Abstract
Adaptive radiotherapy (ART) was introduced in the late 1990s to improve the accuracy and efficiency of therapy and minimize radiation-induced toxicities. ART combines multiple tools for imaging, assessing the need for adaptation, treatment planning, quality assurance, and has been utilized to monitor inter- or intra-fraction anatomical variations of the target and organs-at-risk (OARs). Ethos™ (Varian Medical Systems, Palo Alto, CA), a cone beam computed tomography (CBCT) based radiotherapy treatment system that uses artificial intelligence (AI) and machine learning to perform ART, was introduced in 2020. Since then, numerous studies have been done to examine the potential benefits of Ethos™ CBCT-guided ART compared to non-adaptive radiotherapy. This review will explore the current trends of Ethos™, including improved CBCT image quality, a feasible clinical workflow, daily automated contouring and treatment planning, and motion management. Nevertheless, evidence of clinical improvements with the use of Ethos™ are limited and is currently under investigation via clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
For the majority of courses of external beam radiotherapy today, a typical process begins with a single treatment plan generated at the simulation step and carried through the entire course [1]. Given time and resource constraints, these plans are rarely modified over the course of treatment despite the internal changes within the patient that may lead to errors in dose placement, suboptimal disease responses, and avoidable radiation-induced toxicities. Adaptive radiotherapy (ART) was introduced in the late 1990s as a “closed-loop radiation treatment process where the treatment plan can be modified using a systematic feedback of measurements” [2, 3]. Its primary goal has been to improve the accuracy and efficiency of therapy and minimize radiation-induced toxicities. By 2010, ART was widely described within the radiation oncology literature [4, 5]. ART combines multiple tools for imaging, assessing the need for adaptation, treatment planning, quality assurance, and has been utilized to monitor inter- or intra-fraction anatomical variations of the target and organs-at-risk (OARs). These tools can enable dose escalation or maintaining coverage of target doses while reducing doses to OARs [3, 6,7,8,9].
The implementation of ART is categorized into three major classes. (1) Offline ART, in which scheduled imaging between fractions is used to detect systematic and progressive changes that occur during the treatment course. (2) Online ART entails the adjustment of treatment plans prior to radiation delivery to account for inter-fraction changes, both temporal and stochastic, while the patient remains in the treatment position. (3) Real-time ART, which accounts for intra-fractional variations and allows for automatic plan adjustments during radiation delivery without manual intervention [3]. Both offline [6, 10,11,12,13] and online [14,15,16,17,18,19,20,21,22,23] ART has shown improvements in target coverage and OAR sparing in cancers of the prostate, head and neck, lung, abdomen, and pelvis. Executing ART requires adequate information for target and OAR delineation, accurate dose calculation, and sufficient image quality [3].
Given rapid and recent advancements in online ART technology, most commonly delivered either with linear accelerators combined with onboard magnetic resonance imaging (“MRI-Linac”) or CBCT-guided systems, a review that describes the technical and clinical state of the art is necessary. This comprehensive review will focus on published and presented data on CBCT-guided online ART with the Ethos™ system. We also evaluate the feasibility of applying this system to a greater clinical context and discuss future directions. We located peer-reviewed articles and abstracts on the topic of CBCT-guided online ART published from 2019, which was the first year that Ethos was under investigation, to present day. A search of following terms were conducted in PubMed: ““CBCT-guided” OR “CT-Guided” AND “real-time adaptive” OR “online adaptive” OR “ontable adaptive” OR “Ethos”. We excluded publications regarding proton therapy, brachytherapy, MR-Linac, offline ART, and CT-based ART in which CT is not an integrated part of the treatment system (e.g., CT on rails).
CBCT-based online ART
Before each treatment, the process of CBCT-guided ART begins with the use of a cone shaped X-ray beam where the kilovoltage (kV) source and a flat panel detector rotates around a patient on the treatment table [24, 25]. The acquired CT image is then automatically segmented into various organs and bony structures [26]. Based on this delineation of the day’s anatomy, the system generates a preview of the dose distribution for OARs and the disease target from a prioritized list of clinical goals, followed by creation of multiple deliverable treatment plans [26]. At this time, the best plan deemed by the radiation oncologist was selected as the reference plan and approved through a quality assurance (QA) protocol [26]. Finally, treatment is delivered to the patient, and the process is repeated prior to subsequent treatments.
Conventional CBCT has been widely used for positioning of patients during ART, but has significantly inferior image quality due to increased radiation scatter compared to traditional fan-beam (planning) CT [27]. New image reconstruction algorithms such as iterative CBCT (iCBCT) have enhanced the overall quality CBCT image generation to create more accurate CBCT-based image-guided radiotherapy (IGRT) [27, 28]. Ethos™ (Varian Medical Systems, Palo Alto, CA), a radiotherapy treatment system that uses artificial intelligence (AI) and machine learning to perform ART, was introduced in 2020. The system integrates iCBCT [29], and provides a highly efficient adaptive workflow while the patient is on the treatment couch, allowing a physician to select either the reference plan or the adapted treatment plan within a typical 15–25 min scheduled time slot [29].
kV CBCT image quality
High-quality images that are obtained quickly are necessary for auto-segmentation and on-table adaption during a short treatment time slot. Cai et al. investigated the image quality of kV CBCT on Halcyon™ 2.0, which the Ethos™ kV CBCT was built upon [30]. The kV CBCT can rapidly acquire high quality images with iterative reconstruction that yield high contrast-to-noise ratio (CNR) [30]. In addition, the fast gantry motion allows for single breath-holding imaging, which can minimize artifacts and provide potential for on-table structure delineation [30]. Schiff et al. also reported, the image quality of single breath-hold is acceptable for ART with Ethos™, as the treating physician or medical physicist did not reject any images due to poor quality [31, 32]. However, one limitation of kV CBCT is the narrowfield-of-view (FoV) which is overcome by fusing the daily kV CBCT with the planning CT and deformation as its registration [34].
