Abstract
Background
Cryoglobulinemia with pulmonary involvement is rare, and its characteristics, radiological findings, and outcomes are still poorly understood.
Methods
Ten patients with pulmonary involvement of 491 cryoglobulinemia patients at Peking Union Medical College Hospital were enrolled in this retrospective study. We analyzed the characteristics, radiological features and management of pulmonary involvement patients, and compared with those of non-pulmonary involvement with cryoglobulinemia.
Results
The 10 patients with pulmonary involvement (2 males; median age, 53 years) included three patients with type I cryoglobulinemia and seven patients with mixed cryoglobulinemia. All of 10 patients were IgM isotype cryoglobulinemia. All type I patients were secondary to B-cell non-Hodgkin lymphoma. Four mixed patients were essential, and the remaining patients were secondary to infections (n = 2) and systemic lupus erythematosus (n = 1), respectively. Six patients had additional affected organs, including skin (60%), kidney (50%), peripheral nerves (30%), joints (20%), and heart (20%). The pulmonary symptoms included dyspnea (50%), dry cough (30%), chest tightness (30%), and hemoptysis (10%). Chest computed tomography (CT) showed diffuse ground-glass opacity (80%), nodules (40%), pleural effusions (30%), and reticulation (20%). Two patients experienced life-threatening diffuse alveolar hemorrhage. Five patients received corticosteroid-based regimens, and four received rituximab-based regimens. All patients on rituximab-based regimens achieved clinical remission. The estimated two-year overall survival (OS) was 40%. Patients with pulmonary involvement had significantly worse OS and progression-free survival than non-pulmonary involvement patients of cryoglobulinemia (P < 0.0001).
Conclusions
A diagnosis of pulmonary involvement should be highly suspected for patients with cryoglobulinemia and chest CT-indicated infiltrates without other explanations. Patients with pulmonary involvement had a poor prognosis. Rituximab-based treatment may improve the outcome.
Similar content being viewed by others
Introduction
Cryoglobulinemia is defined as the presence of cryoglobulins in the serum that precipitate in vitro at temperatures below 37 °C and dissolve with rewarming [1]. According to the composition of the immunoglobulin, three types of cryoglobulins can be classified [2]. Type I cryoglobulins consist of monoclonal immunoglobulins, and monoclonal gammopathy of undetermined significance is seen in ∼ 40% of patients with type I cryoglobulinemia, followed by multiple myeloma, Waldenström’s macroglobulinemia or small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) [3, 4]. Type II cryoglobulins are composed of monoclonal immunoglobulins with rheumatoid factor (RF) activity and polyclonal immunoglobulins, and type III cryoglobulins are characterized by polyclonal immunoglobulins. The latter two types are referred to as mixed cryoglobulins. Hepatitis C virus (HCV) infection is predominantly associated with mixed cryoglobulinemia [5]. Other common causes include additional infections, such as human immunodeficiency virus and hepatitis B virus; connective tissue diseases; and lymphoproliferative disorders. Nearly 10% of patients remain classified as essential [6].
Clinical presentations of cryoglobulinemia vary from asymptomatic and fatigue to heart failure and rapidly diffuse alveolar hemorrhage (DAH). The most commonly affected organ is the skin, which presents with purpura (75-90%), Raynaud’s phenomenon (20-30%), and distal ulcers (5-15%). The additional commonly involved systems include joints (50-80%), peripheral nerves (50-75%) and kidney (30-40%) [6,7,8]. Involvement of other major organs, such as the central nervous system (CNS), gastrointestinal (GI) tract, heart, and lung, is rare but associated with poor prognosis [9].
To date, few studies and case series of pulmonary involvement in cryoglobulinemia have been reported because of its rarity. Pulmonary involvement occurs in less than 5% of patients with cryoglobulinemia [3, 6, 7]. Pulmonary symptoms vary from dry cough and mild-to-moderate shortness of breath to acute DAH. Some studies [9, 10] have reported cryoglobulinemia with DAH as life-threatening cryoglobulinemia associated with high mortality. However, there are no studies focusing on patients with pulmonary involvement.
In this study, we described the clinical characteristics, imaging presentation, and management of cryoglobulinemia patients with pulmonary involvement. Furthermore, we compared patients of cryoglobulinemia with and without pulmonary involvement.
