Abstract
Background
Increasing evidence shows the pivotal significance of miRNAs in the pathogenesis of osteoporosis. miR-381-3p has been identified as an inhibitor of osteogenesis. This study explored the role and mechanism of miR-381-3p in postmenopausal osteoporosis (PMOP), the most common type of osteoporosis.
Methods
Bilateral ovariectomy (OVX) rat model was established and miR-381-3p antagomir was administrated through the tail vein in vivo. The pathological changes in rats were assessed through the evaluation of serum bone turnover markers (BALP, PINP, and CTX-1), hematoxylin and eosin (H&E) staining, as well as the expression of osteoblast differentiation biomarkers. Moreover, isolated bone marrow mesenchymal stem cells from OVX-induced rats (OVX-BMMSCs) were utilized to explore the impact of miR-381-3p on osteoblast differentiation. In addition, the target gene and downstream pathway of miR-381-3p were further investigated both in vivo and in vitro.
Results
miR-381-3p expression was elevated, whereas KLF5 was suppressed in OVX rats. miR-381-3p antagomir decreased serum levels of bone turnover markers, improved trabecular separation, promoted osteoblast differentiation biomarker expression in OVX rats. ALP activity and mineralization were suppressed, and levels of osteoblast differentiation biomarkers were impeded after miR-381-3p overexpression during osteoblast differentiation of OVX-BMMSCs. While contrasting results were found after inhibition of miR-381-3p. miR-381-3p targets KLF5, negatively affecting its expression as well as its downstream Wnt/β-catenin pathway, both in vivo and in vitro. Silencing of KLF5 restored Wnt/β-catenin activation induced by miR-381-3p antagomir.
Conclusion
miR-381-3p aggravates PMOP by inhibiting osteogenic differentiation through targeting KLF5/Wnt/β-catenin pathway. miR-381-3p appears to be a promising candidate for therapeutic intervention in PMOP.
Similar content being viewed by others
Introduction
Osteoporosis, a systemic bone metabolic disease closely linked to aging, is characterized by the deterioration of bone mass, strength, and microarchitecture, ultimately rendering bones fragile and predisposed to a heightened risk of fractures [1]. Postmenopausal osteoporosis (PMOP) is the most common type of osteoporosis resulted from significant reduction of hormone estrogen after menopause [2]. While the incidence of fractures varies in different countries, it is estimated that up to half of women aged > 50 years old are at risk of fractures [3]. Currently, several drugs have been approved to manage osteoporosis, however, because of the high cost and side effects of these drugs, osteoporosis cannot be well controlled [3, 4]. Hence, it is urgent to develop new therapeutic strategy to alleviate osteoporosis.
It is well known that the equilibrium between osteoblasts and osteoclasts is essential for sustaining healthy bone tissue; however, the imbalance of them is the primary cause of osteoporosis [5]. Bone marrow mesenchymal stem cells (BMMSCs) a type of multipotent progenitor cells capable of differentiating into osteoblasts, adipocytes, and other multi-directional cells. Increasing evidence suggests that as one of crucial regulators of bone homeostasis and regeneration, BMMSCs’ aberrant differentiation leads to the onset of osteoporosis [6, 7]. Promoting BMMSCs’ osteogenic differentiation is capable of restoring bone homeostasis and assisting the treatment of osteoporosis [6, 8].
MicroRNAs (miRNAs), being a class of non-coding RNA molecules, hold crucial significance in governing gene expression. They typically range from 18 to 25 nucleotides in length and are intricately involved in diverse biological processes, encompassing cell development, proliferation, differentiation and apoptosis [9, 10]. The aberrant expression of some miRNAs was implicated in regulating the progression of osteoporosis. For example, serum miR-206 level was lower in osteoporosis patients than the control without osteoporosis, and it might be a potential diagnostic biomarker for osteoporosis. miR-206 promoted proliferation and inhibited apoptosis of osteoblast cells via mediating HDAC4 [11]. miR-15b was highly expressed in the bone tissue of osteoporotic mice. Through targeting USP7/KDM6B pathway, miR-15b suppressed osteoblast differentiation and autophagy, resulted in the initiation of osteoporosis [12].
miR-381-3p is a conserved miRNA among mammals that regulates cell differentiation, proliferation, apoptosis, and migration [13, 14]. In recent years, miR-381-3p has been identified as an inhibitor of osteogenesis [15, 16]. For example, compared with standard healing patients, an increase of miR-381-3p was exhibited in human atrophic nonunion tissues [15]. miR-381-3p inhibition not only promoted osteogenic differentiation in primary human BMMSCs but also in rat femur fracture models [15]. Qiu et al. [17] found that miR-381-3p inhibited osteogenic differentiation process of BMMSCs derived from multiple myeloma patients. However, whether miR-381-3p functions in osteoporosis remains unclear. Bioinformatics analysis predicted that krüppel-like factor 5 (KLF5) was a potential target of miR-381-3p both in humans and rats. KLF5 has been demonstrated to promote osteogenic differentiation through activation of the Wnt/β-catenin pathway [18]. In this study, miR-381-3p’s function in PMOP were investigated in ovariectomy (OVX)-induced osteoporosis rat model and BMMSCs from OVX rats (OVX- BMMSCs). And related molecular mechanism was mainly focused on KLF5/Wnt/β-catenin pathway.