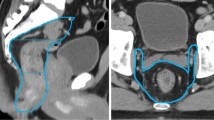
This limitation can be also be overcome by the new HyperSight™ CBCT technology which has novel features such as extended FoV up to 70 cm, as well as an advanced reconstruction techniques for improved image quality [33, 35, 36]. Also, it captures images in less than 6 s, which is 10 times faster than a conventional linear-accelerator-based imaging system (Fig. 1) [35, 37, 38]. This allows tumors that move with respiration to comfortably perform a single breath-hold for the machine to obtain high-quality images needed for daily treatment planning and delivery. It also includes the ability to perform dose calculation directly on the native images. The relevance of this advancement in ontable imaging to CBCT-guided ART is clear; clearer, high-contrast images will improve contouring, speed up the generation of adaptive plans, and enhance confidence in adaptive plan quality.,.
Feasibility of CBCT-based online ART in normal clinical workflow
A typical appointment slot in a ARTtreatment session is generally reserved for 15- 30 min, thought the range could be larger. A large portion of the time will be used for precise positioning of the patient, followed by radiation delivery. The time to complete the adaptive component of the workflow, which is defined as the ART procedural time, includes additional steps between the collection of the CBCT and the first beam on. These steps include an auto-segmentation process, reviewing and optional editing of the autocontours, plan re-optimization, dose calculation, and a quality-assurance check (Fig. 2) [39, 40]. Often, clinician presence is required to assess and adjust online autocontouring and perform plan review [41]. Therefore, the introduction of ART has raised concerns about adding potentially time-consuming steps and clinician involvement into an already time-constrained workflow.
The ART procedural time heavily depends on the disease site (Table 1). For example, the reported Ethos™ ART procedural time on average ranged from 10 to 12 min for prostate cancer [42, 43]. The median overall planning time was mostly dependent on the number of fields (intensity-modulated radiotherapy [IMRT]) and arcs (volumetric modulated arc therapy [VMAT]) that were used: 2.6 min for 7 field IMRT; 3.1 min for 9 field IMRT; 3.4 min for 12 field IMRT; 13.2 min for 2 arc VMAT; and 14 min for 3 arc VMAT [36]. For complex prostate stereotactic body radiotherapy (SBRT), the procedural time is significantly longer [44].
For abdominal and pelvic malignancies, which included reports on the pancreas, liver, bile duct, retroperitoneum, rectum, anus, and abdominal oligometastases, the average procedural time ranged from 17 to 36 min [31, 45,46,47]. The longer procedural time could be explained by the normal peristaltic motion of the gastrointestinal (GI) tract, as well as daily differences in bladder filling and stool burden, leading to a higher tendency for abdominal organs to shift [48]. Therefore, more time is likely required for contouring edits of the PTV and OARs by the treating physician. Specifically for locally advanced pancreatic cancer (LAPC), the emphasis on dose-escalated radiotherapy to improve local control and survival outcomes can further increase the ART procedural time, with OAR contouring being the most time-consuming step [47]. ART for bladder cancer (procedural time ranging from 14 to 32 min) [39, 49, 50] and cervical cancer (16 to 24 min) [40, 51] follows a similar logic with bladder filling and stool burden, while lung malignancies (15 min) [52] is heavily dependent on respiratory motion, all of which would lead to relatively longer procedural time (Tables 1, 2).
In head and neck cancers, a combination of factors such as tumor response, inflammation, muscle atrophy, and weight changes could alter target volumes and shift OARs into radiation fields over the course of a treatment [53]. An average of 20 min is spent on the Ethos™ ART process for head and neck malignancies [53]. For adaptive stereotactic partial breast irradiation (ABPI), the average ART procedural time was 15 min [54]. ART has also been used in hippocampal-avoidance whole brain radiotherapy (HA-WBRT) and has reported an average procedural time of 44 min by Kang et al., with approximately 22 min dedicated to contour adjustments for positional differences between diagnostic and on-table imaging [55]. It is also important to note that adaptation procedural time shortens with site experience and across subsequent fractions in individual patients [47, 49, 56].
Patients undergoing ART currently spend a significantly longer time on the couch during treatment than patients treated without on-table adaptation, with most of the time used for contouring edits and treatment plan review. Nevertheless, most ART sessions can fit into a normal clinical workflow without causing significant delay, as the majority of cases require minimal contouring edits and plan review. Given the potential improvement in target coverage and OAR sparing, the extended treatment time may be worthwhile. With the current state of rapidly improving technology in treatment planning and auto-segmentation [57], it is expected that ART procedural time will continue to decrease. In addition, sites involved in on-table ART have demonstrated that radiation therapists can lead the online ART workflow with minimal input from radiation oncologists, and achieve better efficiency with similar treatment efficacy [58].
