Abstract
Study design
This was a retrospective study.
Objectives
Adjacent segment degeneration (ASD) is a major complication associated with spinal fusion. The lumbar paraspinal muscle is an essential factor influencing the occurrence of ASD. This study aimed to investigate the effect of preoperative lumbar paraspinal muscle quality on L5-S1 adjacent lumbar foraminal stenosis degeneration (ASLFSD) after L4–5 transforaminal lumbar interbody fusion (TLIF).
Methods
A total of 113 patients diagnosed with lumbar spinal stenosis at L4–5 were treated with TLIF. Lumbar paraspinal muscle measurements were obtained preoperatively and bilaterally from axial T2-weighted MR images. The measurements included the total cross-sectional area of psoas (PS-tCSA), of erector spinae (ES-tCSA), and of multifidus (MF-tCSA); and fatty infiltration of psoas (PS-FI), of erector spinae (ES-FI), and of multifidus (MF-FI). Foraminal measurements, including posterior disc height (PDH), disc-to-facet distance (D–F), foraminal height (FH), and foraminal area (FA), were obtained bilaterally using a computed tomography system. The association between lumbar paraspinal muscle quality and changes in foraminal measurements was also studied.
Results
We observed that the FH and FA significantly reduced at 1 year postoperatively at the mean follow-up period of 41.56 ± 8.38 months (range, 43–50 months), and PDH, D–F, FH, and FA all significantly reduced at final follow-up. These changes in foraminal measurements were significantly and negatively correlated with PS-FI, ES-FI, and MF-FI.
Conclusion
During the clinical follow-up, we found that patients with a higher degree of paraspinal muscle FI were more likely to develop L5-S1 ASLFSD after L4–5 TLIF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Lumbar spinal stenosis (LSS) is a common degenerative spinal disease in the older population. After a detailed report on transforaminal lumbar interbody fusion (TLIF) surgery by Harms et al. [1] in 1998, TLIF became the major surgical treatment for LSS. Cole and McCall [1] reported that TLIF is minimally invasive, has less structural exposure, and minimises lamina, facet, and pars dissection compared with posterior lumbar interbody fusion (PLIF). Adjacent segment degeneration (ASD) is a major concern after fusion surgery. However, few studies have discussed ASD of the lumbar foramen [2,3,4,5].
The pathology of lumbar foraminal stenosis was first reported in 1927 [6]. Lumbar foraminal stenosis might have been caused by posterolateral osteophytes, herniated discs, laterally bulging annulus fibrosus, subluxation of the facet, and hypertrophic ligamentum [7]. The concept of foraminal stenosis was defined as lateral spinal stenosis [6]. Notably, the reconstructed sagittal images provide better visualisation of the foramen. The L5-S1 foramen, because of its anatomical and functional features and lumbosacral junction, is more susceptible to significant loading from the trunk and tends to have a higher incidence of degeneration [7].
The lumbar paraspinal muscle plays a vital role in the stability of the entire spine and the effectiveness of spine surgery. Muscle quality can be evaluated using the total cross-sectional area (tCSA) and fatty infiltration (FI). The previous studies have reported that patients with a lower CSA and higher muscle FI are more likely to have low back pain (LBP), ASD, facet joint arthropathy, and spinal misalignment [8,9,10,11,12,13].
To our knowledge, the correlation between paraspinal muscle quality and adjacent segment lumbar foraminal stenosis degeneration (ASLFSD) has not been previously investigated. Consequently, this study aimed to investigate the effects of preoperative paraspinal muscle tCSA and FI on L5-S1 ASLFSD after L4–L5 TLIF.
Patients and methods
Inclusion and exclusion criteria of participants
All participants met the following inclusion criteria: (1) conservative treatment failure after a minimum of 3 months, (2) age ≥ 40 years, and (3) single-level TLIF surgery at L4–L5. The following were the exclusion criteria: (1) any patient with body mass index (BMI) ≥ 30 kg/m2, (2) age < 40 years, (3) multilevel fusion surgery, (4) abnormal muscle activity or ambulation due to parkinsonism or neuromuscular disease, and (5) lumbar spondylolisthesis, lumbar isthmic spondylolysis, spine scoliosis, lumbosacral transitional vertebrae, and lumbar intervertebral instability in L5-S1 (dynamic segment angle change > 5°). Ultimately, 113 patients (54 males and 59 females) diagnosed with L4–5 LSS who underwent single-segment TLIF in our hospital between January 2018 and October 2021 were included in our study.