Methods
Study population
This retrospective study was conducted among patients with cryoglobulinemia at Peking Union Medical College Hospital between January 2015 and December 2022. The inclusion criteria for the study were: (i) detectable cryoglobulins in the serum; (ii) chest computed tomography (CT) findings such as diffuse ground-glass opacity (GGO), reticulation, diffuse nodules, multiple patches, pleural effusions, and DAH; and (iii) exclusion of other diagnoses with pulmonary lesions. Informed consent was obtained from all patients, and the protocol was approved by the Peking Union Medical College Hospital Ethics Committee. The study was performed in accordance with the ethical standard laid down in the 1964 Declaration of Helsinki and its later amendments.
Cryoglobulin detection and laboratory evaluation
The process of testing for cryoglobulins was consistent with those in previous reports [11]. All peripheral blood samples were initially kept at 37 ℃ until serum separation. After warm clotting and centrifugation, the clear serum was preserved at 4 °C for seven days and observed for the formation of cryoprecipitates. The resulting precipitate was washed and rewarmed to 37 °C. All positive cryoglobulins were analyzed by immunoelectrophoresis to determine the type of cryoglobulin according to Brouet’s criteria [2]. Cryoglobulin quantification was analyzed using Wintrobe tubes revealing the cryocrit (packed volume of the precipitate relative to the original serum volume) and direct quantification of immunoglobulins in combination with agarose gel electrophoresis [12].
Laboratory evaluation included complete blood count, liver and kidney function tests, urinalysis, concentration of serum complement 3 (C3), complement 4 (C4) fraction, serum RF level, serum/urine immunofixation electrophoresis (IFE), serum protein electrophoresis (SPE) and 24-hour urine protein.
Clinical presentation and underlying disease
Clinical data included demographic characteristics, clinical manifestations, coexisting medical conditions, chest CT scans and pulmonary function tests (PFTs). Symptoms related to cryoglobulinemia were defined as previous study [13]. The PFT data were interpreted according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) criteria [14].
The underlying diseases included (i) hematological disease [15], including diffuse large B-cell lymphoma (DLBCL) and SLL/CLL, which were compatible with the 2016 version of the World Health Organization classification of neoplastic diseases; (ii) infections, including (a) HCV infection, positivity for anti-HCV antibodies and presence of HCV-RNA in serum by PCR [16], and (b) Epstein‒Barr virus (EBV) infection, presence of EBV-DNA in serum by PCR [17]; and iii) systematic lupus erythematosus (SLE), based on the European League Against Rheumatism (EULAR) 2019 criteria [18].
Treatment and outcomes
Treatment was divided into the following categories: (i) corticosteroid-based treatment and (ii) rituximab-based treatment. The response to treatment was consistent with that reported by previous study [13]. Overall survival (OS) was defined as the time from diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time from the date of diagnosis to the date of underlying disease progression, cryoglobulinemia progression, death from any cause or last follow-up. The last follow-up was January 28, 2023.
Statistical analysis
Clinical and imaging data were analyzed using descriptive statistics. Continuous variables are presented as the median and range, and categorical variables are presented as the number and percentages. The Mann‒Whitney U test was used for continuous variables, whereas Fisher’s exact test was used to compare categorical variables between two groups. Kaplan‒Meier analysis was applied for survival analysis, with survival curves being compared using the log-rank test. Statistical analysis was performed using SPSS 26.0 software (IBM Corp., Armonk, NY, USA), and the significance end point was set to P < 0.05.
Results
Patient characteristics
A total of 491 patients were diagnosed with cryoglobulinemia at Peking Union Medical College Hospital between January 2015 and December 2022. A total of 101 (20.1%) patients had type I cryoglobulinemia, 390 (79.4%) patients had mixed cryoglobulinemia, including 91 (18.5%) with type II, and 299 (60.1%) with type III. A total of 243 (49.5%) patients were asymptomatic, and the remaining patients (50.5%) had at least one organ involved. Overall, 10 (2.0%) patients (2 males and 8 females) fulfilled the inclusion criteria accounted for 2.0% of all cryoglobulinemia patients (n = 491). Patients with pulmonary involvement included 3 with type I cryoglobulinemia and 7 with mixed cryoglobulinemia, including 4 patients with type II and 3 patients with type III. For patients with pulmonary involvement, the median age at diagnosis was 53 years (range, 30–73 years), and the median duration from symptom onset to disease diagnosis was 8 months (range, 2–49 months).