Materials and methods
Animal model establishment and treatment
Twelve-week-old female Sprague-Dawley rats sourced from Vital River Laboratory (Beijing, China) were kept in conditions free of specific pathogens. All animal experiments were approved by the Experimental Animal Ethical Committee of The First Affiliated Hospital of Harbin Medical University (Approval No. 2020052). After acclimation for a week, the rats were randomly allocated to four distinct groups: Sham (n = 6), OVX group (n = 10), OVX + anti-miR-381-3p group (n = 6), and OVX + anti-miR-NC group (n = 6). All surgeries were performed under anesthesia by 50 mg/kg pentobarbital sodium intraperitoneally. Rats in OVX, OVX + anti-miR-381-3p, and OVX + anti-miR-NC groups were carried out bilateral ovariectomy to establish osteoporotic animal models as previously described [19, 20]. While rats in sham group underwent bilateral laparotomy and exposed bilateral ovary. Four weeks after the surgery, rats in OVX + anti-miR-381-3p and OVX + anti-miR-NC groups were administrated with miR-381-3p antagomir (anti-miR-381-3p; RiboBio, Guangzhou, China) or its control antago-NC (anti-NC), respectively, at a dose of 200 nM per rat (200 µL), through the tail vein once a week for 8 weeks [20, 21]. An equal volume of distilled water was given in rats with the sham and OVX groups. After the experimental procedure was completed, rats were euthanized with 100 mg/kg pentobarbital sodium. Following this, blood was extracted from the heart of each rat, and the bilateral femurs and tibiae were surgically removed from the euthanized rats.
Serum biochemical marker analysis
After centrifuging the blood samples at 1000 g for 10 min, the resulting serum was carefully harvested and stored at -20 °C for detection of biochemical markers. To assess bone formation, serum levels of bone alkaline phosphatase (BALP) and N-terminal propeptide of type I procollagen (PINP) were measured using ELISA kits (Cat. No. ml003415 and ml038224, respectively). Similarly, bone resorption was evaluated by the serum levels of C-terminal crosslinked telopeptide of type I collagen (CTX-1) using an ELISA kit (Cat. No. ml003410). All assays were performed following the manufacturer’s guidelines provided by mlbio (Shanghai, China).
Histological analysis via hematoxylin and eosin staining
Prior to histological examination, the femurs obtained from the rats were fixed in 4% paraformaldehyde. Subsequently, they underwent decalcification in ethylene diaminetetra acetic acid. After dehydration in graded ethanol and paraffin embedding, slices of 5 μm thickness were then carefully cut. Hematoxylin and eosin (H&E) staining was carried out to evaluate the morphology of femur. The histological changes of trabecular bone were investigated under a light microscope.
BMMSCs isolation and identification
BMMSCs were isolated from the tibiae and femurs of OVX rats as described previously [22, 23]. Under controlled conditions of 37 °C with 5% CO2, culturing of OVX-BMMSCs was done in α-minimal essential medium (α‐MEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The adherent cells were passaged until 80% confluence. The cells, during the third to fifth passages, were then chosen for further experimentation. Flow cytometry was utilized to authenticate the isolated OVX-BMMSCs using the surface markers of CD34 (MA1-10204, FITC, Invitrogen, Carlsbad, CA, USA), CD45 (MA5-17425, PE, Invitrogen), CD29 (12-0291-82, PE, eBioscience, San Diego, CA, USA) and CD44 (MA5-17522, FITC, Invitrogen) with FITC- or PE-conjugated monoclonal antibodies.
Osteoblast differentiation induction and transfection
After reaching about 80% confluence, the medium of OVX-BMMSCs was changed to osteoblast differentiation medium, which contains α-MEM basal medium with 10% FBS, 1% penicillin/streptomycin, 10 nM dexamethasone, 50 µg/ml ascorbic acid and 10 mM of β-glycerophosphate. Three days following induction [24], the transfection process was performed to deliver 50 nM miR-381-3p agomir (agomir-381-3p)/agomir-NC or 50 nM anti-miR-381-3p/anti-NC ( Ribobio) using Lipofectamine 3000 (Invitrogen). Additionally, for certain experiments, 200 ng of constructed KLF5 silencing plasmid (pLVX-KLF5-shRNA) was co-transfected with 50 nM anti-miR-381-3p at three days after induction, and 200 ng of pLVX-KLF5-shRNA plasmid was transfected again at nine days after induction. The osteoblast differentiation medium was replaced every 3 days. Upon completion of the 14-day induction phase, cells were collected to do the following experiments.
