (LANPC), so as to provide evidence for individualized treatment strategy and designing appropriate clinical trial for different risk LANPC patients.
Methods
Consecutive patients with stage III-IVa (AJCC/UICC 8th) were enrolled in this study. All patients received radical intensity-modulated radiotherapy (IMRT) and concurrent cisplatin chemotherapy (CDDP). The hazard ratios (HRs) of death risk in patients with T3N0 was used as baseline, relative HRs were calculated by a Cox proportional hazard model to classify different death risk patients. Survival curves for the time-to-event endpoints were analyzed by the Kaplan–Meier method and compared using the log-rank test. All statistical tests were conducted at a two-sided level of significance of 0.05.
Results
A total of 456 eligible patients were included. With 12-year median follow-up, 10-year overall survival (OS) was 76%. 10-year loco-regionally failure-free survival (LR-FFS), distant failure-free survival (D-FFS) and failure-free survival (FFS) were 72%, 73% and 70%, respectively. Based on the relative hazard ratios (HRs) of death risk, LANPC patients were classified into 3 subgroups, low-risk group (T1-2N2 and T3N0-1) contained 244 patients with HR < 2; medium-risk group (T3N2 and T4N0-1) contained 140 patients with HR of 2 – 5; high-risk group (T4N2 and T1-4N3) contained 72 patients with HR > 5. The 10-year OS for patients in low-, medium-, and high-risk group were 86%, 71% and 52%, respectively. Significantly differences of OS rates were found between each of the two groups (low-risk group vs. medium-risk group, P < 0.001; low-risk group vs. high-risk group, P < 0.001; and medium-risk group vs. high-risk group, P = 0.002, respectively). Grade 3–4 late toxicities included deafness/otitis (9%), xerostomia (4%), temporal lobe injury (5%), cranial neuropathy (4%), peripheral neuropathy (2%), soft tissue damage (2%) and trismus (1%).
Conclusions
Our classification criteria demonstrated that significant heterogeneity in death risk among TN substages for LANPC patients. IMRT plus CDDP alone maybe suitable for low-risk LANPC (T1-2N2 or T3N0-1), but not for medium- and high-risk patients. These prognostic groupings provide a practicable anatomic foundation to guide individualized treatment and select optimal targeting in the future clinical trials.
Similar content being viewed by others
Background
There were 133,354 new cases of nasopharyngeal carcinoma (NPC) worldwide in 2020, accounting for 0.7% of all cancers [1]. But its geographical global distribution is extremely unbalanced; over 70% of new cases are in east and southeast Asia, with an age-standardized rate (world) of 3.0 per 100,000 in China [2]. Unfortunately, over 75% of patients present with LANPC at the time of diagnosis [3]. Cisplatin-based CCRT has been established as the foundation of treatment strategy for LANPC based on several prospective randomized clinical trials and meta-analysis [4,5,6,7]. Recently, phase III randomized controlled trials have proved that induction chemotherapy (IC) or adjuvant chemotherapy (AC) added to CCRT can significantly prolong survival [4, 8,9,10,11,12,13,14]. However, not all patients of LANPC benefit from IC or AC [15, 16]. In addition, regimens with more chemotherapy has been demonstrated to associate with increasing risk of therapeutic toxicity [17]. Therefore, it is necessary to analysis the treatment strategies in LANPC with different failure risks, so as to facilitate individualized treatment and avoid excessive toxicity causing by overtreatment or treatment failure due to undertreatment.
Because LANPC contains a heterogeneous group of patients, which led to broadly varying disease extent. It is suggested that the current anatomy-based staging system is insufficient for prediction prognosis or treatment benefits. Studies have assessed whether incorporating other clinical factors and non-anatomical factors, such as gross tumor volume [18], 18F-fluorodeoxyglucose (18F-FDG)- positron emission tomography (PET) standardized uptake value (SUV) within primary tumor [19, 20] and plasma Epstein-Barr virus DNA (EBV-DNA) load [21, 22], etc. in LANPC patients would present various disease characteristics, and lead to different outcomes when used a uniform treatment. However, because these parameters had established in different hospitals and laboratories, their cut-off values are not standardized, which limits their clinical application as a general indicator for screening tumor heterogeneity.
