Abstract
Introduction
Acute myocardial infarction (AMI) is a serious, deadly disease with a high incidence. However, it remains unclear how necroptosis affects the pathophysiology of AMI. Using bioinformatic analyses, this study investigated necroptosis in AMI.
Methods
We obtained the GSE66360 dataset related to AMI by the GEO database. Venn diagrams were used to identify necroptosis-related differential genes (NRDEGs). The genes with differential expression in AMI were analyzed using gene set enrichment analysis, and a PPI network was established. A transcription factor prediction and enrichment analysis were conducted for the NRDEGs, and the relationships between AMI, NRDEGs, and immune cells were determined. Finally, in the additional dataset, NRDEG expression levels, immune infiltration, and ROC curve analysis were confirmed, and gene expression levels were further verified experimentally.
Results
GSEA revealed that necroptosis pathways were significantly enriched in AMI. We identified 10 NRDEGs, including TNF, TLR4, FTH1 and so on. Enrichment analysis indicated that the NOD-like receptor and NF-kappa B signaling pathways were significantly enriched. Four NRDEGs, FTH1, IFNGR1, STAT3, and TLR4, were identified; however, additional datasets and further experimental validation are required to confirm their roles. In addition, we determined that a high abundance of macrophages and neutrophils prompted AMI development.
Conclusions
In this study, four potential genes that affect the development of AMI through necroptosis (FTH1, IFNGR1, STAT3, and TLR4) were identified. In addition, we found that a high abundance of macrophages and neutrophils affected AMI. This helps determine the pathological mechanism of necroptosis and immune cells that influence AMI and provides a novel strategy for targeted therapy.
Similar content being viewed by others
Introduction
Cardiovascular disease remains highly prevalent throughout the world, significantly increasing the global health burden [1]. There is a high incidence of acute myocardial infarction (AMI), a severe and fatal ischemic cardiomyopathy characterized by a sudden decrease in myocardial blood flow [2]. Coronary artery disease is often responsible for AMI. When acute occlusion occurs in the coronary arteries due to the rupture of atherosclerotic plaques or other factors, the blood cannot reach the myocardium normally, resulting in severe ischemia and hypoxia in the corresponding myocardium, and eventually cardiomyocyte injury and death [3]. Ventricular remodeling occurs during the acute phase of myocardial ischemia, which reduces heart function, causes heart failure, and increases probability of mortality [4]. Currently, after thrombolysis or surgery to treat AMI, the reopening of blood vessels causes cardiac reperfusion injury, making myocardial injury more serious and further affecting cardiac function [5]. Cardiomyocytes are permanent cells, and once lost they cannot be regenerated [6]; and, unfortunately, the remaining cardiomyocytes after acute myocardial infarction are not sufficient for normal heart function. Therefore, it is necessary to develop efficient drugs to treat AMI.
Necroptosis is a type of regulatory necrosis that is similar to ferroptosis and associated with programmed cell death [7]. The result is loss of integrity of the cell membrane, swelling of organelles, leakage of intracellular fluid, and activation of death receptor ligands [7, 8]. In the classic necroptosis signaling pathway, extracellular signals activate death receptors (TNF-α), which activates receptor-interacting protein kinase 1 (RIP1) and receptor-interacting protein kinase 3 (RIP3) to form necrosome complexes. Activated RIPK3 activates the downstream executor mixed lineage kinase domain-like proteins (MLKL). As phosphorylated MLKL forms oligomers, it promotes membrane pores, ultimately causing necroptosis and inducing inflammation through cell fragmentation and release of intracellular cytokines [8]. Multiple studies have described the relationship between necroptosis and cardiovascular disease, including specifics about necroptosis to AMI [8,9,10,11,12,13,14]. Luedde et al. demonstrate that post-ischemic cardiac remodeling is negatively affected by RIP3 in AMI mice [10].
Recently, there has been an increase in research on tumors and immune cell infiltration. At the same time, some studies have shown that immunity and inflammation were also involved in the pathophysiological changes of AMI. Inflammation and immune-inflammatory imbalances are associated with poor heart failure prognoses, progressive left ventricular remodeling, and heart failure progression [15,16,17]. Bioinformatics studies have demonstrated a link between immune infiltration and AMI [18, 19]; however, the immune infiltration mechanisms of AMI need further clarification.