ALP activity assay and alizarin red staining
An alkaline phosphatase (ALP) Assay Kit (Beyotime) was utilized to measure ALP activity. Briefly, 14 days after osteogenic induction, OVX-BMMSCs were lysed with Cell lysis buffer without inhibitors (Cat. No. P0013J, Beyotime), and then centrifugated at 12,000 g for 5 min. ALP activity of the supernatant was tested in 96-well plates based on the manufacturer’s descriptions.
On the 14th day of induction, the cells in 6-well plates were rinsed with PBS to prepare for mineralization assessment. After fixation with 4% paraformaldehyde for 30 min, Alizarin red S solution (sourced from Solarbio, Beijing, China) was introduced to the wells and allowed to react for 10 min at room temperature. Following this, the cells were rinsed again with PBS, and images were captured under a light microscope.
RNA isolation and real-time PCR
Total RNA from femurs and OVX-BMMSCs was extracted using TRNzol Universal reagent (TIANGEN, Beijing, China). Real-time PCR (qRT-PCR) testing was performed based on miRNA qPCR Assay Kit (CWBIO, Beijing, China) to detect miR-381-3p expression after the synthesis of cDNA with miRNA cDNA Synthesis Kit (CWBIO). For quantifying the expression of mRNAs including Alp, collagen type I alpha 1 (Col1a1), runt-related transcription factor 2 (Runx2), and osteocalcin (Ocn), cDNA synthesis was carried out using the FastKing-RT SuperMix (TIANGEN). For qRT-PCR, SYBR Green PCR Master Mix from Thermo Fisher Scientific (Waltham, MA, USA) was employed. The 2−△△Ct method was applied to calculate the expression of each target gene, normalizing the values to GAPDH (for mRNAs) or U6 small nuclear RNA (for miR-381-3p). The specific primers for qRT-PCR are presented in Table 1.
Western blot
After pulverizing with liquid nitrogen, rat femurs were immersed in the RIPA lysis buffer (Solarbio) supplemented with 1mM phenylmethanesulfonyl fluoride. Subsequently, a BCA protein assay kit (Solarbio) was utilized to examine protein concentrations in the supernatants of the samples, which were obtained through centrifugation at 12,000 g for 5 min. Similarly, total proteins from OVX-BMMSCs were isolated using RIPA lysis buffer. SDS-PAGE was used to separate the same quantity of protein, which was then transferred to PVDF membranes. These membranes were then blocked for 2 h using 5% nonfat milk and exposed to diluted primary antibodies like anti-Runx2 (1:1000, #12556), anti-Col1a1 (1:1000, #91144), anti-Alp (1:800, sc-365765), anti-KLF5 (1:5000, 21017-1-AP), anti-β-catenin (1:1000, #9562), anti-c-Myc (1:1000, #18583), and anti-GAPDH (1:1000, #2118). All primary antibodies were from Cell signaling technology (Danvers, MA, USA) except Alp (from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and KLF5 (from Proteintech, Chicago, IL, USA). In the following, the membranes underwent a triple washing process with TBST, and then incubated with Goat Anti-Rabbit IgG H&L (HRP) secondary antibody from Bioss (Beijing, China) for 1 h. After that, the blots were made visible through a chemiluminescence reaction. The quantification of protein bands was performed using ImageJ software. The protein level of GAPDH was used as control.
Dual-luciferase reporter analysis
Bioinformatics analysis using Starbase (https://rnasysu.com/encori/) and Targetscan (https://www.targetscan.org/vert_80/) algorithms confirmed that KLF5 was a potential target of miR-381-3p both in humans and rats. To further evaluate their relationship, we caried out a dual-luciferase reporter assay. Briefly, the 3’ UTR fragment of KLF5 containing the binding sites of miR-381-3p was subcloned into the downstream of firefly luciferase gene of pmirGLO vector to construct KLF5-WT luciferase reporter plasmid. Moreover, KLF5 mutant type vector (KLF5-MUT) was constructed through mutation of the binding sites. OVX-BMMSCs were seeded into 24-well plates and stimulated to undergo osteogenic differentiation. Three days later, these KLF5 recombinant vectors as well as miR-381-3p mimics or miR-NC mimics were transfected into the induced cells. 48 h later, cells were collected, and their luciferase activity was assessed utilizing a Luciferase Assay System kit (Promega, Madison, WI, USA).
Statistical analysis
The data are represented as mean values ± standard deviation. The statistical evaluations were performed with GraphPad Prism version 8, where one-way ANOVA was applied, followed by a Turkey post-hoc test, to assess significant differences among multiple groups. A P value less than 0.05 was deemed statistically significant.