AJCC/UICC TNM staging classification is an internationally recognized staging system that is widely used for predicting prognosis, guiding treatment strategy for different risk groups and facilitating exchange of experience between oncology centers. But significant heterogeneity is often observed for different T-N subgroups within equivalent clinical TNM stage [23, 24]. It is reasonable to select pertinent chemotherapy scheme for different failure risk levels. Therefore, in order to enhance the power to detect a survival benefit with additional IC or AC, studies excluded those LANPC patients with low relapse risk, some excluded patients with T3-4N0M0 [14, 25], and the other excluded T3-4N0/T3N1M0 [26]. But it is still questioned whether the current division is the optimal scheme, especially within the updated 8th stage groupings. On the basis of this premise, we conducted this retrospective analysis of a series of LANPC patients treated with CCRT. We constructed a framework according to the risk of death after treatment, intended to provide high sensitivity to predict overall survival (OS) risk using the 8th edition T and N stage, so as to provide better guidance of individualized treatment and to serve as a skeleton framework for additional biomarkers be employed.
Methods
Patient eligibility
A total of 456 pathologically diagnosed LANPC patients who received CCRT alone between August 2001 and June 2014 in one attending group of Sun Yat-sen University Cancer Center were included in this study. The pretreatment workup included a complete history and physical examination, hematological and biochemical profiles, nasopharyngoscopy, chest X-ray, abdominal ultrasound, magnetic resonance imaging (MRI) of the head and neck, and whole body emission computed tomography (ECT). Positron emission tomography computed tomography (PET/CT) with 18F-FDG was conducted for 80 (17.5%) patients. Patients were excluded if they had stage I–II disease, distant metastasis (DM) disease, missing medical data, not finish 2-cycle CCRT, received IC or AC. And all patients were restaged according to the AJCC/UICC stage classification system, 8th edition [27].
Treatment
All eligible patients received curative IMRT. The details of the whole IMRT process have been described previously [28]. Briefly, gross tumor volume was determined according to diagnostic MRI as well as physical examination. The nasopharynx gross tumor volume (GTVnx) and the gross tumor volume of metastatic neck lymph nodes (GTVnd) were identified. Two clinical target volumes (CTVs) were delineated: CTV1 and CTV2. CTV1 was defined as the GTVnx plus a 5 to 10 mm margin (2 to 3 mm margin posteriorly) to encompass the high-risk sites of microscopic extension and the whole nasopharynx. CTV2 was defined as the CTV1 plus a 5 to 10 mm margin (2 to 3 mm margin posteriorly) to encompass the low-risk sites of microscopic extension, the level of the lymph node located, and the elective neck area. The prescription dose was 68–70 Gy, 60–66 Gy, 60 Gy, and 54 Gy, in 30 fractions, for the planning target volumes (PTVs) derived from GTVnx, GTVnd, CTV1, and CTV2, respectively. PTVs were generated by a geometric circumferential expansion of 3 mm, as per our institutional protocol that is determined by the aggregation of systematic and random errors. The dose constrains to organs at risk (OARs) were within the tolerance according to the QUANTEC [29]. The dose limitation to OARs has been detailed described in our previous published study [30].
All patients received 2 cycles cisplatin concurrent chemotherapy. The regimen consisted of cisplatin alone at 80 mg/m2/d, intravenous infusion on days 1 and 22 over 2 h. The cumulative cisplatin dose (CCD) was 160 mg/m2.
