Abstract
Background
Osteoporosis (OP) is a high-incidence bone disease that is prone to osteoporotic fractures (OF), so it has attracted widespread attention.
Aim
This study investigated the specific expression and role of miR-331 in patients with OP and OF. The findings have profound implications for the clinical prevention and treatment of these conditions.
Methods
The study included 60 OP patients, 46 OF patients, and 40 healthy controls. The expression level of miR-331-3p was detected using RT-qPCR. BMP2 was used to stimulate differentiation in MC3T3-E1 cells. After induction, the expression activity of osteogenic differentiation-related gene markers was detected using RT-qPCR. The target gene analysis was conducted using a luciferase reporter assay.
Results
The levels of miR-331-3p were significantly elevated, while NRP2 levels were significantly reduced in OF patients. Post-surgery, miR-331-3p levels decreased over time. MiR-331-3p was found to negatively regulate the luciferase activity of NPR2 in MC3T3-E1 cells. Furthermore, overexpression of miR-331-3p inhibited cell proliferation and decreased the levels of osteoblast differentiation markers.
Conclusion
The up-regulation of miR-331-3p can promote OP and might also encourage the occurrence of OF by regulating NRP2. However, this needs further verification.
Similar content being viewed by others
Introduction
Osteoporosis (OP) is a systemic bone disease with various causes. It’s characterized by decreased bone mineral density and quality, damaged bone microstructure, increased bone fragility, and a tendency for fractures [1]. OP is primarily categorized into primary and secondary types. Primary OP is further classified into postmenopausal OP (type I), senile OP (type II), and idiopathic OP [2]. OP’s morbidity and mortality rates are high, and the incidence of OP and osteoporotic fractures (OF) is expected to rise in the future [3, 4]. OF can cause pain, severe disability, and even death, with mortality rates up to 20%. Up to 50% of those bedridden for long periods can become permanently disabled [5,6,7]. OP is a chronic condition and its treatment is a lengthy process. Long-term drug treatment poses a certain risk of adverse reactions [8,9,10,11]. However, the clinical application of some biochemical markers of bone strength is limited due to their low sensitivity and specificity [12,13,14,15,16]. Therefore, it’s necessary to find new and more reliable markers for OP prevention and diagnosis.
The role of non-coding RNAs in OP has been widely studied. Small interfering RNA (siRNAs) has been associated with tendon repair and homeostasis and may be useful in the study of the metabolic process of OP [17,18,19,20,21]. MicroRNAs (miRNAs) can alter gene expression in bone cells, affecting bone remodeling and fracture healing [22, 23]. Abnormally expressed miRNAs may lead to bone diseases such as OP and OF [24]. MiRNAs can regulate osteogenic differentiation of mesenchymal stem cells in OP, but the specific mechanism is still in the early research stage [25, 26]. MiR-331-3p, an essential regulatory miRNA in stem cell differentiation and apoptosis, has been associated with osteoporosis. Studies show that miR-331-3p is negatively correlated with the T score in the Caucasian population [27]. It is also associated with postmenopausal OP in women, and may down-regulate abnormally expressed genes [28]. However, there are relatively few studies on the specific expression and role of miR-331 in OP and OF. Its specific mechanism in OP remains unclear. This study aims to explore its expression and role in OF.
In this study, we first examined the expression level of miR-331-3p in the serum of patients with OP and OF patients. We then investigated the effect of miR-331-3p on the proliferation and differentiation of osteoblasts. We explored the mechanism of miR-331-3p in OF, with the aim of providing new methods and ideas for preventing OP and treating OF.
Materials and methods
Research subjects and sample collection
The study population consisted of 60 OF patients, 46 OP patients, and 40 healthy subjects. These subjects were categorized into three groups: OP, OF, and NC control. The general pathological data of these groups were analyzed. Fasting venous blood was collected from each group a day before testing, and serum was separated for subsequent examinations. The OF patients in this study were later treated with intramedullary nailing. Intramedullary nail fixation is a commonly used surgical treatment that fixes a fracture by inserting a metal needle into the fracture site. The advantages of this approach are less trauma and faster recovery.