Results
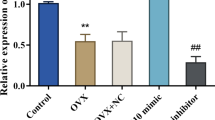
An increase of mir-381-3p in OVX-induced osteoporotic rats
Compared with the sham group, an increase of miR-381-3p was seen in the femoral tissues of rats after OVX treatment (Fig. 1A). Its expression was notably suppressed in the femoral tissues of OVX-induced rats following anti-miR-381-3p administration (Fig. 1A).
Mir-381-3p antagomir alleviates osteoporosis and promotes osteogenic differentiation in vivo
Bone turnover markers (BTMs) are useful tools to monitor osteoporosis [25]. OVX causes the high turnover of bone metabolism [26]. Levels of serum BTMs in rats were detected by ELISA. As revealed in Fig. 2A and C, compared with the sham group, increases of markers of bone formation (BALP, PINP) and resorption (CTX-1) were observed in OVX rats, which indicated a high bone turnover pathology in OVX rats. Administration with anti-miR-381-3p significantly decreased their levels. Moreover, the pathological changes of femurs in each group were evaluated by H&E staining. An obvious decrease of trabecular bone area was seen in OVX rats, which confirmed the successful establishment of an osteoporosis model in rats. Anti-miR-381-3p dramatically increased trabecular bone area compared with OVX rats (Fig. 2D and E). Subsequently, levels of osteoblast differentiation markers were analyzed in the bone tissues. qRT-PCR assay demonstrated the suppression of mRNA expression levels of Runx2, Alp, Col1a1, and Ocn in the femurs of OVX rats, which were facilitated by anti-miR-381-3p (Fig. 2F). The protein levels of Runx2 and Col1a1 were also confirmed by western blot (Fig. 2G and H). These results indicated that anti-miR-381-3p alleviated osteoporosis and stimulated osteogenic differentiation in OVX-induced rats.
miR-381-3p antagomir alleviated osteoporosis and promoted osteogenic differentiation in vivo. Serum levels of BALP (A), PINP (B), and CTX-1 (C) were detected by ELISA. (D) H&E staining of rat femur. Magnification: 20×. (E) Trabecular bone area was quantified. F: The mRNA expression of Runx2, Alp, Col1a1, and Ocn in the femurs of rats was measured by qRT-PCR. G and H: Western blot analysis for the protein levels of Runx2 and Col1a1 in rat femurs. *P < 0.05
Mir-381-3p expression is decreasing during osteogenic differentiation of OVX-BMMSCs
Flow cytometry identified that the isolated OVX-BMMSCs were negative for CD34 and CD45, but positive for CD29 and CD44 (Fig. 3A and B), indicating the successful isolation of BMMSCs from OVX rats. miR-381-3p expression was detected in OVX-BMMSCs during osteogenesis. The results showed that compared with undifferentiated OVX-BMMSCs, miR-381-3p expression was decreasing in differentiated OVX-BMMSCs (Fig. 3C).
miR-381-3p expression was decreasing during osteogenic differentiation of OVX-BMMSCs. A and B: Flow cytometry showed that the isolated OVX-BMMSCs were CD34−, CD45-, and CD29+, CD44+. C: Isolated OVX-BMMSCs were cultured in osteoblast differentiation medium for 7 and 14 days, miR-381-3p expression was determined by qRT-PCR. *P < 0.05 vs. day 0
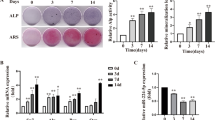
Mir-381-3p inhibits OVX-BMMSCs osteogenesis in vitro
miR-381-3p’s influence on osteogenic differentiation process in osteoporosis were further analyzed in OVX-BMMSCs. The transfection of miR-381-3p agomir led to an elevation in miR-381-3p expression, while transfection with anti-miR-381-3p resulted in a decrease in its expression (Fig. 4A). miR-381-3p agomir inhibited ALP activity and mineralization, whereas anti-miR-381-3p promoted their levels (Fig. 4B and C). Meanwhile, the mRNA levels of osteoblast differentiation markers (Runx2, Alp, Col1a1 and Ocn) were all decreased by miR-381-3p agomir, whereas accelerated after anti-miR-381-3p transfection (Fig. 4D). Consistently, the protein levels of Alp, Runx2 and Col1a1 showed the same tendency as their mRNA expression (Fig. 4E and F). These data suggested that in OVX-BMMSCs, miR-381-3p inhibited the osteogenic differentiation process.