Patient assessment and follow-up
Patients were evaluated at least once per week during IMRT. The first assessment of tumor response was performed one month after completion of radiotherapy by physical examination and flexible nasopharyngoscopy. MRI of the head and neck was performed three months after radiotherapy. Then, patients were required to be evaluated once every 3 months in the first 3 years, once every 6 months for the following 2 years, and once every year thereafter. During each follow-up visit, complete physical exams including indirect nasopharyngeal speculum examinations were performed. Head-neck MRI, hematologic and biochemical profiles, chest radiography and abdominal ultrasonography were required each year. Further investigations would be arranged when clinically indicate. Tumor recurrence or metastasis was confirmed on the basis of the results of biopsy or fine-needle aspiration. For lesions that was not accessible, the clinical diagnosis was based on the presence of at least 2 radiological features on CT, MRI, ECT or 18F-FDG PET/CT. Management of residual disease and tumor relapse, if detected, was determined on a case-by-case basis.
Acute toxicities during CCRT were graded according to the Common Terminology Criteria for Adverse Events (version 3.0); late toxicities were graded according to the Late Radiation Morbidity Scoring Criteria of the Radiation Therapy Oncology Group [31].
Statistical analysis
We calculated failure-free survival (FFS) from the date of first histological diagnosis to the date of treatment failure or death from any cause, whichever was first. Histology biopsy or at least two different examinations (MRI, CT, PET/CT or endoscopy) were used to determine treatment failure. Overall survival (OS) was defined as death due to any cause. For locoregional (LR-FFS) analysis, we recorded the latencies to the first locoregional recurrence, or death from any cause. For distant failure-free survival (D-FFS), we recorded the latencies to the first distant failure, or death from any cause. Statistical analysis was performed using SPSS 22.0 (Chicago, Illinois, USA). Survivals were calculated by the Kaplan–Meier method. The log-rank test was used to calculate the significance of differences between survival curves. Cox regression was used to determine prognostic factors. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by a Cox proportional hazards model. All statistical tests were conducted at a two-sided level of significance of 0.05.
Results
Patient characteristics
From August 2001 and June 2014, 456 patients were enrolled, with the detailed demographic and clinical characteristics summarized in Table 1. The majority were nonkeratinizing (undifferentiated) carcinoma (438, 96.1%). After being restaged using the AJCC/UICC 8th staging system, 302 patients were stage III and 154 were stage IVa. Up to then, 298 (65%) survivors were followed up to at least 10 years.
All patients completed IMRT as planned. The median D95 of PTVnx, PTVnd, PTV1, and PTV2 were 69.2 Gy, 63.6 Gy, 64.8 Gy, and 56.1 Gy, as shown in Additional file 1: Table S1.
Survivals
At the last follow-up on May 30th, 2021, the median follow-up was 12-year (IQR 6 years—14 years). Overall, One hundred and thirteen patients died, among them, 84 (74.3%) were cancer-specific deaths, and 29 (25.7%) patients died of noncancer-related causes. The 5-year and 10-year OS was 81% and 76%, respectively. 137 (30%) of 456 patients had treatment failure. The proportion of patients with FFS at 5-year and 10-year were 74% and 70%, respectively. 35 (25.5%) of 137 patients experienced local recurrence, 8 (5.8%) had regional recurrence, and 72 (52.6%) developed DM. The 10-year LR-FFS and D-FFS were 72% and 73%, respectively.
Relative HRs according to various T and N combination subgroups for death
To evaluate the relative risk of death for different T and N subgroups, the HRs for each subgroup were calculated with death risk as the endpoint. T1N2 was analyzed with T2N2 because of the limited case fold. Similarly, any T with N3 were combined and analyzed. The HR of patients with T3N0 disease was defined at baseline (HR = 1). Host factors (sex, age) were included as covariates, and the Cox regression model was used to calculate HRs in various T and N subgroups. Patients were then divided into 8 subgroups. As T and N combination increased, the HR showed a tendency to gradually increase (Fig. 1).