In this study, AMI datasets were retrieved from the Gene Expression Omnibus (GEO) database. They were analyzed using bioinformatic methods to explore the relationship of AMI with necroptosis and immune infiltration. An essential goal of our study was to identify the key genes and mechanisms underlying necroptosis in AMI so that new insights can be gained into its treatment. We also attempted to find changes in the infiltrating immune cells in patients with AMI to gain new implications for immunotherapy.
Methods
Data source
The Gene Expression Omnibus database (GEO) (http://www.ncbi.nlm.nih.gov/geo) stores a large number of sequencing and microarray data provided by research institutions worldwide. We searched the data sets about myocardial infarction using the keywords “acute myocardial infarction” or “AMI,” and finally, GSE66360 was identified (GPL570-Affymetrix Human Genome U133 Plus 2.0 Array). This datasets was published on February 28, 2015, with the purpose of investigating the application of molecular biomarkers in the differential diagnosis of AMI. Among the GSE66360 dataset, circulating endothelial cells were isolated from 49 patients with AMI and 50 healthy controls followed by gene expression measurements.
Identification of differential genes related to necroptosis
Using the GEO2R online analysis tool (www.ncbi.nlm.nih.gov/geo/ge2r), we identified differentially expressed genes in the dataset GSE66360. The GEO2R tool uses two R packages (“GEOquery” and “Limma”) to conduct online analysis. Since this probe matched no gene, it was excluded. Gene expression corresponding to multiple probes was averaged. Then we performed follow-up gene set enrichment analysis (GSEA) on differential genes with adjusted p-values (< 0.05), and we identified the genes with |logFC| > 1 that were associated with necroptosis. The Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/) contains the most extensive and common data on metabolic pathways. According to the KEGG database, 144 genes are associated with necroptosis. For detailed information on these genes, please refer to Supplementary Material S1. Necroptosis-related differential genes (NRDEGs) were identified using Venn diagrams.
Differential gene enrichment analysis
Comparing the biological functions of patients with AMI and healthy individuals, genes with differential expression were analyzed using GSEA by the “clusterProfiler” R package, which is based on the molecular signature database (MSigDB) [20]. Statistical significance was set at p < 0.05. We used the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/tools.jsp) to perform Gene Ontology (GO) and KEGG pathway enrichment analysis [21]. Biological functions and signaling pathways of NRDEGs were investigated through this approach. Significantly enriched terms were defined as those with an adjust p-value < 0.05.
Network construction of protein-protein interaction (PPI)
Using the Search Tool for the Retrieval of Interacting Genes database (STRING 11.5; https://cn.string-db.org/), the NRDEG interaction relationships were determined [22]. Then we could construct a relationship between proteins or the coexistence of upstream and downstream regulatory pathways. An overall score of > 0.4 was considered to be statistically significant. Cytoscape (version 3.9.1 https://cytoscape.org/) was used to visualize and analyze the PPI network [23], and key functional modules were analyzed using the Cytoscape plug-in MCODE.
Hub gene selection and analysis
For the identification of hub genes, we used the CytoHubba plug-in (Cytoscape, version 3.9.1). In order to select hub genes, we used seven common algorithms: MCC, MNC, Degree, EPC, EcCentricity, Closeness, and Radiality. With GeneMANIA (http://genemania.org/), these hub genes were connected in a co-expression network [24], which identified internal links in the genome and predicted other associated genes.
Transcription factor prediction and verification
NRDEG-related transcription factors (TFs) were predicted using Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining (TRRUST) (https://www.grnpedia.org/trrust/) [25]. TTRUST can predict transcriptional regulatory networks, including 8444 TF targets and the regulatory relationships of 800 human TFs. Through Cytoscape, TFs and NRDEGs were visually interconnected. We verified the expression levels of these TFs with GSE66360.
Immune infiltration and receiver operating characteristic (ROC) curve analyses
Using ImmuCellAI (http://bioinfo.life.hust.edu.cn/ImmuCellAI#!/), gene expression can be used to estimate the number of immune cells [26]. The percentage of infiltrating immune cells was added together to determine the infiltration score. We also conducted an analysis of Pearson’s correlation between NRDEGs and immune cell infiltration. The ROC curve analysis of the datasets was performed based on the “pROC” R package.