Surgical technique
All the patients were placed in the prone position. The segments were located preoperatively using C-arm radiography. Lateral and anteroposterior images were obtained before surgery to determine the pedicle position of the surgical segment. Additionally, a posterior median incision was made, and the natural cleavage plane between the multifidus and longissimus muscles was separated to expose the facet joints bilaterally (Wiltse approach). After identification of the traversing and exiting nerve roots, an aggressive full discectomy was performed in Kambin’s triangle [14]. An appropriate height cage (Medtronic Sofamor Danek, Memphis, USA) filled with bone obtained from laminectomy, bone morphogenetic protein (rhBMP-2,4 mg, Hangzhou Jiuyuan, China) was inserted into the intervertebral space, and pedicle screws and a rob system were implanted. Notably, artificial bone or ilium was not used in any patient. All surgeries were performed by a senior spine surgeon.
Sagittal measurements
We measured the patient's lumbar lordosis (LL), pelvic incidence (PI), pelvic tilt (PT), sacral slope (SS), sagittal vertical axis (SVA), and pelvic incidence–lumbar lordosis mismatch (PI–LL) on standing full-length lateral radiographs of the spine preoperatively.
Foraminal measurements
A 64-row multidetector computed tomography (CT) system (version 3.0; INFINITT Healthcare Co., Ltd., Seoul, South Korea; slice < 5 mm) was used for all patients preoperatively, 1 year postoperatively, and at the final postoperative follow-up.
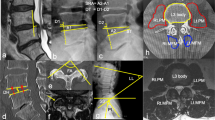
The anatomical boundaries of the foramen were composed of the adjacent superior–inferior vertebral pedicles, posteroinferior margin of the superior vertebral body, intervertebral disc, posterosuperior margin of the inferior vertebral body, ligamentum flavum, and facet joint as the posterior boundaries (Fig. 1a). We selected the level of the bilateral L5-S1 nerve root entrances to the foramen, which appears as the area between the medial edges of the superior and inferior pedicle cortical bone connections in the sagittal plane. Foraminal measurements included the posterior disc height (PDH, mm), disc-to-facet distance (D–F, mm), foraminal height (FH, mm), and foraminal area (FA, mm2) (Fig. 1b).
a The anatomical boundaries of L5-S1 foramen boundaries for CT scan in the sagittal plane. b The measurements made on the disc and intervertebral foramen. Posterior disc height (PDH): The distance between the upper and lower endplates of the involved disc. The disc-to-facet distance (D–F): The vertical distance between the apex of the superior articular process and the vertical line, defined as the caudal end of the bulging intervertebral disc to the inferior endplate in the sagittal plane. Foraminal height (FH): The maximum distance between the inferior margin of the pedicle of the superior vertebra and the superior margin of the pedicle of the inferior vertebra. Foraminal area (FA): FA is bounded by the surfaces of the upper and lower pedicles, the caudal end of the disc, and the anterior edge of the ligamentum flavum (the area circled by the blue line)
Lumbar paraspinal muscle measurements
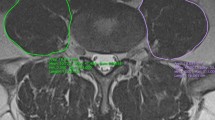
Measurements of the lumbar paraspinal muscles were obtained from T2-weighted images using the ImageJ software. Magnetic resonance imaging (MRI) was conducted with a 1.5-T MRI superconducting imaging system (Siemens, Avanto, Germany). Region of interest (ROI) was used in muscular measurements, including tCSA, in which we excluded the “tent”, which was defined as the region between the fascial plane and erector spinae [15, 16] (Fig. 2). The FI was defined as the area of fatty tissue measured using the thresholding technique (Fig. 3), and they reflect the quality of the lumbar paraspinal muscles.
All measurements were performed bilaterally at the inferior vertebral endplate of L4, including total cross-sectional area of psoas (PS-tCSA), of erector spinae (ES-tCSA), and of multifidus (MF-tCSA); and fatty infiltration of psoas (PS-FI), of erector spinae (ES-FI), and of multifidus (MF-FI). The tCSA of the muscle was standardised as the square of the patient’s height (cm2/m2).