Comparison on treatment outcomes among risk subgroups for LANPC
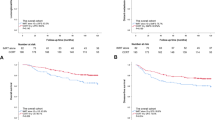
According to the HR analysis, 456 patients were classified into 3 sub-groups: low-risk group (T1-2N2 or T3N0-1) contained 244 patients with HR < 2, medium-risk group (T3N2 or T4N0-1) included 134 patients with HR of 2–5, high-risk group (T4N2 or T1-4N3) involved 78 patients with HR > 5. The 10-year LR-FFS for low-risk, medium-risk and high-risk group were 82%, 69%, and 47%, respectively (P < 0.001). The 10-year D-FFS for low-risk, medium-risk and high-risk group were 83%, 68%, and 50%, respectively (P < 0.001). The 10-year OS for low-risk, medium-risk and high-risk group were 86%, 71%, and 52%, respectively (P < 0.001) (Fig. 2). Further analysis showed that when the OS rate was compared between each of the two groups (low-risk vs. medium-risk, low-risk vs. high-risk, and medium-risk vs. high-risk), significant differences could be found (P < 0.001, P < 0.001, and P = 0.002, respectively) (Table 2).
Failure patterns in risk subgroups
The different failure patterns in risk subgroups were summarized in Table 3. The results showed a direct relationship between an increased distant metastasis rate or death rate and a higher risk subgroup (P < 0.001 and P < 0.001, respectively). More than half of the high-risk patients (52%) died.
Adverse events
Over the entire treatment course, 174 patients (38%) experienced grade 3 or 4 acute adverse events. Leukopenia was the most common severe hematologic event (21%), following by neutropenia (14%). Mucositis was the most common severe nonhematologic event (17%). The incidence of late toxic effects of grade 3 or 4 was 16% (72 patients). There were 40 patients (9%) suffered more than one late toxic effects of grade 3 or 4. Ear damage (8%) was the most common severe late toxic event. The detailed acute and late toxicities are shown in Table 4.
Discussion
As the first study with over 10-year follow-up using CCRT alone for LANPC in the era of IMRT, we established an anatomic-based risk stratification for overall death in LANPC patients, and generated three distinctly different risk groups: low-risk group (T1-2N2 and T3N0-1), medium-risk group (T3N2 and T4N0-1), and high-risk group (T4N2 and T1-4N3). Our results showed that low-risk patients can gain benefit from CCRT, with mild late toxicity; however, the 10-year OS rate was 71% for the middle-risk group, 52% for the high-risk group, which suggested that more advanced treatment strategy were needed for these patients.
Since the current TNM staging system included a heterogeneous group of NPC patients with different prognosis, important non-anatomical prognostic factors, such as EBV-DNA load or primary tumor SUV, are suggested to incorporate into guiding the clinical treatment [19,20,21,22]. However, the variation of cut-off values hamper their clinical applications. For example, in a matched study, the authors defined patients of N2-3 stage with an EBV DNA ≥ 4000 copies/ml as being very high-risk group [32]. Yet the results from a phase III study proved that the EBV DNA level ≥ 6000 copies/ml were significantly associated with poorer FFS and OS[14].
For low-risk group, our study showed a satisfactory 5-year OS (90%). A recent randomized trial selected low-risk LANPC patients treated with different CCD to test the non-inferiority of 2-cycle over 3-cycle concurrent cisplatin regimen. It concluded that 2-cycle concurrent cisplatin yielded comparable survival benefits to 3-cycle, and was associated with less acute and late toxicities and improved quality of life [33], which give us a clue that the satisfactory survival benefits might still be achieved by reducing CCD in concurrent chemotherapy for low-risk LANPC.
Over the past few decades, considerable efforts have been made to improve survival outcomes of LANPC patients by adding IC or AC to CCRT. Several phase 3 studies have shown that the additional IC or AC gained significant improvement in survivals compared with CCRT alone [4, 8,9,10,11,12,13,14]. In our study, the 5-year FFS and OS for medium-risk group was 69% and 78%, which were similar to that (66.4% and 77.7%) of CCRT alone group in Li et al. study[14]. Further, Li and colleagues proved that after adding IC to CCRT, a significant improvement of 5-year FFS and OS (77.4% and 85.6%, respectively) (P = 0.042) were noted [14], despite the complete rates of IMRT and ≥ 200 mg/m2 CDDP were lower than CCRT alone group (97.9% vs. 100%, and 85.9% vs. 98.3%, respectively). Therefore, for the medium-risk group, further investigation may be needed to evaluate the utility of IC or AC.