Treatment planning workflow
Multiple studies have shown that the treatment plan quality generated by the Intelligent Optimization Engine (IOE) is consistent with that of manually optimized treatment plans. Calmels et al., demonstrated that highly consistent IMRT plans were generated by the IOE in 60 pelvic cases with equivalent coverage and OAR sparing compared to manually optimized plans [36]. Roover et al., observed that the automated treatment plans for prostate SBRT cases achieves similar plan quality as those that were manually optimized, citing inter-planner variation as the main cause of dosimetric differences [53].
The Ethos™ automated treatment planning process differs from the traditional steps that are utilized in conventional treatment planning. The most notable difference comes from the automated optimization process that is conducted by the IOE. The IOE takes the input of an ordered and ranked list of clinical goals as the physician’s intent. This intent is converted into traditional optimization objectives with additional heuristics and optimization structures the user does not control [29]. Careful consideration of these ranked and ordered goals are important as they are also used for generating the online adaptive treatment plans which account for dosimetric tradeoffs that can be seen in the patient anatomy of the day. The process of optimizing the plans via clinical goals allows clinicians to make intuitive judgments when comparing the dosimetric results between the scheduled and adapted plans.
The Ethos™ treatment unit utilizes Mobius 3D-Adapt as an independent plan QA check for online adaptive radiation therapy treatments. A retrospective study by Zhao et al. performed patient specific QA for 16 adaptive plan sessions and found the gamma passing rate to be 99% (± 0.7%) [59].
Auto-contouring impact on plan quality
High-quality, high-efficiency ART sessions require a robust auto-contouring and auto-planning system. In a study of 25 prostate cancer patients, the quality of Ethos™ autocontours before and after manual editing were studied [60]. Moazzezi et al. reported that for most patients even without manual edits, the target coverage and OAR doses met clinical goals after adaption [60]. Mao et al. in a study of 10 locally advanced lung patients (and 290 total fractions), also compared the dosimetric consequences of adapting with unedited vs. edited autocontours [61]. They found that clinical target volume (CTV) coverage was improved by adapting with autocontouring, while further improvements were made with manually edited structures. OAR doses were sometimes decreased but not cumulatively across the treatment course. They concluded that “Accuracy of Ethos™ automatic contouring is considered clinically acceptable”. Byrne et al. also reported that the adaptive plan was selected in 95% of the delivered fractions over the scheduled plan; 11% of the auto-generated contours needed no changes and 81% required only minor edits [44]. Nevertheless, physician review of daily auto-segmentation was still necessary for all patients in the case of outliers [60].
The consistency of Ethos™ auto-contouring and auto-planning functionalities has been positively reported in the literature. Chapman et al. used deformations of a pelvic phantom to evaluate the robustness and reproducibility of Ethos™ auto-segmentation and planning [57]. High reproducibility and accuracy were observed for structures such as femoral heads, bowel, and rectum; reproducibility was less consistent for prostate and bladder, both of which more often required additional editing [57]. Despite the large deformations in the target and surrounding OARs, auto-generated plans met all clinical constraints [57]. Limitations with regard to auto-contouring can occasionally provide dosimetrically less accurate plans. For example, air-gaps between bolus and skin are often filled in during auto-contouring and assumed to be excess tissue unless corrected by user, or large changes in bowel gas leading to inaccurate deformations [62].
Ethos-driven single visit palliative treatment
In palliative treatment, a fast workflow is ideal to hasten relief, reduce anxiety, and minimize inconvenience for the patient. In one study of bony metastases in the spine and pelvis, 47 patients were treated in a single visit without a planning CT scan [63]. A “rough” plan was generated based on diagnostic images prior to the visit, and the plan was then adapted online using the Ethos™ CBCT [63, 64]. Adaptative plans were selected for all patients because of significant improvements in target coverage (PTV/CTV V95%, p value < 0.005) compared to the diagnostic based non-clinical reference plan and the majority of patients (~ 63%) required no or only minor contouring edits [63]. The study met its main goal to implement a simulation free workflow for single visit delivery of CBCT-based ART delivery within 2 h, leveraging diagnostic CT for pre-planning; median time for the workflow was 85 min, with 30 min spent in the treatment room. Most importantly, patients reported satisfaction with length of the consultation and treatment session, with 80% of patients stating they would choose future radiation procedures in the same treatment pathway [63].
OAR sparing and target coverage improvements
Over a course of treatment, normal physiologic organ shifts and radiation-induced tumor responses may lead to anatomical changes in the disease target and surrounding tissues. Significant dosimetric improvements are derived using Ethos™ CBCT-guided online ART. In abdominal oligiometastases, according to Schiff et al., 75% (30 out of 40) of the fractions had OAR constraint violations without plan adaption, while only 5% (2 out of 40) of the fractions had violations with adaptation [31]. Similar improvements were achieved in pancreatic cancer, in which 97.5% (39 out of 40) non-adapted fractions had OAR constraint violations, compared to 0% (0 out of 40) in adapted fractions [47]. OAR dose reductions were also seen with Ethos™ in breast [65], bladder [39], cervical [40, 51], prostate [42,43,44, 60, 66], and head and neck [67] malignancies.