CT-based classification system of LFS
Figure 4 shows the CT-based grading of lumbar foraminal stenosis (LFS) as proposed by Haleem et al. [17].
CT-based classification system of lumbar foraminal stenosis, normal foramen—grade 0, anteroposterior/superior–inferior fat compression—grade 1, both anteroposterior/superior–inferior compressions with no distortion of nerve root—grade 2, and grade 2 with additional distortion of nerve root—grade 3 [17]
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26 software (SPSS Inc., IBM Company Headquarters, Chicago, IL, USA). Correlations between the paraspinal muscle and lumbar foraminal measurements were computed using Pearson’s correlation analysis. An independent sample t-test and Chi-square test were performed to compare the differences between the groups. Statistical significance was set at p < 0.05. Notably, all parameters above were measured by an experienced orthopaedic surgeon.
Results
Patients characteristics
Table 1 summarises the basic characteristics of the included patients and lumbar paraspinal muscle measurements. Overall, 113 patients underwent L4–5 TLIF (right surgical approach: 54 and left surgical approach: 59), composed of 54 males and 59 females, and the mean follow-up period was 41.56 ± 8.38 months (range, 43–50 months). In total, 226 foramina were studied. The mean age of these patients was 62.49 ± 8.68 years, and the mean BMI was 24.49 ± 2.84 kg/m2.
Foraminal measurements and correlations
The lumbar foramen measurements at 1 year postoperatively all reduced compared to those values preoperative, but only the FH (20.81 ± 2.71 and 20.31 ± 2.56, p < 0.05) and FA (63.00 ± 22.97 and 58.0 2 ± 20.41, p < 0.05) were significant. Compared with the preoperative values, PDH, FH, D–F, and FA were all significantly reduced at 1 year postoperatively, indicating the occurrence of L5-S1 foraminal stenosis after TLIF in the 1st year postoperatively (Tables 2 and 3).
Table 4 shows the correlation between the lumbar foramen measurement change and the lumbar paraspinal muscle. Compared with preoperative measurements, changes in foramen measurements, including PDH, D–F, FH, and FA at the final postoperative follow-up, were positively correlated with preoperative PS-FI, ES-FI, and MF-FI. Therefore, the higher the muscle fat content, the more likely ASLFSD was to occur, whereas there was no significant correlation between preoperative paraspinal muscle tCSA and foramen measurement changes. Similarly, in the comparison of lumbar foramen measurements between the final follow-up and 1 year postoperatively, there was a positive correlation between D–F, FH, and FA changes and preoperative PS-FI, ES-FI, and MF-FI, while PDH did not show any significant correlation.
Table 5 shows that in the final follow-up postoperatively, according to the CT-based classification system of LFS, we compared paraspinal muscle quality between patients graded 3 and patients graded 0, 1, and 2. We found that there was a significant difference in the FI of the paraspinal muscle between the two groups of patients, and patients who with severe LFS have a higher degree of FI. However, interestingly, these patients also had a larger tCSA of paraspinal muscle, although no significant differences were observed.
Discussion
ASD is common after lumbar fusion surgery, and adjacent foramen segment stenosis is often observed. Ryu et al. [18] reported that reoperation is most likely associated with foraminal stenosis in patients with ASD (p = 0.001). Our study aimed to investigate the relevance of preoperative paraspinal muscle quality in the occurrence of L5-S1 ASLFSD after L4–5 TLIF.
Changes in PDH, D–F, and FH could potentially lead to a reduction in FA owing to the anatomical structure of the intervertebral foramen. In our study, FH and FA were significantly reduced at 1 year postoperatively compared to preoperative foraminal measurements, and PDH, D–F, FH, and FA were all significantly reduced at the final postoperative follow-up compared to 1 year postoperatively. Although our study follow-up period was short, foraminal stenosis did occur after surgery. The reasons for the occurrence of L5-S1 ASLFSD after L4–5 TLIF also varied. The previous studies have shown that fusion surgery increases pressure in the intervertebral disc and facet joint in adjacent segments [1, 19,20,21,22,23]. The increase in biomechanical pressure promotes disc degeneration, further disc herniation, extrusion of the lumbar foramen, and a decrease in foraminal height [20, 24,25,26]. The accelerated degeneration of facet joints after fusion surgery may be another contributing factor to the change in foramen morphology [9,10,11,12].