This study received approval from the ethics committee of The Sixth Affiliated Hospital of Xinjiang Medical University. All subjects and their families provided informed consent to participate.
RNA extraction and PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, USA). Its purity and concentration were assessed using a NanoDrop-2000 (Thermo Fisher, USA). We used the RNA to generate cDNA with the Takara reverse transcription kit (Takara, Japan). Then, we employed real-time fluorescent quantitative PCR (RT-qPCR) to detect the expression of miR-331-3p in the serum of patients from the three groups. We also monitored the changes in miR-331-3p expression after 24 h, and after 1, 2, 3, and 4 weeks of surgical treatment. The expression level was calculated using the 2−ΔΔCt method.
Cell culture
In this study, we used MC3T3-E1 cells from the Chinese Academy of Sciences Cell Bank. These cells are pluripotent osteoblast precursor cells derived from mouse skulls. We induced their differentiation using Bone Morphogenetic Protein 2 (BMP2). Using RT-qPCR, we monitored the expression of miR-331-3p and osteogenic differentiation-related gene markers at three different time points: immediately after induction, 7 days post-induction, and 14 days post-induction.
Dual-luciferase reporter assay
The binding sites of miR-331-3p and NRP2 were identified using the TargetScan database (http://www.targetscan.org/vert_72/). The identified binding site was cloned into the pGL3 reporter vector to create the wild-type NRP2 vector (NRP2-wt). A mutant NRP2 vector (NRP2-mut) was then created by mutating the binding site. The established vectors were co-transfected with miR-331-3p mimic, miR-331-3p inhibitor and mimic NC into BMP2-induced MC3T3-E1 cells using Lipofectamine 2000. The relative luciferase activity was estimated with the Dual-Luciferase Reporter Assay System (Promega, USA). The results were normalized to the Renilla luciferase.
Cell transfection
MC3T3-E1 cells were cultured in DMEM medium with 10% FBS bovine serum and placed in a 5% CO2 incubator at 37℃. For cell transfection, Lipofectamine 2000 (Thermo-Fisher, USA) was used. The controls received 125 µL DEPC water, miR-331-3p mimic, and its negative control (mimic NC) (Med Chemexpress, USA). Additionally, 125µL DEPC was added to the miR-331-3p inhibitor and inhibitor NC. Based on the different treatments, they were categorized into five groups: blank control, miR-331-3p mimic, mimic NC, miR-331-3p inhibitor, and inhibitor NC. To further examine the expression level of NRP2 in MC3T3-E1 cells, the NRP2 vector, pcDNA3.1-NRP2 (OE-NRP2), or pcDNA3.1 vector (OE-NC) and miR-331-3p mimic or mimic NC were transfected simultaneously using Lipofectamine 2000.
CCK-8 assay
Cell proliferation was detected using CCK-8 (Dojindo, Japan). Cells from the untreated control group, mimic NC group, miR-331 mimic group, miR-331 mimic + OE-NC group, and miR-331 mimic + OE-NRP2 group were cultured in 96-well plates. At 0, 24, 48, and 72 h, 10 µl of CCK-8 was added to each well and mixed. After culturing for an additional 2 h, the absorbance (OD) value of cells in each well was measured at 450 nm with a microplate reader.
Statistical analysis
In this study, each experiment was conducted three times. Data obtained was analyzed using SPSS26.0 and GraphPad Prism 9.0 software. Differences between two groups were analyzed by using the Student t-test. Differences between multiple groups were analyzed by using the one-way ANOVA test. Pearson correlation test was applied for assessing the relationship between serum levels of miR-331-3p and NRP2. The results are presented as mean ± standard deviation. A statistical difference is indicated by p < 0.05.
Results
General information about the study subjects
Here is the general pathological data for the three groups: In the NC control group, there were 14 males and 26 females, averaging 66.93 ± 9.42 years old. Their bone mineral density (BMD) T-values were greater than − 1, indicating normal BMD. In the OP group, 13 males and 33 females were present, with an average age of 65.72 ± 9.69 years. Their BMD T-values ranged from − 1 to 2.5, showing a loss of bone mass. In the OF group, there were 23 males and 37 females, averaging 65.53 ± 8.30 years old. Their BMD was less than − 2.5, indicating osteoporosis. There were no significant differences in age, gender, and BMI between the groups (p > 0.05) (Table 1).