In the current study, the 10-year OS for the high-risk group (52%) were much lower than the low-risk group (86%) and medium-risk group (71%) (P < 0.001, P = 0.002, respectively). In addition, A series of phase 3 studies indicated that the IC + CCRT cannot effectively improve the prognosis of high-risk LANPC patients [13, 14, 34], further intensification treatment is needed. Recently, two phase 3 trials have focusing on the efficacy and safety profile of additional metronomic adjuvant capecitabine in patients with high-risk LANPC [26, 35]. Both trials proved that adjuvant capecitabine was well tolerance. Miao and colleagues [35] reported an estimated 7.7% (88.8% for adjuvant capecitabine vs 81.1% for CCRT) better 3-year DMFS and an estimated 11.5% (91.5% for adjuvant capecitabine vs 80% for CCRT) better 3-year LRRFS with the addition of capecitabine. Similarly, Chen and colleagues [26] reported an estimated 9.6% better 3-year FFS with the addition of AC (85.3% for metronomic capecitabine vs 75.7% for IC + CCRT), suggesting a potential role for capecitabine as an adjuvant therapy in the treatment of high-risk LANPC.
Anti-programmed death (PD) therapy has become the backbone of cancer immunotherapy and a major modality of cancer treatment. Since studies have proved that anti-PD1 therapy is a potential treatment option for patients with recurrent or metastatic (R/M) NPC [36,37,38,39]. It is reasonable to assume that it might also workable in LANPC. Several randomized trials are currently underway to evaluated therapeutic benefits of adding PD-1 antibody in high-risk LANPC, and the results are worth expected.
In this study, we summarized patients’ acute and late toxicities, especially the latter. Most of patients’ late toxicities were in grade 1–2, only 8% patients had grade 3–4 deafness or otitis, 5% patients had grade 3 temporal lobe injury, 4% patients had grade 3 dry mouth, 4% patients had grade 3 cranial neuropathy. Other grade 3–4 late toxicities were lower than 3%.
Besides, there are some limitations in this study. First, the data were derived from one single institution in endemic area, whether the findings can be reproduced and are generalizable to other patient populations remains to be demonstrated. External validation in multicenter hospitals is warranted. Second, some may argue that we did not integrate non-anatomical factors, such as EBV-DNA, into the model. However, routine detection of plasma EBV-DNA was not widely used for our patients treated before 2009, and the methodology has not been standardized so far. Our model presents a practical way for evaluating risk of death, and provides a relatively stable anatomic framework that partitions according to a death risk hierarchy.
Conclusions
This article proved that our classification criteria are practicable and useful for LANPC. Cisplatin-based CCRT satisfied survival outcomes for low-risk LANPC patients. For medium-risk and high-risk patients, more effective systematic treatment strategies and treatment sequences need to be explored.
Availability of data and materials
Not applicable.
Abbreviations
- NPC:
-
Nasopharyngeal carcinoma
- HRs:
-
Relative hazard ratios
- RT:
-
Radiation therapy
- IMRT:
-
Intensity-modulated radiotherapy
- 2D-CRT:
-
2-Dimensional conventional radiotherapy
- CCRT:
-
Concurrent chemo-radiotherapy
- AC:
-
Adjuvant chemotherapy
- IC:
-
Induction chemotherapy
- LR-FFS:
-
Locoregional failure-free survival
- D-FFS:
-
Distant failure-free survival
- DMFS:
-
Distant metastasis-free survival
- FFS:
-
Failure-free survival
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- DM:
-
Distant metastasis
- CT:
-
Contrast-enhanced computed tomography
- MRI:
-
Magnetic resonance imaging
- ECT:
-
Emission computed tomography
- PET:
-
Positron emission tomography
- SUV:
-
Standardized uptake value
- EBV-DNA:
-
Epstein-Barr virus DNA
- GTVnx:
-
The gross tumor volume of nasopharynx
- GTVnd:
-
The gross tumor volume of the involved neck area
- CTV1:
-
The high-risk sites of microscopic extension
- CTV2:
-
The low-risk sites of microscopic extension
- PTVs:
-
Planning target volumes
- OARs:
-
Organs at risk
- CCD:
-
Cumulative cisplatin dose
- CIs:
-
Confidence intervals
References
Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2020, 70(4):313.