miR-381-3p inhibited OVX-BMMSCs osteogenesis in vitro. A: OVX-BMMSCs was transfected with indicated vector and cultured in osteoblast differentiation medium. 14 days after induction, miR-381-3p expression was tested by qRT-PCR. B: ALP activity was measured using an ALP Assay Kit. C: Alizarin red staining of OVX-BMMSCs at 14 days after induction. D: Osteoblast differentiation marker genes, including Runx2, Alp, Col1a1 and Ocn, were detected in OVX-BMMSCs after designated treatment. E and F: Western blot analysis for the protein levels of Alp, Runx2 and Col1a1 in OVX-BMMSCs at 14 days after induction
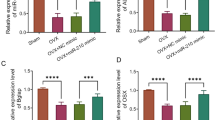
Mir-381-3p directly targets KLF5 during osteoporosis
Through bioinformatics software analysis, it was discovered that the 3′UTR of KLF5 includes a site that complements the seed region of miR-381-3p both in humans and rats. The RNA sequence alignment between them in rats was shown in Fig. 5A. The luciferase activity analysis verified the direct binding between miR-381-3p and KLF5 in OVX-BMMSCs (Fig. 5B). The decreases of KLF5 mRNA and protein levels in the femurs of OVX rats were significantly upregulated by anti-miR-381-3p administration (Fig. 5C and E). In OVX rats, a significant negative correlation emerged between the expression levels of miR-381-3p and KLF5 (Fig. 5F). Moreover, KLF5 expression in OVX-BMMSCs was inhibited by miR-381-3p agomir, and enhanced by anti-miR-381-3p during osteogenic differentiation induction (Fig. 5G and I). These data verified miR-381-3p directly targeting KLF5 during osteoporosis.
miR-381-3p directly targeted KLF5 during osteoporosis. A: The diagram of miR-381-3p binding sites on the 3′UTRs of KLF5 in rats. B: The relative luciferase activity of OVX-BMMSCs co-transfected with miR-381-3p mimics/miR-NC and KLF5 wild-type (KLF5-WT)/mutant (KLF5-MUT) luciferase reporter plasmid. KLF5 mRNA (C) and protein (D and E) expression in rats in different groups was detected by qRT-PCR and western blot, respectively. F: The correlation between KLF5 mRNA and miR-381-3p in OVX rats. The mRNA (G) and protein (H and I) levels of KLF5 in OVX-BMMSCs transfected with designated vector were examined. *P < 0.05
Mir-381-3p suppresses Wnt/β-catenin signaling pathway via targeting KLF5
The protein levels of Wnt3a, β-catenin, and c-Myc were both suppressed in the femurs of OVX rats. Administration of anti-miR-381-3p in OVX rats significantly enhanced their levels (Fig. 6A and B). Moreover, during osteogenic differentiation in OVX-BMMSCs, transfection with miR-381-3p agomir led to a reduction in the levels of Wnt3a, β-catenin and c-Myc, whereas transfection with anti-miR-381-3p resulted in their upregulation (Fig. 6C and D). We also found that downregulation of KLF5 reversed the influences of anti-miR-381-3p on Wnt3a, β-catenin and c-Myc levels during osteogenic differentiation in OVX-BMMSCs (Fig. 6C and F). These results confirmed that miR-381-3p inactivated Wnt/β-catenin signaling pathway via targeting KLF5 during osteogenic differentiation in osteoporosis.
miR-381-3p suppressed Wnt/β-catenin signaling pathway via targeting KLF5. A and B: The protein levels of Wnt3a, β-catenin and c-Myc in rat femur were detected by western blot. C and D: Wnt3a, β-catenin and c-Myc protein expression in OVX-BMMSCs at 14 days after induction were measured by western blot. E and F: OVX-BMMSCs were transfected with KLF5 shRNA (shKLF5) or its control (shNC), and KLF5 protein level was determined by western blot. *P < 0.05
Discussion
Estrogen-deficient animal model is commonly used to mimic PMOP in women [27]. Increased bone turnover, comprising both bone formation and resorption, constitutes a crucial factor contributing to osteoporosis in postmenopausal women [28]. Our investigation revealed a rise in bone turnover and trabecular separation in rats subjected to OVX, suggesting PMOP rat model was successfully established. Here, we utilized in vivo and in vitro strategies to explore the association and molecular mechanism of miR-381-3p in OVX-induced osteoporosis models.
miR-381-3p has emerged as a potential target for cancer therapy [29]. Although miR-381-3p is an inhibitor of osteogenesis [15, 16], until now, limited study investigated its role in osteoporosis. In our study, intravenous injection of miR-381-3p antagomir inhibited the increase of bone turnover markers, alleviated histological changes of femurs in OVX rats. This suggested that miR-381-3p improved bone loss in osteoporotic rats. Promoting osteogenic differentiation is a promising strategy to treat osteoporosis [7, 30]. Here, we observed a decrease in osteoblast differentiation markers in rats subjected to OVX. Notably, inhibition of miR-381-3p was found to elevate the levels of these markers that were inhibited by OVX. OVX led to the decreased osteogenic differentiation in BMMSCs, and increased its adipogenic differentiation [31]. Therefore, the impact of miR-381-3p in PMOP was further explored in OVX-BMMSCs in vitro. We demonstrated that the augmentation of miR-381-3p hindered ALP activity and mineralization, and impeded the expression of osteoblast differentiation biomarkers during osteoblast differentiation of OVX-BMMSCs. Contrarily, suppression of miR-381-3p enhanced ALP activity and mineralization, augmented the expression of markers related to osteoblast differentiation. These data confirmed that miR-381-3p inhibition relieved OVX-induced osteoporosis through enhancing osteogenesis of BMMSCs.