In terms of target coverage, gross tumor volume (GTV) V100% and D95% improved in 62.5% (25 out of 40) and 50% (20 out of 40) of abdominal oligometastases plans with the use of Ethos™ CBCT-guided online ART [31]. Adaptive planning also achieved better planning target volume (PTV) coverage in studies involving diseases sites in breast [54, 65], bladder [39, 49, 68], brain [55], head and neck [53, 67], lung [52, 61], pancreas [47], pelvis [46, 69], and prostate [42,43,44, 66]. However, for cervical and rectal cancers, the reported target coverage was similar between adapted and non-adapted plans [40, 70]. In one study of prostate cancer by Moazzezi et al., the improvement in CTV D98% with ART was minimal [60].
During treatment planning, narrower margins help reduced OAR doses while preserving target coverage. Ethos™ CBCT-guided ART accounts for interfraction changes and, due to the treatment speed, minimizes the impact of intrafraction motions allowing for margin reductions. Ray et al. reported that prostate margins could be reduced to 3 to 4 mm symmetrically without altering the CTV coverage with the use of Ethos™ [71]. In treated bladder cancer, ART plans achieved a median 42% primary PTV reduction and 24–30% V45Gy reduction to the bowel cavity compared to non-ART plans [46]. Significant PTV bladder and head and neck volume reduction were also achieved by Aström et al. [39] and Dohopolski et al. [67], respectively.
Motion management
Target and OAR motion during the ART procedure may result in errors and reduce the quality of adaptive plans. Surface-guided radiation therapy (SGRT), which uses optical surface scanning for patient positioning, can be used with ART for intra-fraction motion monitoring and respiratory gating especially for lung and abdominal malignancies [72]. It provides real-time motion monitoring of the patient surface throughout the whole treatment fraction. The beam can be held if parts of the patient’s surface deviate from the reference position based on the planning CT set-up or if the calculated isocentric deviations exceed a certain threshold [72].
Nevertheless, the actual magnitude of internal organ movement during procedure treatment was previously unknown. Storm et al. studied intra-fraction bladder motion during the adaptive procedure in 17 patients treated with Ethos™ by comparing the patient positions from the initial CBCT, prior to the ART process, to the CBCT directly before beam on time and observed only small changes in bladder volume and center of mass position, with a median time of 14 mintues between the two scans [50]. Zwart et al. compared prostate position on the CBCT taken just prior to the beam on time to that on the CBCT immediately after treatment, with a mean time of 4.2 ± 0.6 min between scans [73]. The 95th percentile of prostate motion ranged from 1.7 mm in the x-direction to 3.2 mm in the z-direction, which suggests that smaller PTV margins can be safely implemented in clinical practice. Storm et al. captured a median 8.5 cm3 increase in bladder filling volume with a second CBCT after the adaptive planning CBCT and prior to treatment delivery [50]. Finally, Jong et al. also obtained a second CBCT to validate with respect to the CTV coverage immediately prior to treatment delivery, which took on average 20 min, and reported excellent motion management in rectal cancer in the vast majority of cases except for two incidence of workflow interruption due to the second CBCT having insufficient target coverage [26].
Future directions
The studies summarized in this review have shown improvements in radiotherapy by reducing doses to normal tissues, improving target coverage, and increasing the potential for dose escalation. However, the question that naturally follows is, do these technical advances translate into improved clinical outcomes? Currently, there are ongoing clinical trials investigating clinical outcomes and PROs obtained with the use of Ethos™ CBCT-based ART across a wide range of disease sites including cancers of the head and neck (NCT04883281, NCT04379505), lung (NCT05488626), pancreas (NCT05764720), bladder (NCT05295992, NCT05700227), cervix (NCT05197881), and anus (NCT05438836). These studies are designed to demonstrate meaningful improvements in treatment-related side effects, such as acute GI toxicity in the treatment of bladder cancer and advanced cervical cancers, by comparing Ethos™ CBCT-based ART to the standard of care.
Conclusion
Online ART entailing treatment plans adjusted prior to delivery to account for temporal and stochastic changes observed in a single treatment fraction while the patient remains in the treatment position is feasible with Ethos™ CBCT-guided ART system. The auto-segmentation tool performs well with some editing and the procedural time with learning can be reduced to 15–30 min based on the complexity of anatomical site. There are dosimetric gains seen in either reduced dose to OARs, improved target coverage, or dose escalation while maintaining OAR tolerance doses. Ongoing prospective studies will help define clinical gain in terms of local control, reduced morbidities, and better patent-reported outcomes.
Availability of data and materials
Research data is stored in Varian repository and will be shared upon request to the corresponding author.