Other datasets validated NRDEGs and immune infiltration
Another datasets about acute myocardial infarction, GSE60993 (Illumina GPL6884 platform, Illumina HumanWG-6 v3.0, expression beadchip), was obtained from the GEO database.This datasets was was published on May 23, 2015, with the aim of early screening for biomarkers of ST-elevation myocardial infarction. This datasets included peripheral blood transcriptome sequencing data from seven ST-segment elevations during myocardial infarction and seven healthy human. GSE60993 was used to verify the expression of NRDEGs. To ensure the robustness of the results, another dataset, GSE61144 (Sentrix GPL6106 platform, Sentrix Human-6 v2 Expression BeadChip), was utilized for further validation of NRDEGs. This datasets included peripheral blood transcriptome sequencing data from seven ST-segment elevations during myocardial infarction and ten healthy human. ImmuCellAI and ROC curve analysis were also used to estimate the immune cell abundance in GSE60993 and GSE61144.
Animal model
This study is a randomized controlled study. The 30 C57BL/6 male mice were separated into two groups at 6–8 weeks of age by online random number generators: the control group (n = 15) and AMI group (n = 15). Randomization, surgical procedures, and data statistics were performed by each of the three fellows. After feeding all mice in a pathogen-free environment for one week, 2.5% isoflurane was used for induction and 1.0% for maintenance of anesthesia in the two groups. As the mice were opened and their chests were exposed, the heart was visible through the left side’s fourth intercostal space. Within the AMI group, the left anterior descending branch was ligated with a nylon thread 7 − 0. The control group received the same treatment, except that they were ligated. All surviving animals were included in subsequent studies (the control group = 15 and AMI group = 15). One week later, we euthanized the two groups of mice and collected heart tissue samples. Gene expression was assessed by subsequent experiments. This animal experiment had been approved by the Animal Ethics Committee of Fujian Medical University (IACUC FJMU 2023-0052), and all the experiments met relevant ARRIVE guidelines and regulations.
Quantitative real-time PCR
Total cellular RNA was extracted from mouse heart tissue using TRIzol reagent. The levels of mRNA were determined by quantitative real-time PCR (qRT-PCR) (Takara Bio) and TB Green real-time PCR. LightCycler 480 release 1.5.0 (Roche LC480) was used for amplification. The primer sequences are shown in Supplementary material S2.
Immunohistochemistry staining
Paraffin was used to encapsulate the heart tissues, which were then cut into 4 μm slices. Paraffin tissue slices were baked in an oven (60 °C) for 1 h and then dewaxed by dipping them in different concentrations of xylene and ethanol. An Immunohistochemical Kit (UItraSensitive SP mouse/rabbit; MXB) was used to deal with the tissue slices after dewaxing, and the corresponding primary antibody was selected to incubate slices overnight (4 °C). Finally, the DAB staining kit (DAB-0031; MXB) was used for staining. We used the Nikon fluorescence microscope (Nikon ECLIPSE Ni) to obtain images of the slices and Image-Pro Plus 6.0 (IPP 6.0) to analyze the results.
Statistical analysis
The statistical analysis was conducted using GraphPad Prism 9.0 (GraphPad Software, USA). The experimental results were expressed as the average ± standard error of measurement (SEM). Student’s t-tests after F-tests were used to analyze the two groups of data. P-values < 0.05 indicate statistical significance.Taking into account multiple testing, we applied Bonferroni correction for both multiple correlations and t-tests. We set the significance threshold at p < 0.05/n (n represents the number of tests conducted). Specifically, for TFs, n = 12; for immune cells, n = 24; and for NRDEGs, n = 10.
Results
Identification of differential genes and NRDEGs
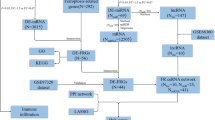
The flowchart of this study can be seen in Fig. 1. GSE66360 gene expression profiles identified 2647 differential genes with an adjusted p-value < 0.05, including 1274 upregulated genes and 1373 downregulated genes. These genes were used for GSEA analysis [20]. When we set the adjusted p-value to < 0.05 and |logFC|≥1, there were 657 differentially expressed genes, of which 463 increased and 194 decreased. Through overlap between the necroptosis gene set and differential genes in GSE66360, 10 NRDEGs with an increase in expression were identified (Fig. 3b, c and d), including TNF, TLR4, FTH1, STAT3, IL1B, GLUL, NLRP3, TNFAIP3, IL1A, and IFNGR1.