KLF5 is an important transcription factor that is associated with multiple cellular functions, including embryonic development, cell differentiation, proliferation, apoptosis, autophagy, stemness and migration [32, 33]. Existing evidence has demonstrated that KLF5 is intimately linked to the process of osteogenic differentiation [34]. For example, KLF5 level was accelerated during osteogenesis of human periodontal ligament cells (hPDLCs), and its silencing reduced osteogenic differentiation of hPDLCs [34]. Huang et al. [18] found that KLF5 elevation was responsible for miR-19b-induced osteogenic differentiation and fracture healing. Li and co-workers showed that OVX-induced osteoporotic mice and H2O2-treated BMMSCs, KLF5 expression was diminished. KLF5 could stimulate osteogenic differentiation of BMMSCs [35]. Consistently, our study also found a decrease of KLF5 in OVX-induced rats. Studies have verified that KLF5 is a target of many miRNAs [34, 36, 37]. However, the relationship between miR-381-3p and KLF5 remains unknown. A negative correlation between them was exhibited in the femurs of OVX-induced rats. Bioinformatic analysis predicted that KLF5 was a potential target for miR-381-3p both in humans and rats. Subsequently, dual-luciferase reporter assay confirmed their direct binding. Furthermore, anti-miR-381-3p promoted KLF5 levels in vivo and in vitro both at transcription and translation levels. And miR-381-3p upregulation decelerated KLF5 expression during osteogenic differentiation of OVX-BMMSCs. These data showed miR-381-3p directly targeting KLF5 during osteogenic differentiation in osteoporosis.
Wnt/β-catenin signaling serves as a crucial regulator of the initiation and development of osteoporosis, in which, it functions in bone formation through promoting the differentiation of BMMSCs into mature osteoblasts [38, 39]. c-Myc stands as a crucial downstream effector of the Wnt/β-catenin signaling cascade [40]. In our study, we found Wnt3a, β-catenin and c-Myc levels were enhanced after administration of anti-miR-381-3p in OVX rats. In OVX-BMMSCs, their levels were restrained after miR-381-3p upregulation, but elevated after miR-381-3p inhibition, during osteogenic differentiation. This suggested that in the context of osteogenic differentiation in osteoporosis, miR-381-3p exerted an inhibitory effect on the Wnt/β-catenin signaling pathway. It has been reported that in the osteogenic differentiation process of BMMSCs, KLF5 triggered the activation of Wnt/β-catenin pathway [18]. We further evaluated whether miR-381-3p regulated Wnt/β-catenin pathway through targeting KLF5. Here, we found that downregulation of KLF5 abrogated the effects of anti-miR-381-3p on Wnt3a, β-catenin and c-Myc expression in OVX-BMMSCs. Collectively, above data indicated that miR-381-3p suppressed Wnt/β-catenin signaling pathway via targeting KLF5 during osteogenic differentiation in osteoporosis. However, our study still has some limitations. First, given that osteoclast-induced bone resorption is another determinant factor in osteoporosis, miR-381-3p’s influence on osteoclastogenesis needs further investigation [41]. Moreover, miR-381-3p might also target other genes except KLF5 in osteoporosis, which also needs further exploration. Additionally, miR-381-3p expression profile needs to be evaluated in PMOP patients.
In conclusion, this is the first time to delve the connection between miR-381-3p and osteoporosis. miR-381-3p inhibited OVX-induced osteoporosis by inhibiting osteogenic differentiation through targeting KLF5/Wnt/β-catenin signaling pathway (Fig. 7). miR-381-3p emerges as a potential therapeutic target for the management of PMOP.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14–21.
Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis, Nature reviews. Disease Primers. 2016;2:16069.
Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu W, Peng Y. Dietary natural N-Acetyl-d-Glucosamine prevents bone loss in Ovariectomized Rat Model of Postmenopausal osteoporosis. Molecules 23 (2018).
Zhou Y, Xu Z, Wang Y, Song Q, Yin R. LncRNA MALAT1 mediates osteogenic differentiation in osteoporosis by regulating the miR-485-5p/WNT7B axis. Front Endocrinol. 2022;13:922560.
An F, Wang X, Wang C, Liu Y, Sun B, Zhang J, Gao P, Yan C. Research progress on the role of lncRNA-miRNA networks in regulating adipogenic and osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporosis. Front Endocrinol. 2023;14:1210627.