Abbreviations
- CT:
-
Computed tomography
- CBCT:
-
Cone beam computed tomography
- iCBCT:
-
Iterative cone beam computed tomography
- ART:
-
Adaptive radiotherapy
- OAR:
-
Organs-at-risk
- kV:
-
Kilovoltage
- QA:
-
Quality assurance
- IGRT:
-
Image-guided radiotherapy
- AI:
-
Artificial Intelligence
- MRI:
-
Magnetic Resonance Imaging
- FDA:
-
Food and Drug Administration
- IMRT:
-
Intensity-modulated radiotherapy
- VMAT:
-
Volumetric modulated arc therapy
- SBRT:
-
Stereotactic body radiotherapy
- GI:
-
Gastrointestinal tract
- LAPC:
-
Locally advanced pancreatic cancer
- ABPI:
-
Adaptive stereotactic partial breast irradiation
- HA-WBRT:
-
Hippocampal-avoidance whole brain radiotherapy
- IOE:
-
Intelligent optimization Engine
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- GTV:
-
Gross tumor volume
- SGRT:
-
Surface-guided radiation therapy
References
Lim-Reinders S, Keller BM, Al-Ward S, Sahgal A, Kim A. Online adaptive radiation therapy. Int J Radiat Oncol. 2017;99(4):994–1003. https://doi.org/10.1016/j.ijrobp.2017.04.023.
Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42(1):123–32. https://doi.org/10.1088/0031-9155/42/1/008.
Glide-Hurst CK, Lee P, Yock AD, et al. Adaptive radiation therapy (ART) strategies and technical considerations: a state of the ART review from NRG oncology. Int J Radiat Oncol Biol Phys. 2021;109(4):1054–75. https://doi.org/10.1016/j.ijrobp.2020.10.021.
Sonke J-J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 2010;20(2):94–106. https://doi.org/10.1016/j.semradonc.2009.11.003.
Yan D. Adaptive radiotherapy: merging principle into clinical practice. Semin Radiat Oncol. 2010;20(2):79–83. https://doi.org/10.1016/j.semradonc.2009.11.001.
Vargas C, Yan D, Kestin LL, et al. Phase II dose escalation study of image-guided adaptive radiotherapy for prostate cancer: use of dose-volume constraints to achieve rectal isotoxicity. Int J Radiat Oncol Biol Phys. 2005;63(1):141–9. https://doi.org/10.1016/j.ijrobp.2004.12.017.
Liu M, Wang Z, Zhou T, et al. Individual isotoxic radiation dose escalation based on V20 and advanced technologies benefits unresectable stage III non-small cell lung cancer patients treated with concurrent chemoradiotherapy: long term follow-up. Oncotarget. 2017;8(31):51848–58. https://doi.org/10.18632/oncotarget.16288.
Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45(7):856–64. https://doi.org/10.1080/02841860600936369.
Green OL, Henke LE, Hugo GD. Practical clinical workflows for online and offline adaptive radiation therapy. Semin Radiat Oncol. 2019;29(3):219–27. https://doi.org/10.1016/j.semradonc.2019.02.004.
Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(5):1297–308. https://doi.org/10.1016/j.ijrobp.2004.12.052.
Spoelstra FOB, Pantarotto JR, van Sörnsen de Koste JR, Slotman BJ, Senan S. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2009;75(4):1092–7. https://doi.org/10.1016/j.ijrobp.2008.12.027.
Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–93. https://doi.org/10.1016/j.ijrobp.2011.08.017.
Li X, Quan EM, Li Y, et al. A fully automated method for CT-on-rails-guided online adaptive planning for prostate cancer intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(5):835–41. https://doi.org/10.1016/j.ijrobp.2013.04.014.
Ahunbay EE, Peng C, Godley A, Schultz C, Li XA. An on-line replanning method for head and neck adaptive radiotherapy. Med Phys. 2009;36(10):4776–90. https://doi.org/10.1118/1.3215532.
Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2018;126(3):519–26. https://doi.org/10.1016/j.radonc.2017.11.032.
El-Bared N, Portelance L, Spieler BO, et al. Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol. 2019;9(1):e46–54. https://doi.org/10.1016/j.prro.2018.08.010.
Li XA, Liu F, Tai A, et al. Development of an online adaptive solution to account for inter- and intra-fractional variations. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;100(3):370–4. https://doi.org/10.1016/j.radonc.2011.08.027.
Liu F, Erickson B, Peng C, Li XA. Characterization and management of interfractional anatomic changes for pancreatic cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(3):e423–9. https://doi.org/10.1016/j.ijrobp.2011.12.073.
Court LE, Dong L, Lee AK, et al. An automatic CT-guided adaptive radiation therapy technique by online modification of multileaf collimator leaf positions for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;62(1):154–63. https://doi.org/10.1016/j.ijrobp.2004.09.045.
Ahunbay EE, Peng C, Holmes S, Godley A, Lawton C, Li XA. Online adaptive replanning method for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(5):1561–72. https://doi.org/10.1016/j.ijrobp.2009.10.013.
Mohan R, Zhang X, Wang H, et al. Use of deformed intensity distributions for on-line modification of image-guided IMRT to account for interfractional anatomic changes. Int J Radiat Oncol Biol Phys. 2005;61(4):1258–66. https://doi.org/10.1016/j.ijrobp.2004.11.033.
Heijkoop ST, Langerak TR, Quint S, et al. Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT. Int J Radiat Oncol Biol Phys. 2014;90(3):673–9. https://doi.org/10.1016/j.ijrobp.2014.06.046.