Expression of mir-331-3p in serum of OP patients and OF patients
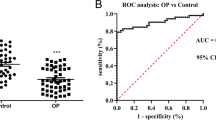
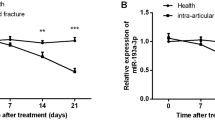
The serum levels of miR-331-3p in both OP patients and OF patients were significantly higher than those in healthy controls, with the highest level observed in OF patients (Fig. 1A). After treatment with intramedullary nail fixation,, a gradual decrease in the expression level of miR-331-3p was observed in OF patients over time, reaching its lowest point in the fourth week (Fig. 1B).
Changes of mir-331-3p level and mRNA of osteoblast-related genes in cell lines after BMP2 treatment
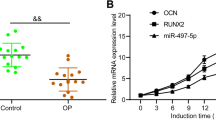
Following BMP2 induction, the level of miR-331-3p declined over time (Fig. 2A). Concurrently, the expression activity of osteogenesis-related genes, such as ALP, RUNX2, ODF, and OCN, increased over time (Fig. 2B).
The BMP2-treated cell lines showed changes in miR-331-3p expression and mRNA levels of osteogenesis-related genes. The expression of miR-331-3p gradually decreased over time post-induction (A), while the expression of osteogenic genes ALP, RUNX2, ODF, and OCN gradually increased over time post-induction (B). * p < 0.05, ** p < 0.01
Validation and interaction evaluation of the targeting relationship between mir-331-3p and NRP2
The miR-331-3p level was regulated through cell transfection. Compared to the control group, the miR-331-3p mimic transfection significantly increased miR-331-3p expression levels in cells (p < 0.001). Conversely, the miR-331-3p inhibitor transfection significantly decreased these levels (p < 0.01) (Fig. 3A). The miR-331-3p binding site to NRP2 is displayed in Fig. 3B. In MC3T3-E1 cells, miR-331-3p up-regulation significantly inhibited NRP2-wt’s luciferase activity, and miR-331-3p knockdown had an opposite effect. The luciferase activity of NRP2-mut was not affected by miR-331-3p regulation (Fig. 3C). MiR-331-3p up-regulation inhibited NRP2 expression level (p < 0.05), while its downregulation increased NRP2 expression level (p < 0.01) (Fig. 3D). Among the three groups, OF patients had the lowest NRP2 expression (Fig. 3E). Furthermore, there was a significant negative correlation between the expression levels of miR-331-3p and NRP2, with a Pearson r-value of -0.7081 (Fig. 3F). After BMP2 induction, the expression level of NRP2 in the cell line significantly increased over time (p < 0.01) (Fig. 3G).
The targeting relationship between miR-331-3p and NRP2 was confirmed. After transfection with the miR-331-3p mimic, the expression level of miR-331-3p significantly increased. Conversely, it significantly decreased after transfection with the miR-331-3p inhibitor, indicating a successful transfection (A). MiR-331-3p binds to NRP2 (B). The up-regulation of miR-331-3p inhibited the luciferase activity of NRP2-wt, while the knockdown of MiR-331-3p accelerated its activity. The luciferase activity of NRP2-mut was not regulated by miR-331-3p (C). NRP2 expression decreased with the miR-331 mimic transfection, and increased significantly with the miR-331 inhibitor (D). Among the three groups, OF patients had the lowest NRP2 expression (E). A significant negative correlation was observed between miR-331-3p and NRP2 expression in OF patients, with an r-value of -0.7081 (F). After BMP2 induction, NRP2 expression in cell lines increased over time (G). * p < 0.05, ** p < 0.01, *** p < 0.001
NRP2 partly reversed the effects of mir-331-3p in MC3T3-E1 cells
In MC3T3-E1, transfection with miR-331 mimic significantly increased the expression of mir-331-3p (Fig. 4A), while it significantly decreased the expression of NPR2 (Fig. 4B). This transfection inhibited cell growth and decreased the expression of osteogenic differentiation-related gene markers. However, co-transfection of miR-331 mimic with OE-NRP2 reduced this growth inhibition and reversed the inhibitory effect of miR-331-3p on osteogenic differentiation in MC3T3-E1 cells (Fig. 4C and D).