Zeng C, Wang S, Chen F, Wang Z, Li J, Xie Z, Ma M, Wang P, Shen H, Wu Y. Alpinetin alleviates osteoporosis by promoting osteogenic differentiation in BMSCs by triggering autophagy via PKA/mTOR/ULK1 signaling, phytotherapy research. PTR. 2023;37:252–70.
Wang X, Chen T, Deng Z, Gao W, Liang T, Qiu X, Gao B, Wu Z, Qiu J, Zhu Y, Chen Y, Liang Z, Zhou H, Xu C, Liang A, Su P, Peng Y, Huang D. Melatonin promotes bone marrow mesenchymal stem cell osteogenic differentiation and prevents osteoporosis development through modulating circ_0003865 that sponges miR-3653-3p. Stem Cell Res Ther. 2021;12:150.
Plotkin LI, Wallace JM. MicroRNAs Osteocytes Bone. 2021;150:115994.
Lee AY. The role of MicroRNAs in epidermal barrier. Int J Mol Sci 21 (2020).
Lu Z, Wang D, Wang X, Zou J, Sun J, Bi Z. MiR-206 regulates the progression of osteoporosis via targeting HDAC4. Eur J Med Res. 2021;26:8.
Li K, Chen S, Cai P, Chen K, Li L, Yang X, Yi J, Luo X, Du Y, Zheng H. MiRNA-483-5p is involved in the pathogenesis of osteoporosis by promoting osteoclast differentiation. Mol Cell Probes. 2020;49:101479.
Wang J, Sun N, Ju Y, Ni N, Tang Z, Zhang D, Dai X, Chen M, Wang Y, Gu P, Ji J. Mir-381-3p cooperated with Hes1 to regulate the proliferation and differentiation of retinal progenitor cells. Front cell Dev Biology. 2022;10:853215.
Zhu XS, Zhou HY, Yang F, Zhang HS, Ma KZ. Mir-381-3p inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1, the journal of gene medicine 23 (2021) e3274.
Long H, Zhu Y, Lin Z, Wan J, Cheng L, Zeng M, Tang Y, Zhao R. miR-381 modulates human bone mesenchymal stromal cells (BMSCs) osteogenesis via suppressing wnt signaling pathway during atrophic nonunion development. Cell Death Dis. 2019;10:470.
Zhu Y, Zhao S, Cheng L, Lin Z, Zeng M, Ruan Z, Sun B, Luo Z, Tang Y, Long H. Mg(2+) -mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J Orthop Research: Official Publication Orthop Res Soc. 2022;40:1563–76.
Qiu L, Cai J, Zhang N, Ma L, Fan FY, Li XM. Effect of miR-381-3p/FGF7 axis on the osteogenic differentiation of bone marrow mesenchymal stem cells through MEK/ERK signaling pathway. Tissue Cell. 2022;76:101791.
Huang Y, Xu Y, Feng S, He P, Sheng B, Ni J. miR-19b enhances osteogenic differentiation of mesenchymal stem cells and promotes fracture healing through the WWP1/Smurf2-mediated KLF5/beta-catenin signaling pathway. Exp Mol Med. 2021;53:973–85.
Liang J, Chen J, Ye Z, Bao D. Cathelicidin LL-37 improves bone metabolic balance in rats with ovariectomy-induced osteoporosis via the Wnt/beta-catenin pathway. Physiol Res. 2022;71:369–77.
Ren LJ, Zhu XH, Tan JT, Lv XY, Liu Y. MiR-210 improves postmenopausal osteoporosis in ovariectomized rats through activating VEGF/Notch signaling pathway. BMC Musculoskelet Disord. 2023;24:393.
Li Z, Zhang W, Huang Y. MiRNA-133a is involved in the regulation of postmenopausal osteoporosis through promoting osteoclast differentiation. Acta Biochim Biophys Sin. 2018;50:273–80.
He Q, Qin R, Glowacki J, Zhou S, Shi J, Wang S, Gao Y, Cheng L. Synergistic stimulation of osteoblast differentiation of rat mesenchymal stem cells by leptin and 25(OH)D(3) is mediated by inhibition of chaperone-mediated autophagy. Stem Cell Res Ther. 2021;12:557.
Xu L, Song C, Ni M, Meng F, Xie H, Li G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRalpha-induced beta-catenin degradation. Int J Biochem Cell Biol. 2012;44:612–9.
Zhang Y, Dong Y, Wei Q, Zhuang Z, Liu Y, Yuan Q, He W, Jing Z, Li J, Li P, Zhang L, Hong Z, Zhang N, Wang H, Li W. miR-126 mitigates the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting the ERK1/2 and Bcl-2 pathways. Acta Biochim Biophys Sin. 2023;55:449–59.
Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63:464–74.