Henke LE, Olsen JR, Contreras JA, et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Adv Radiat Oncol. 2019;4(1):201–9. https://doi.org/10.1016/j.adro.2018.10.003.
Kumar M, Shanavas M, Sidappa A, Kiran M. Cone beam computed tomography—know its secrets. J Int oral Heal JIOH. 2015;7(2):64–8.
Srinivasan K, Mohammadi M, Shepherd J. Applications of linac-mounted kilovoltage cone-beam computed tomography in modern radiation therapy: a review. Pol J Radiol. 2014;79:181–93. https://doi.org/10.12659/PJR.890745.
de Jong R, Visser J, van Wieringen N, Wiersma J, Geijsen D, Bel A. Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer. Radiat Oncol. 2021;16(1):136. https://doi.org/10.1186/s13014-021-01866-7.
Mao W, Liu C, Gardner SJ, et al. Evaluation and clinical application of a commercially available iterative reconstruction algorithm for CBCT-Based IGRT. Technol Cancer Res Treat. 2019;18:1533033818823054. https://doi.org/10.1177/1533033818823054.
Gardner SJ, Mao W, Liu C, et al. Improvements in CBCT image quality using a novel iterative reconstruction algorithm: a clinical evaluation. Adv Radiat Oncol. 2019;4(2):390–400. https://doi.org/10.1016/j.adro.2018.12.003.
Archambault Y, Boylan C, Bullock D, et al. Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning. Med Phys Int J. 2020;8(2).
Cai B, Laugeman E, Mazur TR, et al. Characterization of a prototype rapid kilovoltage x-ray image guidance system designed for a ring shape radiation therapy unit. Med Phys. 2019;46(3):1355–70. https://doi.org/10.1002/mp.13396.
Schiff JP, Stowe HB, Price A, et al. In silico trial of computed tomography-guided stereotactic adaptive radiation therapy (CT-STAR) for the treatment of abdominal oligometastases. Int J Radiat Oncol Biol Phys. 2022;114(5):1022–31. https://doi.org/10.1016/j.ijrobp.2022.06.078.
Henke LE, Fischer-Valuck BW, Rudra S, et al. Prospective imaging comparison of anatomic delineation with rapid kV cone beam CT on a novel ring gantry radiotherapy device. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2023;178:109428. https://doi.org/10.1016/j.radonc.2022.11.017.
Holewijn RA, Bot M, van den Munckhof P, Schuurman PR. Implementation of intraoperative cone-beam computed tomography (O-arm) for stereotactic imaging during deep brain stimulation procedures. Oper Neurosurg (Hagerstown, Md). 2020;19(3):E224–9. https://doi.org/10.1093/ons/opaa110.
Bojechko C, Hua P, Sumner W, Guram K, Atwood T, Sharabi A. Adaptive replanning using cone beam CT for deformation of original CT simulation. J Med Radiat Sci. 2022;69(2):267–72. https://doi.org/10.1002/jmrs.549.
Fonseca GP, Lustermans D, Bogowicz M, Taasti V, Vaassen F, Canters R, Theeuwen K, van Elmpt W, Verhaegen F. PO-1668 An evaluation of a novel CBCT system: image quality, extended FoV and metal artefact reduction. In: European Society of Radiotherapy and Oncology. 2023. https://www.estro.org/Congresses/ESTRO-2023/1377/imagingacquisitionandprocessing/15208/anevaluationofanovelcbctsystem-imagequality-extend.
Fallone C, MacDonald L, Cherpak A, Chytyk-Praznik K, Robar J. PD-0662 Evaluation of Ethos HyperSight imaging performance compared to standard CBCT and FBCT. In: European Society of Radiotherapy and Oncology 2023. https://www.estro.org/Congresses/ESTRO-2023/1389/imaging/12842/evaluationofethoshypersightimagingperformancecompa.
Haertter A, Koger B, Salerno M, Kennedy C, Teo B-KK, Alonso-Basanta M, Dong L, Li T. PO-1835 Scanning mode parameters to improve image fidelity on an ultrafast ring-gantry Linac kVCBCT system. In: European Society of Radiotherapy and Oncology 2023.
Hyer DE, Cai B, Rong Y. Future mainstream platform for online adaptive radiotherapy will be using on-board MR rather than on-board (CB) CT images. J Appl Clin Med Phys. 2021;22(7):4–9. https://doi.org/10.1002/acm2.13352.
Åström LM, Behrens CP, Calmels L, et al. Online adaptive radiotherapy of urinary bladder cancer with full re-optimization to the anatomy of the day: Initial experience and dosimetric benefits. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2022;171:37–42. https://doi.org/10.1016/j.radonc.2022.03.014.
Branco D, Mayadev J, Moore K, Ray X. Dosimetric and feasibility evaluation of a CBCT-based daily adaptive radiotherapy protocol for locally advanced cervical cancer. J Appl Clin Med Phys. 2022. https://doi.org/10.1002/acm2.13783.
Chin S, Eccles CL, McWilliam A, et al. Magnetic resonance-guided radiation therapy: a review. J Med Imaging Radiat Oncol. 2020;64(1):163–77. https://doi.org/10.1111/1754-9485.12968.