Moreover, correlations between foraminal parameter changes, paraspinal muscle tCSA, and FI were analysed. Regardless of whether it was 1 year or the final postoperative follow-up, the FI of the paraspinal muscles was negatively correlated with changes in the foraminal measurements, though there was no significant correlation with PDH. Our results indicate that the tCSA of the paraspinal muscle is not a decisive factor affecting the degeneration of the intervertebral foramen and that the degree of muscular FI is a risk factor for the occurrence of ASLFSD. To further validate our hypothesis, we compared the difference in paraspinal muscle quality between patients with severe spinal stenosis (grade 3, based on the CT classification system of LFS) and general patients (grades 0, 1, and 2, based on the CT classification system of LFS) during the final follow-up. The patients of the grade 3 have higher degree of paraspinal muscle FI. However, we also found that tCSA of the paraspinal muscle was larger in these patients. These increases in tCSA have not brought improvement to the patients. So, we believe that muscular FI is the more valuable for predicting L5-S1 ASLFSD after L4–5 TLIF. Then, how do the paraspinal muscles work?
Paraspinal muscle quality influences surgical efficacy. The previous studies have reported that a smaller tCSA is associated with a poorer fusion rate in patients undergoing PLIF [27, 28]. Wang et al. [29] demonstrated that a smaller multifidus tCSA and higher multifidus FI on preoperative MRI scans were significantly associated with higher ODI scores both preoperatively and postoperatively. In the lumbar muscle system, the psoas attached directly to the vertebral bodies anterolaterally acts as the primary flexor muscle group, whereas the multifidus and erector spinae act as strong extensor muscle groups [19]. Additionally, McGill et al. [30] showed that the erector spinae reduce the compression force from 20 to 35% in a body experiment under external compression. When the multifidus was studied as an individual muscle, it acted more as a segmental stabiliser to enable the separate control of individual vertebrae [31]. Electromyography studies have confirmed this result and revealed that the multifidus plays a role in controlling intersegmental motion [32, 33]. Thus, we strongly believe that, with a higher paraspinal muscle, the FI was more likely to develop ASLFSD after fusion surgery.
Why did we choose the L5-S1 level as our research subject? Regarding anatomical factors, the L5-S1 disc is at the lowermost part of the spine and is the most variable area of lumbar spine activity. The disc of L5-S1 is also more prone to degeneration in lumbar fusion and LBP patients [34, 35]. However, the presence of preoperative disc degeneration did not show a significant correlation with the development of postoperative ASD [36]. A study on the degenerative stenosis of the L3–4 intervertebral foramen after L4–5 TLIF surgery can be further investigated.
This study has some limitations, including a relatively small sample size and short follow-up period. Furthermore, this study did not include the intervertebral foramen of L3–4. Undoubtedly, with longer follow-up times, the incidence of ASLFSD following TLIF surgery will increase, and with a larger sample size, the association between ASLFSD and paraspinal muscle quality will become more apparent. Therefore, a long-term and large-scale study can be extended in future. Additionally, since we only considered preoperative MRI appearance of the paraspinal muscles, postoperative muscle atrophy and fatty infiltration of the patients were not further discussed. Finally, spinal sagittal balance is another influencing factor that cannot be ignored, we will further verify its relationship with ASLFSD in our subsequent research.
Nevertheless, this study has several strengths. All surgical operations were performed in the natural cleavage plane between the multifidus and longissimus muscles to minimise the damage to the muscle [37]. This approach also has the advantages of less blood loss, fewer ASD rates, less damage to paraspinal muscle, and fewer additional surgical procedures [38, 39]. Moreover, our measurements of the foramen area were comprehensive, including foraminal height and width, which could help us understand ASLFSD in a three-dimensional way. Additionally, this study was the first to evaluate spinal muscle quality as a prognosticator of ASLFSD after TLIF surgery; thus, this study could be a cornerstone for further studies analysing the factors influencing postoperative radiological foraminal stenosis in fusion surgery.
In the previous studies, research on ASLFSD after lumbar fusion surgery was scarce. Our research can make that clinical physicians have a deeper understanding of ASLFSD and pay more attention to this issue, and provide some theoretical basis for future research.