Enrichment analysis of differential genes and NRDEGs
GSEA analysis was performed to identify the signaling pathways associated with the differentially expressed genes. The result included 734 GO projects and 70 KEGG pathways (Supplementary Materials S3 and S4). GSEA showed that the necroptosis pathway (p.adjust = 0.004) was significantly enriched among the differentially expressed genes, suggesting that acute myocardial infarction was indeed associated with necroptosis (Fig. 2). Moreover, GSEA showed that the IL-17 signaling pathway, Toll-like receptor signaling pathway and NF-kappa B signaling pathway were significantly enriched in AMI (Fig. 2). The enrichment scores of the 10 terms in the upper parts of GSEA based on KEGG and GO, are shown in Fig. 3a. The potential function and metabolic pathways of NRDEGs were explored by GO and KEGG analyses [21]. 90 GO projects and 62 KEGG pathways were identified (Supplementary Materials S5 and S6). GO enrichment analysis showed that protein binding, identical protein binding, cytokine activity, and interleukin-1 receptor binding were enriched in molecular functions, whereas the cytosol, plasma membrane, extracellular region, and cell surface were enriched in cellular components. More importantly, the inflammatory response, cellular response to lipopolysaccharide, positive regulation of interleukin-6 production, positive regulation of NF-kappa B transcription factor activity, and immune response were involved in the biological process of AMI (Fig. 4a). According to KEGG pathway enrichment analyses, NRDEGs were involved in signaling pathways, such as necroptosis, the NOD-like receptor, NF-kappa B, and AGE-RAGE signaling pathway (Fig. 4b).
Acquisition of necroptosis-related differential genes (NRDEGs) in acute myocardial infarction (AMI). (A) Top and bottom 20 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment scores in gene set enrichment analysis (GSEA). (B) Venn diagram of NRDEGs from differential genes in GSE66360 differential versus necroptosis-related genes. (C) Volcano plot of NRDEGs. (D) Heatmap of NRDEGs
Results of the enrichment analysis of necroptosis-related differential genes (NRDEGs). (A) Results of the gene ontology (GO) enrichment analysis. (B) Results of the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. BP: biological process; CC: cellular component; MF: molecular function
Analyzing the PPI network and its construction
PPI networks of NRDEGs were constructed using the STRING database (Fig. 5a), which was then analyzed using Cytoscape [23]. The key modules of the PPI network were identified using the Cytoscape MCODE plug-in, and then a module with seven nodes and 21 edges was obtained (Fig. 5b). In addition, identification of hub genes was performed using cytoHubba. The results of the seven cytoHubba algorithms are presented in Table 1. The interactions of all the hub genes were shown in Fig. 5c, including TNF, TLR4, STAT3, IL1B, NLRP3, TNFAIP3, IL1A, and IFNGR1. The GeneMANIA database was used to construct the co-expression network of these hub genes [24]. It revealed a complex PPI network with 70.49% of co-expression, 16.46% of shared protein domains, 5.95% of physical interactions, 2.69% of pathways, and 4.23% of co-localizations (Fig. 5d).
Protein-protein interaction (PPI) network of necroptosis-related differential genes (NRDEGs). (A) The PPI network was derived from the STRING database. (B) The key modules of the PPI network explored by Cytoscape’s MCODE function. (C) Hub genes explored by CytoHubba. (D) Co-expression network of hub genes
Prediction of NRDEG-related transcription factors
Twelve TFs were predicted to regulate hub genes based on the TRRUST database [25]. The interactions between these TFs and their corresponding hub genes are shown in Fig. 6a. By verifying their expression in GSE66360 cells, six TFs were found to be highly expressed in AMI cells (Fig. 6b). Together, they regulated seven hub genes, including TNF, TLR4, STAT3, IL1B, TNFAIP3, IL1A, and IFNGR1 (Table 2).