We compared the baseline characteristics of type I cryoglobulinemia patients with (n = 3) and without pulmonary involvement (n = 98). We also compared the baseline characteristics of mixed cryoglobulinemia patients with (n = 7) and without pulmonary involvement (n = 383). The demographic and clinical characteristics are presented in Tables 1 and 2. Compared to type I patients without pulmonary involvement, patients with pulmonary involvement tended to have a higher RF titer (7.0 vs. 4.6 IU/ml, P = 0.065). Out of 390 mixed cryoglobulinemia patients, those with pulmonary involvement had higher frequencies of skin involvement (71.4% vs. 29.2%, P = 0.046), renal involvement (57.1% vs. 20.9%, P = 0.041), and peripheral nerve involvement (42.9% vs. 6.8%, P = 0.011) compared to those without pulmonary involvement. Furthermore, mixed cryoglobulinemia patients with pulmonary involvement showed a markedly higher RF titer than those without pulmonary involvement (226.1 vs. 98.0 IU/ml, P = 0.048).
All patients with pulmonary involvement experienced persistent pulmonary symptoms, which included dyspnea (50%), dry cough (30%), chest tightness (30%), and hemoptysis (10%). The other most common clinical manifestations were purpura (50%) and proteinuria (50%), followed by renal function impairment (40%), hematuria (40%), sensory neuropathy (30%), arthralgia (20%), heart failure (20%), and ulcer (10%). The detailed clinical characteristics of patients with pulmonary involvement are shown in Table 3. Six patients had additional organs affected by cryoglobulinemia. Skin (n = 6) was the most commonly involved organ, followed by kidney (n = 5), peripheral nerves (n = 3), joints (n = 2), and heart (n = 2). Furthermore, both patients with cardiac involvement presented with heart failure. Echocardiography or cardiac magnetic resonance revealed decreased ejection fraction, and massive pericardial effusion in these patients. All type I patients were secondary to B-cell non-Hodgkin lymphoma, including DLBCL (n = 2) and SLL/CLL (n = 1). Among the 7 patients with mixed cryoglobulinemia, 4 patients were essential, and the remaining 3 patients were secondary to infections (n = 2), including HCV (n = 1) and EBV (n = 1), and SLE (n = 1).
Laboratory findings
The median concentration of cryocrit for patients with pulmonary involvement was 100.9 mg/L (range, 24.3–3463 mg/L), all of which was IgM isotype, including 6 with IgMκ, 1 with IgMλ, and 3 with poly IgM. Serum complement levels were evaluable in all patients with pulmonary involvement: seven patients had a decrease in C3 serum levels, and the median concentration was 0.489 g/L (range, 0.283–0.681 g/L), and five patients had a decrease in C4 serum levels, and the median concentration was 0.016 g/L (range, 0.002–0.076 g/L). Out of 9 patients with pulmonary involvement, 6 patients exhibited elevated titers of RF, and the median level of RF was 463.4 IU/ml (range, 41.5-19489.2 IU/ml).
In the 4 patients with renal function impairment, the median serum creatinine level was 176 µmol/L (range, 99–299 µmol/L). Five patients experienced proteinuria, with a median 24-hour urine protein level was 1.47 g/L (0.62–1.84 g/L), while four patients presented with hematuria. Additionally, serum IFE was positive in 3 patients (#2, #6, and #7) of 9 evaluable patients, all of which were IgMκ isotype, and urine IFE was positive in 2 patients (#1, #2) of 5 evaluable patients, including one light chain of κ and one light chain of λ. Furthermore, among 9 patients who underwent SPE, two patients (#6, #7) were positive, with M protein levels of 10.50 g/L and 21.60 g/L, respectively.
Radiological presentation
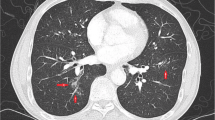
The radiological presentation of patients and examples of typical radiologic findings are shown in Table 4; Fig. 1. Both patients (#1, #2) with DAH showed diffuse bilateral GGO and multiple patches, and patient #2 also presented with pleural thickening and pleural effusions (Fig. 2A-C). Among the other 8 patients, 6 patients showed diffuse bilateral patchy GGOs, which were distributed mainly in the bilateral lower lobes of two patients (#5 and #10). Multiple, diffuse, small nodules were observed in four patients, two of whom (#8 and #9) had a large solitary nodule (maximum diameter > 10 mm) (Fig. 1C). Moreover, the CT scans revealed pleural thickening (50%), pleural effusions (30%), reticulations mainly in the bilateral lower lobes (20%), and bilateral interlobular septal thickening (10%).