Chen Y, Chan A, Le Q, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (London, England). 2019;394(10192):64–80.
Pan J, Ng W, Zong J, Lee S, Choi H, Chan L, Lin S, Guo Q, Sze H, Chen Y et al: Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016, 122(21):3307–15.
Blanchard P, Lee A, Marguet S, Leclercq J, Ng W, Ma J, Chan A, Huang P, Benhamou E, Zhu G, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55.
Chan A, Teo P, Ngan R, Leung T, Lau W, Zee B, Leung S, Cheung F, Yeo W, Yiu H, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–44.
Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, Wu SX. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol. 2005;23(33):8461–8.
Yi J, Huang X, Gao L, Luo J, Zhang S, Wang K, Qu Y, Xiao J, Xu G. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2014;9:56.
Chen Y, Ismaila N, Chua M, Colevas A, Haddad R, Huang S, Wee J, Whitley A, Yi J, Yom S, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II–IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021;39(7):840–59.
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, Jin F, Shi M, Chen YP, Hu WH, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–35.
Xia W, Liang H, Lv X, Wang L, Ye Y, Ke L, Xu L, Guo X, Xiang Y. Stage-specific concurrent chemoradiotherapy with or without induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective, population-based study. Cancer Manag Res. 2019;11:9813–27.
Cao S, Yang Q, Guo L, Mai H, Mo H, Cao K, Qian C, Zhao C, Xiang Y, Zhang X, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer (Oxford, England : 1990) 2017; 75:14–23.
Yang Q, Cao S, Guo L, Hua Y, Huang P, Zhang X, Lin M, You R, Zou X, Liu Y et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. European journal of cancer (Oxford, England : 1990) 2019;119:87–96.
Sun Y, Li W-F, Chen N-Y, Zhang N, Hu G-Q, Xie F-Y, Sun Y, Chen X-Z, Li J-G, Zhu X-D, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20.
Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, Hu CS, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145(1):295–305.
Lan X, Xiao Y, Zou X, Zhang X, OuYang P, Xie F. Outcomes of adding induction chemotherapy to concurrent chemoradiotherapy for stage T3N0-1 nasopharyngeal carcinoma: a propensity-matched study. Onco Targets Ther. 2017;10:3853–60.
Xu C, Zhang S, Li W, Chen L, Mao Y, Guo Y, Liu Q, Ma J, Tang L. Selection and validation of induction chemotherapy beneficiaries among patients with T3N0, T3N1, T4N0 nasopharyngeal carcinoma using epstein-barr virus DNA: a joint analysis of real-world and clinical trial data. Front Oncol. 2019;9:1343.
Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, Huang PY, Zhu G, Chua DTT, Chen Y, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35(5):498–505.
Xue F, Ou D, Ou X, Zhou X, Hu C, He X. Prognostic efficacy of extensive invasion of primary tumor volume for T3–4 nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. Oral Oncol. 2020;100:104478.
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu S, Shi W, Zhu B, Zhang Y, Hu C. Pretreatment (18)F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma–a retrospective study. Radiat Oncol. 2015;10:4.
Xiao W, Xu A, Han F, Lin X, Lu L, Shen G, Huang S, Fan W, Deng X, Zhao C. Positron emission tomography-computed tomography before treatment is highly prognostic of distant metastasis in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy treatment: a prospective study with long-term follow-up. Oral Oncol. 2015;51(4):363–9.