Prediction of the transcription factors (TFs). (A) Interactions of hub genes with related TFs. Circles represent the hub genes, and squares represent the transcription factors. Color depth represents the importance. (B) Expression of the TFs was verified in GSE66360. #, indicate results that survived multiple testing correction
Immune infiltration and NRDEGs
Through the enrichment analysis mentioned above, NRDEGs and immune-related pathways are closely related. ImmuCellAI was used to evaluate the relationship between NRDEGs and immune cell infiltration in patients with AMI [26]. In the AMI group, a significant difference was observed in many types of immune cells compared with the normal control group. Specifically, higher levels of macrophages and neutrophils were observed in AMI. However, CD4 + T, gamma delta (yδ)-T, Tr2, Tfh, exhausted-T and central-memory-T were lowly expressed in AMI (Fig. 7a and b, and 8a). We also evaluated the correlations between NRDEGs and immune cells to determine their connections and potential roles. We found that NRDEGs were positively correlated with macrophages, neutrophils, and cytotoxicity. In addition, they were negatively correlated with Th2, Th17, CD4 + T, and gamma delta (yδ)-T cells (Fig. 8b).
Validation of NRDEGs and immune infiltration
To make the results of the NRDEGs and immune infiltration more credible, we used another AMI dataset, GSE60993, for validation. Finally, we found that four genes in the NRDEGs were upregulated, such as in GSE66360, including FTH1, IFNGR1, STAT3, and TLR4 (Fig. 9a). We used ImmuCellAI to analyze immune cell abundance in GSE60993. Based on the results, it can be concluded that, when compared to healthy controls, the enrichment of macrophages and neutrophil was higher in AMI, and that of CD4 + T, CD8 + T, gamma delta (yδ)-T cell, Tfh, exhausted-T cell and effector-memory-T cell was lower (Fig. 9b). For the four significant genes identified, we performed ROC curve analysis to confirm their diagnostic strength. The results showed that the AUC values for all four genes were greater than 0.7 (Fig. 9c). To further ensure the robustness of the validation, these four genes were validated in a new dataset, GSE61144, with both immune infiltration and ROC analyses performed. The same results were observed. This suggests that the aforementioned four genes may influence the progression of AMI through necroptosis and have high sensitivity and specificity for diagnosis. Through verification, there was evidence that necroptosis and immune cell infiltration contribute to the pathogenesis of AMI.
Validation of necroptosis-related differential genes (NRDEGs) and immune cell abundance. (A) The expression level of NRDEGs was observed in GSE60993. (B) Box-plots of the immune cell proportion in GSE60993. (C) The ROC curve analysis of four hub genes in GSE60993. (D) The expression level of four hub genes in GSE61144. (E) Box-plots of the immune cell proportion in GSE61144. (F) The ROC curve analysis of four hub genes in GSE61144. AUC > 0.7 indicated that the model had a good fitting effect. #, indicate results that survived multiple testing correction
Experimental verification of NRDEG expression
Aiming at the obtained NRDEGs, including FTH1, IFNGR1, STAT3, and TLR4, we established animal models of myocardial infarction in mice (Fig. 10a) and verified levels of these gene expression. Using qPCR to detect the gene expression levels in the hearts of AMI and control mice, we found that as compared with the control group, the mRNA expression levels of FTH1, IFNGR1, STAT3, and TLR4 were higher in AMI mice (Fig. 10b). To further determine the expression of these NRDEGs at the protein level, we performed immunohistochemical (IHC) analyses of heart tissues from the two groups of mice. The IHC results were as expected. In contrast with the healthy control group, AMI increased the levels of FTH1, IFNGR1, STAT3, and TLR4 proteins in cardiomyocytes (Fig. 10c). Based on these results, inhibiting the expression of these genes may be beneficial in treating AMI.
Experimental validation of the vital necroptosis-related differential genes (NRDEGs) in model mice. (A) Heart anatomical cross sections of representative mice. (B) The expression levels of the mRNA obtained by qPCR, n = 4 in each group. (C) The expressions of vital NRDEGs were observed by immunohistochemistry (IHC), n = 4 in each group. The results were expressed as mean ± SEM. The two groups of data were statistically analyzed by double-tailed Student’s t-test. * p < 0.05; ** p < 0.01; *** p < 0.001
Discussion
Necroptosis is vital in severe cardiac pathological conditions such as myocardial ischemia-reperfusion injury, heart failure, and AMI [9]. To date, the process of necroptosis signal transduction has been described in many studies, but the upstream activation targets of the RIP1-RIP3-MLKL and RIP3-CaMKII-mPTP pathways are not clear [7,8,9]; therefore, it is not easy to achieve targeted therapy for myocardial infarction. In this study, we identified hub genes that may affect the development of AMI through necroptosis, providing potential therapeutic targets. We then identified the immune cells associated with AMI using immune cell correlation analysis to provide support for immunotherapy.