Following transfection with the miR-331-3p mimic, there was a significant increase in the expression level of miR-331-3p. However, there was no difference in the expression level of miR-331-3p between the co-transfected miR-331-3p mimic + OE-NRP2 cells and the negative control (A). In cells transfected with the miR-331-3p mimic, the expression level of NRP2 decreased. However, in co-transfected miR-331-3p mimic + OE-NRP2 cells, the expression of NRP2 was significantly higher than in the negative control cells (B). Cell growth was inhibited after transfection with the miR-331-3p mimic. Yet, cells co-transfected with miR-331-3p mimic + OE-NRP2 gradually resumed growth over time, compared to the negative control (C). Furthermore, the expression activity of osteogenic differentiation-related gene markers was notably decreased in cells transfected with the miR-331-3p mimic. In contrast, co-transfection of miR-331-3p mimic + OE-NRP2 reversed the inhibitory effect of miR-331-3p on the osteogenic differentiation of MC3T3-E1 cells, compared to the negative control (D). * p < 0.05, ** p < 0.01, ***p < 0.001
Discussion
OP is a widespread complex bone disease that can lead to osteoporotic fractures [29, 30]. In clinical practice, X-ray bone mineral density measurements are frequently used to deduce the severity of osteoporosis [31]. If the T-value of the bone mineral density measurement is greater than − 1, it’s considered normal. If the T-value falls between − 1 and − 2.5, it indicates osteopenia. A T-value below − 2.5 is indicative of OP. In instances where a T-value below − 2.5 coexists with a fragility fracture, a diagnosis of severe OP is made. This aligns with our findings. The role of miRNA in the growth and differentiation of osteoclasts and osteoblasts has been widely researched [32, 33]. Yuan et al. utilized a miR-214 inhibitor to suppress miR-214 expression in osteoclasts, thereby reversing bone resorption and treating OP [34]. MiR-331-3p is known to regulate cell proliferation, transformation, migration, and invasion, but its exact role in OP remains unclear [35, 36]. We found that miR-331-3p expression was substantially higher in OP patients. After surgery, the expression of miR-331-3p decreased gradually over time.
Research has shown that miR-331-3p inhibits various human diseases by targeting different genes [37]. For instance, by targeting NRP2, miR-331-3p suppressed the proliferation and E6/E7 expression in cervical cancer cells [38]. In a further examination of miR-331-3p’s regulatory role in OP, the Targetscan database was used to predict its direct target genes. The results indicated a direct binding between miR-331-3p and the NRP2 3 ‘-UTR, suggesting NRP2 as a potential direct target gene. This was corroborated by a luciferase reporter assay. Neuropilin-2 (NRP2) knockout mice, characterized by low bone mass, increased osteoclast count, and decreased osteoblast count, were also studied [39]. Verlinden et al.‘s research showed male and female NRP2 knockout mice displaying decreased bone mass, reduced osteoblasts, and increased osteoclast count, all of which are strongly associated with OP [40]. In line with these studies, our research found a significant reduction in NRP2 expression in the serum of OP and OF patients. In MC3T3-E1, NRP2 expression increased over time with osteogenic induction. Furthermore, NRP2 expression decreased significantly in cell lines transfected with miR-331-3p mimics, while it increased considerably in those transfected with miR-331-3p inhibitors.