Lee A, Lee V, Ng W, Strojan P, Saba N, Rinaldo A, Willems S, Rodrigo J, Forastiere A, Ferlito A. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur J Cancer (Oxford, England: 1990) 2021, 153:109–122.
Kim K, Le Q, Yom S, Ng R, Chan K, Bratman S, Welch J, Divi R, Petryshyn R, Conley B. Clinical utility of Epstein-Barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2017;98(5):996–1001.
Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, Tan T, Chua DT, O’Sullivan B, Tung R, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):15–22.
Su SF, Han F, Zhao C, Huang Y, Chen CY, Xiao WW, Li JX, Lu TX. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer. 2011;30(8):565–73.
Chen L, Hu C-S, Chen X-Z, Hu G-Q, Cheng Z-B, Sun Y, Li W-X, Chen Y-Y, Xie F-Y, Liang S-B, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13(2):163–71.
Chen Y, Liu X, Zhou Q, Yang K, Jin F, Zhu X, Shi M, Hu G, Hu W, Sun Y et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet (London, England) 2021.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: A Cancer J Clin. 2017; 67(2):93–99.
Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, Deng XW, Lu TX, Cui NJ, Zhao C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874–83.
Bentzen S, Constine L, Deasy J, Eisbruch A, Jackson A, Marks L, Ten Haken R, Yorke E. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3-9.
Miao J, Di M, Chen B, Wang L, Cao Y, Xiao W, Wong K, Huang L, Zhu M, Huang H, et al. A prospective 10-year observational study of reduction of radiation therapy clinical target volume and dose in early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2020;107(4):672–82.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–6.
Guo SS, Tang LQ, Chen QY, Zhang L, Liu LT, Guo L, Mo HY, Luo DH, Huang PY, Xiang YQ, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in stage III-IVb nasopharyngeal carcinoma patients with Epstein-Barr virus DNA >/=4000 copies/ml: a matched study. Oncotarget. 2016;7(20):29739–48.
Mai H-Q, Li XY, Mo H-Y, Ling G, Luo D-H, Sun R, Liu L, Guo S-S, Yang J-H, Sun X-S et al. De-intensified chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma based on plasma EBV DNA: A phase 2 randomized noninferiority trial. J Clin Oncol 2021, 39(15_suppl):110.
Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, Lou PJ, Wang HM, Tsai MH, Lai SC, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol. 2018;29(9):1972–9.
Miao J, Wang L, Tan SH, Li J-G, Yi J, Zhang Y, Gong X, Yanqun X, Chen Q-Y, Chen M, et al. Adjuvant capecitabine in locoregionally advanced nasopharyngeal carcinoma: a multicenter randomized controlled phase III trial. J Clin Oncol. 2021;39:15_suppl:6005–6005.
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–50.
Ma B, Lim W, Goh B, Hui E, Lo K, Pettinger A, Foster N, Riess J, Agulnik M, Chang A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol. 2018;36(14):1412–8.
Hsu C, Lee S, Ejadi S, Even C, Cohen R, Le Tourneau C, Mehnert J, Algazi A, van Brummelen E, Saraf S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–6.
Mai H, Chen Q, Chen D, Hu C, Yang K, Wen J, Li J, Shi Y, Jin F, Xu R et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation of China [No. 81872469, 82073330, and 82203966], Guangdong Provincial Medical Science and Technology Research Fund [A2022355]. The funding provided the resources for the study design, data collection and data analysis, and manuscript polish.
Author information
Authors and Affiliations
Contributions
AAX was major contributor in writing the manuscript. JJM, ACL and LW analyzed the patient data. SMH, ZWM and XFS were responsible for the statistical data. FH and CZ performed the follow-up examination. YWY, XWD and CZ was responsible for the study design. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Volume and dosimetry data of target volumes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, AA., Miao, JJ., Wang, L. et al. Efficacy of concurrent chemoradiotherapy alone for loco-regionally advanced nasopharyngeal carcinoma: long-term follow-up analysis. Radiat Oncol 18, 63 (2023). https://doi.org/10.1186/s13014-023-02247-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02247-y