In this study, the activation of necroptosis in AMI was determined by GSEA. Four hub genes were found to most likely affect AMI by necroptosis: FTH1, IFNGR1, STAT3, and TLR4. Surprisingly, FTH1 was not included in the results of the cytoHubba algorithm but was highly expressed in additional datasets and our experiments. We believe that the results of gene expression verification are more credible than those of the cytoHubba algorithm and that the role of FTH1 in AMI cannot be ignored. NRDEGs were associated with NOD-like receptors and NF-kappa B signaling pathways based on the KEGG enrichment analysis. Correlation analysis between NRDEGs and immune cells revealed that macrophages, neutrophils, cytotoxic cells, and NRDEGs exhibited positive correlations. Meanwhile, the immune infiltration analysis also suggested the high abundance of macrophages and neutrophils in AMI.
FTH1 encoded the ferritin heavy chain, which was the main iron storage protein within cells. Ferritin played a crucial role in regulating iron homeostasis in the body. Iron overload could lead to oxidative stress and damage in cardiomyocytes, [27] while FTH1 might have reduced the accumulation of free iron, thereby alleviating oxidative stress and myocardial injury. IFNGR1 mediated the signaling of interferon-gamma (IFN-γ). IFN-γ could promote the activation and migration of macrophages and other immune cells, participating in the repair and remodeling of the damaged site. However, excessive IFN-γ signaling might have led to an excessive inflammatory response and exacerbation of myocardial injury [28]. STAT3 was a transcription factor that played a key role in various cell signaling pathways. On one hand, STAT3 activation could promote cell survival, anti-apoptosis, and myocardial protection; on the other hand, STAT3 might have also promoted inflammatory responses under certain conditions. Therefore, the regulation of STAT3 activity had a potential double-edged sword effect in the treatment of myocardial infarction [29].
In addition, GSEA revealed that the Toll-like receptor signaling pathway is related to AMI. Toll-like receptors (TLRs) are innate immune receptors that mediate platelet activation. High TLR expression during AMI causes platelet activation, enhances coronary thrombosis, and causes myocardial injury, and TLR3/4 and TNF can activate RIP1-RIP3-MLKL, causing necroptosis [30, 31]. TLR4 recognized DAMPs released by necrotic cells and activated downstream inflammatory signaling pathways, leading to the recruitment and activation of inflammatory cells. This response might have helped to clear necrotic tissue in the early stages, but excessive TLR4 activation could have resulted in sustained inflammation and exacerbation of myocardial injury [32]. The complement and coagulation cascade pathways were also suggested by GSEA, indicating that there may be problems with the coagulation system in patients with AMI, facilitating the formation of blood vessel thrombosis.
Using ImmuCellAI, we found that macrophages and neutrophils were higher in patients with AMI.The macrophages levels in AMI patients suggest an active inflammatory response, as macrophages are typically involved in the removal of dead cells and tissue remodeling after myocardial infarction. Prior studies have shown that when the myocardium was damaged after AMI, the immune system became activated with a monocyte imbalance [33]. Macrophages are recruited to the infarcted site after AMI, showing pro-inflammatory and anti-inflammatory responses and promoting vascular and scar formation. The different activities of macrophages originate from different subtypes and polarizations, showing a pro-inflammatory phenotype in the early stage and an anti-inflammatory phenotype in the late stage of AMI [34, 35]. However, the early inflammatory response of the heart plays a vital role in later cardiac recovery and scar formation [36, 37]. Therefore, targeted intervention with macrophages and monocytes may be beneficial for treating injury after AMI.