As we all know, the regulation of osteoporosis is in fact a function of both osteoblasts and osteoclasts. In general, osteoblast is the ability to change the cell of osteogenesis, and osteoclasts is able to cell of bone tissue destruction. When bone is damaged or even fractured, osteoblasts begin to function [24]. Over time, it becomes soft, then harder, and finally calcifies. It forms new bone that can cover the injury or rejoin the fracture. Osteoclasts, which are present on the surface of the bones in varying amounts depending on age, health, specific illnesses and conditions, are responsible for the partial dissolution of decalcified bones. Du et al. reported that miR-375 regulates RUNX2, inhibiting osteoblast differentiation and reducing related osteoblast differentiation markers’ activity [41]. Sun et al. showed an upregulation of miR-338-3p inhibits the expression of these markers, thus reducing osteoblast differentiation [42]. Zhang et al. demonstrated that miR-331-3p inhibits the invasion and migration of colorectal cancer cells by targeting NRP2 [36]. Similarly, we chose to study osteoblast differentiation, and the results showed that overexpression of miR-331-3p significantly reduces the expression of osteogenesis-related genes such as ALP, RUNX2, ODF, and OCN, inhibiting cell growth and proliferation. Upregulation of NRP2 counteracts the miR-338-3p’s inhibitory effect on MC3T3-E1 cells’ osteogenic differentiation and lessens the inhibitory effect on cell growth. These results suggest that miR-331-3p negatively regulates osteogenic differentiation of MC3T3-E1 by targeting NRP2, promoting osteoporosis progression. Therefore, miR-331-3p could be a potential target for treating various diseases and is closely associated with the onset and development of OP.
Notably, our results indicated that miR-331-3p expression was decreased during osteoblast differentiation, and it negatively regulated MC3T3-E1 cells osteogenic differentiation by targeting NRP2. However, further experiments are needed to prove whether miR-331-3p can regulate osteoblast differentiation and its regulatory role in osteoblast differentiation. We will continue to investigate the role of miR-331-3p in regulating osteoblast differentiation in the future.
In summary, the expression of miR-331-3p gradually decreased over time during osteogenic differentiation, suggesting it could be a diagnostic marker for fractures. Additionally, miR-331-3p negatively regulated the osteogenic differentiation of MC3T3-E1 cells by targeting NRP2, thereby advancing osteoporosis. As such, an increase in miR-331-3p is associated with the onset of osteoporosis and fractures through the regulation of NRP2 expression. This provides a crucial theoretical foundation for exploring the molecular mechanism of fractures.
Data availability
No datasets were generated or analysed during the current study.
References
Sun P, Huang T, Huang C, Wang Y, Tang D. Role of histone modification in the occurrence and development of osteoporosis. Front Endocrinol (Lausanne). 2022;13964103.
Miyakoshi N, Kobayashi T, Suzuki T, Kikuchi K, Kasukawa Y, Shimada Y. Perioperative Medical Complications after posterior Approach spinal instrumentation surgery for osteoporotic vertebral collapse: a comparative study in patients with primary osteoporosis and those with secondary osteoporosis. Asian Spine J. 2017;11(5):756–62.
Li X, Jiang Y, Huo L, Wu H, Liu Y, Jin J, et al. Nonremission and recurrent Tumor-Induced Osteomalacia: a retrospective study. J Bone Min Res. 2020;35(3):469–77.
Qadir A, Liang S, Wu Z, Chen Z, Hu L, Qian A. Senile osteoporosis: the involvement of differentiation and senescence of bone marrow stromal cells. Int J Mol Sci. 2020;21(1).
Helmrich G. Screening for osteoporosis. Clin Obstet Gynecol. 2013;56(4):659–66.
Wang H, Qi LL, Shema C, Jiang KY, Ren P, Wang H et al. Advances in the role and mechanism of fibroblasts in fracture healing. Front Endocrinol (Lausanne). 2024;151350958.
Zhao JG, Zeng XT, Wang J, Liu L. Association between Calcium or vitamin D supplementation and fracture incidence in Community-Dwelling older adults: a systematic review and Meta-analysis. JAMA. 2017;318(24):2466–82.
Musette P, Brandi ML, Cacoub P, Kaufman JM, Rizzoli R, Reginster JY. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21(5):723–32.