Morgan HE, Wang K, Yan Y, et al. Preliminary evaluation of PTV margins for online adaptive radiotherapy of the prostatic fossa. Pract Radiat Oncol. 2022. https://doi.org/10.1016/j.prro.2022.11.003.
Zwart LGM, Ong F, Ten Asbroek LA, et al. Cone-beam computed tomography-guided online adaptive radiotherapy is feasible for prostate cancer patients. Phys Imaging Radiat Oncol. 2022;22:98–103. https://doi.org/10.1016/j.phro.2022.04.009.
Byrne M, Archibald-Heeren B, Hu Y, et al. Varian ethos online adaptive radiotherapy for prostate cancer: early results of contouring accuracy, treatment plan quality, and treatment time. J Appl Clin Med Phys. 2022;23(1):e13479. https://doi.org/10.1002/acm2.13479.
Calmels L, Sibolt P, Åström LM, et al. Evaluation of an automated template-based treatment planning system for radiotherapy of anal, rectal and prostate cancer. Tech Innov Patient Support Radiat Oncol. 2022;22:30–6. https://doi.org/10.1016/j.tipsro.2022.04.001.
Sibolt P, Andersson LM, Calmels L, et al. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region. Phys Imaging Radiat Oncol. 2021;17:1–7. https://doi.org/10.1016/j.phro.2020.12.004.
Schiff JP, Price AT, Stowe HB, et al. Simulated computed tomography-guided stereotactic adaptive radiotherapy (CT-STAR) for the treatment of locally advanced pancreatic cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2022;175:144–51. https://doi.org/10.1016/j.radonc.2022.08.026.
Mostafaei F, Tai A, Omari E, et al. Variations of MRI-assessed peristaltic motions during radiation therapy. PLoS One. 2018;13(10):e0205917. https://doi.org/10.1371/journal.pone.0205917.
Azzarouali S, Goudschaal K, den Boer D, Visser J, Hulshof M, Bel A. PD-0235 AI-based online adaptive CBCT-guided radiotherapy for bladder cancer using SIB and fiducial markers. Radiother Oncol. 2022;170:S196–7. https://doi.org/10.1016/S0167-8140(22)02790-6.
Storm KS, Åström LM, Sibolt P, Serup-Hansen E, Behrens CP, Persson G. PO-1719 Intra-fractional variation during daily online adaptive radiotherapy of bladder cancer. Radiother Oncol. 2022;170:S1518–9. https://doi.org/10.1016/S0167-8140(22)03683-0.
Yock AD, Ahmed M, Ayala-Peacock D, Chakravarthy AB, Price M. Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum. J Appl Clin Med Phys. 2021;22(10):210–21. https://doi.org/10.1002/acm2.13425.
Gonzalez Y, Meng B, Parsons DDM, et al. Initial clinical experience of CBCT-based adaptive online radiotherapy for SAbR of thoracic malignancies. Int J Radiat Oncol Biol Phys. 2022;114(3):e591–2. https://doi.org/10.1016/j.ijrobp.2022.07.2276.
Yoon SW, Lin H, Alonso-Basanta M, et al. Initial evaluation of a novel cone-beam CT-based semi-automated online adaptive radiotherapy system for head and neck cancer treatment—a timing and automation quality study. Cureus. 2020;12(8):e9660. https://doi.org/10.7759/cureus.9660.
Montalvo SK, Kim N, Nwachukwu C, et al. On the feasibility of improved target coverage without compromising organs at risk using online adaptive stereotactic partial breast irradiation (A-SPBI). J Appl Clin Med Phys. 2022. https://doi.org/10.1002/acm2.13813.
Kang KH, Price A, Reynoso FJ, et al. In-silico trial of simulation-free hippocampal-avoidance whole brain radiotherapy using diagnostic MRI-based and online adaptive planning. Int J Radiat Oncol Biol Phys. 2022;114(3):e535. https://doi.org/10.1016/j.ijrobp.2022.07.2143.
Kim T, Kiser K, Laugeman E, et al. Surface imaging-guided adaptive radiotherapy (ART): Imaging protocol and verification for inter- and intra-fractional motion managements. Int J Radiat Oncol Biol Phys. 2022;114(3):e193–4. https://doi.org/10.1016/j.ijrobp.2022.07.1107.
Chapman JW, Lam D, Cai B, Hugo GD. Robustness and reproducibility of an artificial intelligence-assisted online segmentation and adaptive planning process for online adaptive radiation therapy. J Appl Clin Med Phys. 2022;23(8):e13702. https://doi.org/10.1002/acm2.13702.
Shepherd M, Graham S, Ward A, et al. Pathway for radiation therapists online advanced adapter training and credentialing. Tech Innov Patient Support Radiat Oncol. 2021;20:54–60. https://doi.org/10.1016/j.tipsro.2021.11.001.
Zhao X, Stanley DN, Cardenas CE, Harms J, Popple RA. Do we need patient-specific QA for adaptively generated plans? Retrospective evaluation of delivered online adaptive treatment plans on Varian Ethos. J Appl Clin Med Phys. 2023;24(2):e13876. https://doi.org/10.1002/acm2.13876.
Moazzezi M, Rose B, Kisling K, Moore KL, Ray X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J Appl Clin Med Phys. 2021;22(10):82–93. https://doi.org/10.1002/acm2.13399.