Moreover, it has been shown that a high proportion of neutrophils in the body increases the risk of death in patients with AMI [38]. These are the first responders to inflammation and are essential in the acute phase of the immune response. Their increased presence in AMI indicates an immediate immune reaction to myocardial injury, contributing to the inflammatory milieu. Immune cell infiltration and necroptosis have a complex relationship. Necroptosis may cause the death of immune cells, aggravate the inflammatory response, and exacerbate myocardial injury. In contrast, cells undergoing necroptosis are recognized by immune cells and cleaved, which in turn causes a more severe inflammatory response [39]. NRDEGs were positively correlated with macrophages, neutrophils, and cytotoxicity. This implies that the genes differentially expressed in AMI are associated with the activation and function of these immune cells, suggesting a link between NRDEG expression and the heightened inflammatory and cytotoxic response observed in AMI. In summary, necroptosis may affect the immune response, and in turn, the occurrence of AMI. CD4 + T cells, γδ-T cells, Tr2 cells, Tfh cells, exhausted-T cells, and central memory T cells were expressed at lower levels. These helper T cells were crucial in participating in both innate and adaptive immunity and coordinating immune responses. The reduced expression of these T cell subtypes indicated that various aspects of the adaptive immune response were generally impaired during the occurrence of AMI. NRDEGs were negatively correlated with these cells, suggesting that these genes might have inhibited this immune response, contributing to the progression of AMI.
This study had some limitations. First, the data analyzed by bioinformatics came from a public database; therefore, the rigor of the data is difficult to guarantee. Second, regarding the experimental verification of the screened NRDEGs, we used the heart tissue of AMI mice rather than that of humans, which is difficult to obtain. Therefore, the experimental results may differ between humans. Finally, elucidating the relationship between immune infiltration and necroptosis using transcriptome data proved challenging. A deeper investigation in the future is needed to confirm these findings.
Conclusions
In this study, through bioinformatics analysis and related experiments, we identified four genes (FTH1, IFNGR1, STAT3, and TLR4) that may affect necroptosis, thereby affecting AMI occurrence and development. In addition, through correlation analysis of immune infiltration, we determined that a high abundance of macrophages and neutrophils affected AMI. The results of this study can help determine the pathological mechanism of necroptosis and immune cells that influence AMI and provide new insights for targeted therapy.
Data availability
The dataset(s) supporting the conclusions of this article is(are) available in the Gene Expression Omnibus database (GEO) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi. GSE66360, GSE60993 and GSE61144).
Abbreviations
- AMI:
-
Acute Myocardial Infarction
- GEO:
-
Gene Expression Omnibus Database
- NRDEGs:
-
Necroptosis-Related Differential Genes
- GSEA:
-
Gene Set Enrichment Analysis
- PPI:
-
Protein-Protein Interaction
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- DAVID:
-
Database for Annotation, Visualization, and Integrated Discovery
- GO:
-
Gene Ontology
- STRING:
-
Search Tool for the Retrieval of Interacting Genes database
- TFs:
-
Transcription Factors
- TRRUST:
-
Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining
- ROC:
-
Receiver Operating Characteristic
- IHC:
-
Immunohistochemistry Staining
References
Virani SS, et al. Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Ibanez B, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J. 2005;26(23):2493–519.
Nian M, et al. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94(12):1543–53.
Galiuto L, DeMaria AN, Iliceto S. Microvascular damage during myocardial ischemia-reperfusion: pathophysiology, clinical implications and potential therapeutic approach evaluated by myocardial contrast echocardiography. Ital Heart J. 2000;1(2):108–16.
Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364(9429):183–92.
Linkermann A, Green DR. Necroptosis N Engl J Med. 2014;370(5):455–65.
Choi ME et al. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight, 2019. 4(15).
Zhang T, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22(2):175–82.
Luedde M, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103(2):206–16.
Zhe-Wei S, Li-Sha G, Yue-Chun L. The role of necroptosis in Cardiovascular Disease. Front Pharmacol. 2018;9:721.
DeRoo E, Zhou T, Liu B. The role of RIPK1 and RIPK3 in Cardiovascular Disease. Int J Mol Sci, 2020. 21(21).
Chakraborty A, et al. Programmed cell death in aortic aneurysm and dissection: a potential therapeutic target. J Mol Cell Cardiol. 2022;163:67–80.
Karunakaran D, et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2(7):e1600224.
Anzai A, Ko S, Fukuda K. Immune and Inflammatory networks in myocardial infarction: current research and its potential implications for the clinic. Int J Mol Sci, 2022. 23(9).
Wang XH, et al. Mechanism of complement activation on cardiac immune and inflammatory response caused by ischemic postconditioning in acute myocardial infarction. J Biol Regul Homeost Agents. 2020;34(5):1763–9.