Conclusion
In our clinical follow-up, we found that patients with a higher degree of paraspinal muscle FI were more likely to develop L5-S ASLFSD after L4–5 TLIF.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LSS:
-
Lumbar spinal stenosis
- ASD:
-
Adjacent segment degeneration
- TLIF:
-
Transforaminal lumbar interbody fusion
- PLIF:
-
Posterior lumbar interbody fusion
- tCSA:
-
Total cross-sectional area
- FI:
-
Fatty infiltration
- ASLFSD:
-
Adjacent segment lumbar foraminal stenosis degeneration
- PS-tCSA:
-
Psoas total cross-sectional area
- ES-tCSA:
-
Erector spinae total cross-sectional area
- MF-tCSA:
-
Multifidus total cross-sectional area
- PS-FI:
-
Psoas fatty infiltration rate
- ES-FI:
-
Erector spinae fatty infiltration rate
- MF-FI:
-
Multifidus fatty infiltration rate
- PDH:
-
Posterior disc height
- D–F:
-
Disc-to-facet distance
- FH:
-
Foraminal height
- FA:
-
Foraminal area
- ROI:
-
Region of interest
- LFS:
-
Lumbar foraminal stenosis
- LL:
-
Lumbar lordosis
- PI:
-
Pelvic incidence
- PT:
-
Pelvic tilt
- SS:
-
Sacral slope
- SVA:
-
Sagittal vertical axis
- PI–LL:
-
Pelvic incidence–lumbar lordosis mismatch
References
Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik. Orthop Traumatol. 1998;10:90–102.
Xia XP, Chen HL, Cheng HB. Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta-analysis. Spine. 2013;38(7):597–608.
Poh SY, Yue WM, Chen J, et al. P148. clinical and radiological review of adjacent segment degeneration after transformaminal lumbar interbody fusion. Spine J. 2008;8(5):172S.
Okuda S, Yamashita T, Matsumoto T, et al. Adjacent segment disease after posterior lumbar interbody fusion: a case series of 1000 patients. Glob Spine J. 2018;8(7):722–7.
Wang T, Ding W. Risk factors for adjacent segment degeneration after posterior lumbar fusion surgery in treatment for degenerative lumbar disorders: a meta-analysis. J Orthop Surg Res. 2020;15(1):1–16.
Putti V. New conceptions in the pathogenesis of sciatic pain. Lancet. 1927;2:53–60.
Orita S, Inage K, Eguchi Y, et al. Lumbar foraminal stenosis, the hidden stenosis including at L5/S1. Eur J Orthop Surg Traumatol. 2016;26(7):685–93.
Demoulin C, Crielaard JM, Vanderthommen M. Spinal muscle evaluation in healthy individuals and low-back-pain patients: a literature review. Joint Bone Spine. 2007;74(1):9–13.
Kalichman L, Hodges P, Li L, et al. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J. 2010;19(7):1136–44.
Han G, Jiang Y, Zhang B, et al. Imaging evaluation of fat infiltration in paraspinal muscles on MRI: a systematic review with a focus on methodology. Orthop Surg. 2021;13(4):1141–8.
Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. BioMed Res Int. 2017;2017:1–14.
Kalichman L, Klindukhov A, Li L, Linov L. Indices of paraspinal muscles degeneration: reliability and association with facet joint osteoarthritis. Feasibility study. Clin Spine Surg. 2016;29(9):465–70.
Kim JY, Paik HK, Ahn SS, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine J. 2016;16(7):867–75.
Kambin P. Arthroscopic microdiscectomy of the lumbar spine. Clin Sports Med. 1993;12:143–50.
Crawford RJ, Cornwall J, Abbott R, et al. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord. 2017;18(1):1–11.
Berry DB, Padwal J, Johnson S, et al. Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord. 2018;19(1):1–9.
Haleem S, Malik M, Guduri V, et al. The Haleem–Botchu classification: a novel CT-based classification for lumbar foraminal stenosis. Eur Spine J. 2021;30:865–9.
Ryu DS, Park JY, Kuh SU, et al. Surgical outcomes after segmental limited surgery for adjacent segment disease: the consequences of makeshift surgery. World Neurosurg. 2018;110:e258–65.