Mao W, Riess J, Kim J, et al. Evaluation of auto-contouring and dose distributions for online adaptive radiation therapy of patients with locally advanced lung cancers. Pract Radiat Oncol. 2022;12(4):e329–38. https://doi.org/10.1016/j.prro.2021.12.017.
Kisling K, Keiper TD, Branco D, Kim GG-Y, Moore KL, Ray X. Clinical commissioning of an adaptive radiotherapy platform: results and recommendations. J Appl Clin Med Phys. 2022;23(12):e13801. https://doi.org/10.1002/acm2.13801.
JoshuaNelissen K, Versteijne E, Senan S, et al. Same-day adaptive palliative radiotherapy without prior CT simulation: Early outcomes in the FAST-METS study. Radiother Oncol. 2023. https://doi.org/10.1016/j.radonc.2023.109538.
Nelissen KJ, Versteijne E, Senan S, Slotman BJ, Verbakel WFAR. Clinical implementation of single visit palliative adaptive radiotherapy without prior CT simulation. Int J Radiat Oncol Biol Phys. 2022;114(3):e594–5. https://doi.org/10.1016/j.ijrobp.2022.07.2283.
Stanley DN, McConnell K, Cardenas CE, et al. Stereotactic breast radiotherapy delivered via external beam daily online adaptive replanning. Int J Radiat Oncol Biol Phys. 2022;114(3):e597. https://doi.org/10.1016/j.ijrobp.2022.07.2288.
De Roover R, Crijns W, Poels K, et al. Automated treatment planning of prostate stereotactic body radiotherapy with focal boosting on a fast-rotating O-ring linac: plan quality comparison with C-arm linacs. J Appl Clin Med Phys. 2021;22(9):59–72. https://doi.org/10.1002/acm2.13345.
Dohopolski M, Choi B, Meng B, et al. Dosimetric impact of simulated daily adaptive radiotherapy with significantly reduced setup margins in the definitive treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2022;114(3):e590. https://doi.org/10.1016/j.ijrobp.2022.07.2273.
Zwart L, ten Asbroek L, van Dieren E, Dasselaar J, Ong F. PO-1673 Correction of target shape changes in bladder cancer patients using online adaptive radiotherapy. Radiother Oncol. 2022;170:S1472–3. https://doi.org/10.1016/S0167-8140(22)03637-4.
Musunuru HB, Lynch C, Katipally RR, et al. Developing a CBCT-guided online adaptive radiotherapy approach for isotoxic delivery of ultra-high dose to oligometastatic disease in combination with immunotherapy. Int J Radiat Oncol Biol Phys. 2022;114(3):e594. https://doi.org/10.1016/j.ijrobp.2022.07.2282.
de Jong R, Crama KF, Visser J, et al. Online adaptive radiotherapy compared to plan selection for rectal cancer: quantifying the benefit. Radiat Oncol. 2020;15(1):162. https://doi.org/10.1186/s13014-020-01597-1.
Ray X, Moazzezi M, Bojechko C, Moore KL. Data-driven margin determination for online adaptive radiotherapy using batch automated planning. Int J Radiat Oncol Biol Phys. 2020;108(3):e370. https://doi.org/10.1016/j.ijrobp.2020.07.2378.
Freislederer P, Kügele M, Öllers M, et al. Recent advances in surface guided radiation therapy. Radiat Oncol. 2020;15(1):187. https://doi.org/10.1186/s13014-020-01629-w.
Zwart L, Jasper J, Vrieze E, et al. PO-1691 Intrafraction prostate motion during CBCT-guided online adaptive radiotherapy. Radiother Oncol. 2022;170:S1491. https://doi.org/10.1016/S0167-8140(22)03655-6.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HL reviewed and interpreted the existing literature, created tables, and wrote the manuscript. DS reviewed and interpreted the existing literature, created figures, and wrote the manuscript. HC reviewed and interpreted the existing literature and wrote the manuscript. RC reviewed and interpreted the existing literature and wrote the manuscript. AM reviewed and interpreted the existing literature and wrote the manuscript. PK reviewed and interpreted the existing literature and wrote the manuscript. DK reviewed and interpreted the existing literature and wrote the manuscript. SB reviewed and interpreted the existing literature, provided guidance, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr Hefei Liu reports temporary employment at Varian Medical Systems. Dr David Schaal employment at Varian Medical Systems. Dr Heather Curry reports employment at Varian Medical Systems. Mr Ryan Clark reports employment at Varian Medical Systems. Mr Anthony Magliari reports employment at Varian Medical Systems. Dr Patrick Kupelian reports employment at Varian Medical Systems. Dr Deepak Khuntia has a leadership role as the Senior Vice President and Chief Medical Officer at Varian Medical Systems. Dr Sushil Beriwal has a leadership role as the Vice President of Medical Affairs at Varian Medical Systems, reports grant as an Elsevier consultant, and reports participation in advisory board at Xoft DSMB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, H., Schaal, D., Curry, H. et al. Review of cone beam computed tomography based online adaptive radiotherapy: current trend and future direction. Radiat Oncol 18, 144 (2023). https://doi.org/10.1186/s13014-023-02340-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02340-2