Wu ATH et al. Identification of a Novel Theranostic signature of metabolic and Immune-Inflammatory Dysregulation in myocardial infarction, and the potential Therapeutic properties of Ovatodiolide, a Diterpenoid Derivative. Int J Mol Sci, 2022. 23(3).
Xie Y, et al. Identification of potential biomarkers and immune cell infiltration in acute myocardial infarction (AMI) using bioinformatics strategy. Bioengineered. 2021;12(1):2890–905.
Zhao E, Xie H, Zhang Y. Predicting Diagnostic Gene biomarkers Associated with Immune Infiltration in patients with Acute myocardial infarction. Front Cardiovasc Med. 2020;7:586871.
Yu G, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–7.
Sherman BT, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50(W1):W216–21.
Szklarczyk D, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–12.
Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Franz M, et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46(W1):W60–4.
Han H, et al. TRRUST: a reference database of human transcriptional regulatory interactions. Sci Rep. 2015;5:11432.
Miao YR, et al. ImmuCellAI: a Unique Method for Comprehensive T-Cell subsets abundance prediction and its application in Cancer Immunotherapy. Adv Sci (Weinh). 2020;7(7):1902880.
Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33(4):457–63.
O’Shea J.J., Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–31.
Boengler K, et al. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120(2):172–85.
Cao M, et al. c-Jun N-terminal kinases differentially regulate TNF- and TLRs-mediated necroptosis through their kinase-dependent and -independent activities. Cell Death Dis. 2018;9(12):1140.
Hally KE, et al. Platelet toll-like receptor (TLR) expression and TLR-mediated platelet activation in acute myocardial infarction. Thromb Res. 2017;158:8–15.
Oyama J, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109(6):784–9.
Lafuse WP, Wozniak DJ, Rajaram MVS. Role of Cardiac macrophages on Cardiac inflammation, fibrosis and tissue repair. Cells, 2020. 10(1).
Luo RY, et al. ProBDNF promotes sepsis-associated encephalopathy in mice by dampening the immune activity of meningeal CD4(+) T cells. J Neuroinflammation. 2020;17(1):169.
Hu ZL, et al. Brain-derived neurotrophic factor precursor in the immune system is a novel target for treating multiple sclerosis. Theranostics. 2021;11(2):715–30.
Shen WY, et al. Up-regulation of proBDNF/p75(NTR) signaling in antibody-secreting cells drives systemic lupus erythematosus. Sci Adv. 2022;8(3):eabj2797.
Li JN et al. Brain-derived neurotrophic factor Precursor contributes to a Proinflammatory Program in Monocytes/Macrophages after Acute myocardial infarction. J Am Heart Assoc, 2023: p. e028198.
Wang W, et al. The neutrophil-lymphocyte ratio to predict poor prognosis of critical acute myocardial infarction patients: a retrospective cohort study. Biochem Med (Zagreb). 2023;33(1):010702.
Liu F, et al. Role of Necroptosis and Immune Infiltration in Human Stanford Type A aortic dissection: Novel insights from Bioinformatics analyses. Oxid Med Cell Longev. 2022;2022:p6184802.
Acknowledgements
Not applicable.
Funding
This work was supported by the Fujian Provincial Special Reserve Talents Fund [2021-25] and the National Natural Science Foundation of China [82370470].
Author information
Authors and Affiliations
Contributions
LK.Ma and YK.Chen contributed to the conceptualization. LK.Ma and YK.Chen contributed to data curation and writing of the original draft. LK.Ma and LF.Xie contributed to the formal analysis. JK.Li, ZF.Zhang, M.Zarif contributed to the investigation. LK.Ma, YK.Chen, TC.Chai and QS.Wu contributed to the methodology and the visualization. ZH.Qiu and LW.Chen contributed to the writing, review, and editing. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Research ethics and patient consent
In this study, there was no direct human participation, and all human-related data was taken from GEO database. This animal experiment had been approved by the Animal Ethics Committee of Fujian Medical University (IACUC FJMU 2023-0052). All methods were carried out in accordance with relevant guidelines and regulations. All methods were reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
All authors provided their consent for publication.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, L., Chen, K., Li, J. et al. Identification of potential therapeutic targets from bioinformatics analysis of necroptosis and immune infiltration in acute myocardial infarction. J Cardiothorac Surg 19, 524 (2024). https://doi.org/10.1186/s13019-024-03038-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-03038-6