Shen Y, Wang N, Zhang Q, Liu Y, Wu Q, He Y, Wang Y, Wang X, Zhao Q, Zhang Q, Qin L, Zhang Q. Jin-Tian-Ge ameliorates ovariectomy-induced bone loss in rats and modulates osteoblastogenesis and osteoclastogenesis in vitro. Chin Med. 2022;17:78.
Hartke JR. Preclinical development of agents for the treatment of osteoporosis. Toxicol Pathol. 1999;27:143–7.
Zheng H, Qi S, Chen C. Salidroside improves bone histomorphology and prevents bone loss in Ovariectomized Diabetic rats by upregulating the OPG/RANKL ratio. Molecules 23 (2018).
Sha H, Gan Y, Xu F, Zhu Y, Zou R, Peng W, Wu Z, Ma R, Wu J, Feng J. MicroRNA-381 in human cancer: its involvement in tumour biology and clinical applications potential. J Cell Mol Med. 2022;26:977–89.
You WL, Xu ZL. Curculigoside promotes osteogenic differentiation of ADSCs to prevent ovariectomized-induced osteoporosis. J Orthop Surg Res. 2021;16:279.
Qi M, Zhang L, Ma Y, Shuai Y, Li L, Luo K, Liu W, Jin Y. Autophagy maintains the function of bone marrow mesenchymal stem cells to prevent Estrogen Deficiency-Induced osteoporosis. Theranostics. 2017;7:4498–516.
Chen Z, Zhang Q, Wang H, Li W, Wang F, Wan C, Deng S, Chen H, Yin Y, Li X, Xie Z, Chen S. Klf5 mediates odontoblastic differentiation through regulating dentin-specific Extracellular Matrix Gene expression during mouse tooth development. Sci Rep. 2017;7:46746.
Luo Y, Chen C. The roles and regulation of the KLF5 transcription factor in cancers. Cancer Sci. 2021;112:2097–117.
Wangzhou K, Lai Z, Lu Z, Fu W, Liu C, Liang Z, Tan Y, Li C, Hao C. MiR-143-3p inhibits osteogenic differentiation of Human Periodontal Ligament cells by targeting KLF5 and inactivating the Wnt/beta-Catenin pathway. Front Physiol. 2020;11:606967.
Li L, Wang H, Chen X, Li X, Wang G, Jie Z, Zhao X, Sun X, Huang H, Fan S, Xie Z, Wang J. Oxidative stress-Induced Hypermethylation of KLF5 promoter mediated by DNMT3B impairs Osteogenesis by diminishing the Interaction with beta-catenin. Antioxid Redox Signal. 2021;35:1–20.
Feng J, He W, Xia J, Huang Q, Yang J, Gu WP, Zhang N, Liu YH. Human umbilical cord mesenchymal stem cells-derived exosomal circDLGAP4 promotes angiogenesis after cerebral ischemia-reperfusion injury by regulating miR-320/KLF5 axis, FASEB journal: official publication of the Federation of American Societies for Experimental Biology 37 (2023) e22733.
Dang X, Yang L, Guo J, Hu H, Li F, Liu Y, Pang Y. Mir-145-5p is associated with smoke-related chronic obstructive pulmonary disease via targeting KLF5. Chemico-Biol Interact. 2019;300:82–90.
Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–32.
Gao Y, Chen N, Fu Z, Zhang Q. Progress of wnt signaling pathway in osteoporosis. Biomolecules 13 (2023).
Li Z, Yang Z, Liu W, Zhu W, Yin L, Han Z, Xian Y, Wen J, Tang H, Lin X, Yang Y, Wang J, Zhang K. Disheveled3 enhanced EMT and cancer stem-like cells properties via Wnt/beta-catenin/c-Myc/SOX2 pathway in colorectal cancer. J Translational Med. 2023;21:302.
Yang JG, Sun B, Wang Z, Li X, Gao JH, Qian JJ, Li J, Wei WJ, Zhang P, Wang W. Exosome-targeted delivery of METTL14 regulates NFATc1 m6A methylation levels to correct osteoclast-induced bone resorption. Cell Death Dis. 2023;14:738.
Funding
None.
Author information
Authors and Affiliations
Contributions
Yansong Wang and Yingwei Zhao designed this study and guaranteed for the integrity of the study. Yingwei Zhao, Jingsong Liu, and Yubo Zhang performed the experiments. Yingwei Zhao, Min Liang, Rui Li, and Yindong Song collected and analyzed the data. Yingwei Zhao written the original manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Liu, J., Zhang, Y. et al. Mir-381-3p aggravates ovariectomy-induced osteoporosis by inhibiting osteogenic differentiation through targeting KLF5/Wnt/β-catenin signaling pathway. J Orthop Surg Res 19, 480 (2024). https://doi.org/10.1186/s13018-024-04992-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04992-6