Hansen L, De Zee M, Rasmussen J, et al. Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine. 2006;31(17):1888–99.
Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938–44.
Chang UK, Kim DH, Lee MC, et al. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7(1):33–9.
Zhou C, Cha T, Wang W, et al. Investigation of alterations in the lumbar disc biomechanics at the adjacent segments after spinal fusion using a combined in vivo and in silico approach. Ann Biomed Eng. 2021;49(2):601–16.
Jiang S, Li W. Biomechanical study of proximal adjacent segment degeneration after posterior lumbar interbody fusion and fixation: a finite element analysis. J Orthop Surg Res. 2019;14:135.
Ou C-Y, Lee T-C, Lee T-H, Huang Y-H. Impact of body mass index on adjacent segment disease after lumbar fusion for degenerative spine disease. Neurosurgery. 2015;76:396–402.
Liang J, Dong Y, Zhao H. Risk factors for predicting symptomatic adjacent segment degeneration requiring surgery in patients after posterior lumbar fusion. J Orthop Surg Res. 2014;9:97–102.
Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine (Phila Pa 1976). 2000;25:1617–24.
Choi MK, Kim SB, Park BJ, et al. Do trunk muscles affect the lumbar interbody fusion rate?: correlation of trunk muscle cross sectional area and fusion rates after posterior lumbar interbody fusion using stand-alone cage. J Korean Neurosurg Soc. 2016;59(3):276–81.
Choi MK, Kim SB, Park CK, et al. Cross-sectional area of the lumbar spine trunk muscle and posterior lumbar interbody fusion rate. Clin Spine Surg. 2017;30(6):E798–803.
Wang W, Sun Z, Li W, et al. The effect of paraspinal muscle on functional status and recovery in patients with lumbar spinal stenosis. J Orthop Surg Res. 2020;15(1):1–6.
McGill SM, Norman RW. Effects of an anatomically detailed erector spinae model on L4L5 disc compression and shear. J Biomech. 1987;20(6):591–600.
Aspden RM. Review of the functional anatomy of the spinal ligaments and the lumbar erector spinae muscles. Clin Anat. 1992;5(5):372–87.
Donisch EW, Basmajian JV. Electromyography of deep back muscles in man. Am J Anat. 1972;1:25–36.
Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27:E29-36.
Roberts S, Gardner C, Jiang Z, et al. Analysis of trends in lumbar disc degeneration using kinematic MRI. Clin Imaging. 2021;79:136–41.
Bonnheim NB, Wang L, Lazar AA, et al. The contributions of cartilage endplate composition and vertebral bone marrow fat to intervertebral disc degeneration in patients with chronic low back pain. Eur Spine J. 2022;102:1–7.
Conaway W, Karamian BA, Mao JZ, et al. The effect of L5-S1 degenerative disc disease on outcomes of L4–L5 fusion. Clin Spine Surg. 2022;35(5):E444–50.
Jin Y, Chen Q, Chen C, et al. Clinical research and technique note of TLIF by Wiltse approach for the treatment of degenerative lumbar. Orthop Surg. 2021;13(5):1628–38.
Ulutaş M, Yaldız C, Seçer M, et al. Comparison of Wiltse and classical methods in surgery of lumbar spinal stenosis and spondylolisthesis. Neurol Neurochir Pol. 2015;49(4):251–7.
Street JT, Glennie RA, Dea N, et al. A comparison of the Wiltse versus midline approaches in degenerative conditions of the lumbar spine. J Neurosurg Spine. 2016;25(3):332–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MHC and HLT participated in the design of the study and wrote the main body of the paper, PZ collected the data, JXL, WJY, and SKF analysed and supplemented the data, and MHC provides tables and figures, and all authors had read the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Wenzhou Medical University. The procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki and approved by the local ethics committee. All participants provided informed consent to participate in this study. Participants’ personal information was anonymised and deidentified before analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, M., Zhang, P., Lai, J. et al. A correlation study of preoperative lumbar paraspinal muscle quality and L5-S1 lumbar foraminal stenosis degeneration after L4–5 TLIF. J Orthop Surg Res 18, 731 (2023). https://doi.org/10.1186/s13018-023-04196-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04196-4