Conti V, Russomanno G, Corbi G, Toro G, Simeon V, Filippelli W, et al. A polymorphism at the translation start site of the vitamin D receptor gene is associated with the response to anti-osteoporotic therapy in postmenopausal women from southern Italy. Int J Mol Sci. 2015;16(3):5452–66.
Migliorini F, Colarossi G, Eschweiler J, Oliva F, Driessen A, Maffulli N. Antiresorptive treatments for corticosteroid-induced osteoporosis: a bayesian network meta-analysis. Br Med Bull. 2022;143(1):46–56.
Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, Giorgino R. Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):351.
Zheng ZZ, Xu JH, Dai Y, Jiang B, Tu ZM, Li L et al. Circulating miR-107 as a diagnostic biomarker of osteoporotic vertebral compression fracture increases bone formation in vitro and in vivo. Life Sci. 2023;323121693.
Migliorini F, Colarossi G, Baroncini A, Eschweiler J, Tingart M, Maffulli N. Pharmacological management of postmenopausal osteoporosis: a Level I evidence based - Expert Opinion. Expert Rev Clin Pharmacol. 2021;14(1):105–19.
Migliorini F, Giorgino R, Hildebrand F, Spiezia F, Peretti GM, Alessandri-Bonetti M et al. Fragility fractures: risk factors and management in the Elderly. Med (Kaunas). 2021;57(10).
Migliorini F, Maffulli N, Colarossi G, Eschweiler J, Tingart M, Betsch M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a bayesian network meta-analysis. J Orthop Surg Res. 2021;16(1):533.
Migliorini F, Maffulli N, Spiezia F, Tingart M, Maria PG, Riccardo G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):318.
Wang T, Zhang C, Xu L, Li X. Roles of circular RNAs in osteogenic/osteoclastogenic differentiation. BioFactors. 2024;50(1):6–15.
Zhang C, Pan L, Zhang H, Ke T, Yang Y, Zhang L et al. Osteoblasts-derived exosomal lncRNA-MALAT1 promotes osteoclastogenesis by targeting the miR-124/NFATc1 Signaling Axis in Bone Marrow-Derived macrophages. Int J Nanomed. 2023;18781–95.
Gargano G, Asparago G, Spiezia F, Oliva F, Maffulli N. Small interfering RNAs in the management of human osteoporosis. Br Med Bull. 2023;148(1):58–69.
Gargano G, Oliva F, Oliviero A, Maffulli N. Small interfering RNAs in the management of human rheumatoid arthritis. Br Med Bull. 2022;142(1):34–43.
Gargano G, Oliviero A, Oliva F, Maffulli N. Small interfering RNAs in tendon homeostasis. Br Med Bull. 2021;138(1):58–67.
Giordano L, Porta GD, Peretti GM, Maffulli N. Therapeutic potential of microRNA in tendon injuries. Br Med Bull. 2020;133(1):79–94.
Oliviero A, Della Porta G, Peretti GM, Maffulli N. MicroRNA in osteoarthritis: physiopathology, diagnosis and therapeutic challenge. Br Med Bull. 2019;130(1):137–47.
Yao J, Xin R, Zhao C, Yu C. MicroRNAs in osteoblast differentiation and fracture healing: from pathogenesis to therapeutic implication. Injury. 2024;55(4):111410.
Waki T, Lee SY, Niikura T, Iwakura T, Dogaki Y, Okumachi E et al. Profiling microRNA expression during fracture healing. BMC Musculoskelet Disord. 2016;1783.
Wang J, Liu S, Li J, Zhao S, Yi Z. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2019;10(1):197.
Gautvik KM, Günther CC, Prijatelj V, Medina-Gomez C, Shevroja E, Rad LH, et al. Distinct subsets of noncoding RNAs are strongly Associated with BMD and fracture, studied in weight-bearing and non-weight-bearing human bone. J Bone Min Res. 2020;35(6):1065–76.
Liu Y, Wang Y, Yang N, Wu S, Lv Y, Xu L. In silico analysis of the molecular mechanism of postmenopausal osteoporosis. Mol Med Rep. 2015;12(5):6584–90.
Karinkanta S, Piirtola M, Sievänen H, Uusi-Rasi K, Kannus P. Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol. 2010;6(7):396–407.
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049–102.
Wong CP, Gani LU, Chong LR. Dual-energy X-ray absorptiometry bone densitometry and pitfalls in the assessment of osteoporosis: a primer for the practicing clinician. Arch Osteoporos. 2020;15(1):135.
Hensley AP, McAlinden A. The role of microRNAs in bone development. Bone. 2021;143115760.
Mohanapriya R, Akshaya RL, Selvamurugan N. A regulatory role of circRNA-miRNA-mRNA network in osteoblast differentiation. Biochimie. 2022;193137–47.
Yuan Y, Guo J, Zhang L, Tong X, Zhang S, Zhou X, et al. MiR-214 attenuates the osteogenic effects of mechanical loading on osteoblasts. Int J Sports Med. 2019;40(14):931–40.
Tian QQ, Xia J, Zhang X, Gao BQ, Wang W. Mir-331-3p inhibits Tumor Cell Proliferation, Metastasis, Invasion by Targeting MLLT10 in Non-small Cell Lung Cancer. Cancer Manag Res. 2020;125749–58.
Zhang H, Wang R, Wang M. Mir-331-3p suppresses cell invasion and migration in colorectal carcinoma by directly targeting NRP2. Oncol Lett. 2019;18(6):6501–08.
Buranjiang G, Kuerban R, Abuduwanke A, Li X, Kuerban G. MicroRNA-331-3p inhibits proliferation and metastasis of ovarian cancer by targeting RCC2. Arch Med Sci. 2019;15(6):1520–29.
Fujii T, Shimada K, Asano A, Tatsumi Y, Yamaguchi N, Yamazaki M et al. MicroRNA-331-3p suppresses cervical Cancer Cell Proliferation and E6/E7 expression by targeting NRP2. Int J Mol Sci 2016;17(8).
Verlinden L, Kriebitzsch C, Beullens I, Tan BK, Carmeliet G, Verstuyf A. Nrp2 deficiency leads to trabecular bone loss and is accompanied by enhanced osteoclast and reduced osteoblast numbers. Bone. 2013;55(2):465–75.
Verlinden L, Doms S, Janssens I, Meyer MB, Pike JW, Carmeliet G et al. Neuropilin 2 in osteoblasts regulates trabecular bone mass in male mice. Front Endocrinol (Lausanne). 2023;141223021.
Du F, Wu H, Zhou Z, Liu YU. microRNA-375 inhibits osteogenic differentiation by targeting runt-related transcription factor 2. Exp Ther Med. 2015;10(1):207–12.
Sun Q, Zhang B, Zhu W, Wei W, Ma J, Tay FR. A potential therapeutic target for regulating osteoporosis via suppression of osteoclast differentiation. J Dent. 2019;8291–97.
Acknowledgements
Not applicable.
Funding
The study is supported by the Xinjiang Uygur Autonomous Region “Tianshan Talents” Training Program for High-level Talents in Medicine and Health (No:TSYC202301B077)
Author information
Authors and Affiliations
Contributions
Conceptualization, C.N. and H.C.; Data curation, C.N. and D.A.; Formal analysis, C.N. and D.A.; Funding acquisition, H.C.; Investigation, D.A.; Methodology, C.N. and D.A.; Project administration, H.C.; Resources, C.N. and D.A.; Software, C.N. and D.A.; Supervision, H.C.; Validation, C.N. and D.A.; Visualization, H.C.; Roles/Writing - original draft, C.N.; Writing - review & editing, H.C.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in line with the principles of the Declaration of Helsinki. All protocols of this investigation were approved by the Ethics Committee of The Sixth Affiliated Hospital of Xinjiang Medical University. The participants’ right to be informed about the study was ensured and written informed consent was obtained from all individual participants included in the study.
Consent for publication
not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Na, C., Ao, D. & Chen, H. MiR-331-3p facilitates osteoporosis and may promote osteoporotic fractures by modulating NRP2 expression. J Orthop Surg Res 19, 487 (2024). https://doi.org/10.1186/s13018-024-04959-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04959-7