A-E, CT imaging findings associated with cryoglobulinemia and lung involvement. A, Diffuse, bilateral, patchy GGOs. B, Diffuse alveolar hemorrhage. C, Solitary large nodule in the right upper lobe. D, Diffuse, bilateral, multiple, small nodules. E, Bilateral pleural thickening. F, Bilateral pleural effusions
CT scans during the treatment of patient #2. A-C, Bilateral multiple patchy and diffuse GGOs along with symptoms of acute dyspnea and hemoptysis. D-F, Remarkable improvement of pulmonary infiltrates after high-dose corticosteroids and rituximab for 4 courses. G-I, Stability of symptoms and infiltrates on repeat chest CT 6 months after the episode of hemoptysis
(A)
Pulmonary function tests
PFTs were performed in three patients (#6, #7, and #10) (Table 4). The baseline PFTs of patient #6 revealed restrictive ventilatory dysfunction and diffusing-capacity defects. Likewise, patient #7 was subjected to baseline PFTs and demonstrated mild restrictive ventilatory dysfunction. Patient #10 showed mild restrictive ventilatory dysfunction with normal forced expiratory volume in 1 s (FEV1) and diffusing-capacity defects. After treatment with corticosteroids and mycophenolate mofetil (MMF), the predicted diffuse lung capacity for carbon monoxide (DLCO) value of patient #10 improved (from 46.8 to 67.2%) significantly along with normal restrictive ventilatory function.
Treatment and outcomes
The treatments and outcomes of the 10 patients are listed in Table 3. One patient (#3) gave up treatment and died from underlying disease progression. Five (50%) patients received a corticosteroid-based regimen as first-line treatment, including corticosteroids alone (n = 3), corticosteroids and cyclophosphamide (CTX) (n = 1), and corticosteroids and MMF (n = 1). Four (40%) patients were treated with a rituximab-based regimen, including rituximab and corticosteriods (n = 1), R-CHOP (rituximab, cyclophosphamide, vincristine, and prednisone) (n = 1), DRC (rituximab, cyclophosphamide and dexamethasone) (n = 1), and antiviral agents and DRC (n = 1).
Of these 9 treated patients, 7 patients achieved clinical remission, consisting of one (#5) with complete remission (CR) and six with partial remission (PR). Furthermore, all 4 patients treated with rituximab-based regimens achieved clinical remission, while both patients (#4, #8) with no remission (NR) received corticosteroid treatment alone. Of the 5 patients evaluable for laboratory response, three patients (#1, #9 and #10) achieved CR, one (#5) achieved PR, and one (#2) showed NR. Moreover, patient #7 was treated with three lines of therapy and has been stable until now, which we previously reported [19].
The median follow-up was 16 months (range, 2–67 months) for patients with pulmonary involvement. Five patients (50%) with pulmonary involvement died during follow-up. The causes of death included underlying disease progression (n = 3) and therapy-related infection (n = 2). The estimated three-year OS and PFS for all patients with cryoglobulinemia were 94.8% and 84.5%, respectively (Fig. 3A). Patients with pulmonary involvement had significantly worse OS and PFS than those without pulmonary involvement (two-year OS 40% vs. 95.6%, P < 0.0001; two-year PFS 30% vs. 87.7%, P < 0.0001; Fig. 3B, C).
Comparison of survival among patients of cryoglobulinemia. (A) Overall survival (OS) and progression-free survival (PFS) of the whole cohort (n = 491). (B) OS of cryoglobulinemia patients with pulmonary involvement (n = 10) and those without pulmonary involvement (n = 481). (C) PFS of cryoglobulinemia patients with pulmonary involvement (n = 10) and those without pulmonary involvement (n = 481)
Discussion
Cryoglobulinemia with pulmonary involvement is extremely rare. To our knowledge, our study is the first reported cohort of cryoglobulinemia patients with pulmonary involvement compared with non-pulmonary involvement patients. This study provides new insights into the clinical characteristics, radiological findings, and management of cryoglobulinemia with pulmonary involvement.
Pulmonary involvement occurred in less than 5% of patients and seemed to be exceedingly rare in type I cryoglobulinemia [3, 6,7,8]. Trejo O et al. [6] reviewed 443 cryoglobulinemia patients and found 6 patients (1%) had pulmonary involvement with hemoptysis or pulmonary infiltrates on X-ray. According to our research, we found 10 patients diagnosed with pulmonary involvement accounted for 2.0% of all cryoglobulinemia patients. Patients with pulmonary involvement included 3 with type I cryoglobulinemia and 7 with mixed cryoglobulinemia. Most patients with pulmonary involvement were asymptomatic, dry cough and mild-to-moderate dyspnea. DAH was relatively uncommon, defined as life-threatening cryoglobulinemia and associated with extremely high mortality (up to 80-100%) [9, 20]. Consistent with previous studies, the pulmonary symptoms in our study included dyspnea (50%), dry cough (30%), chest tightness (30%), and hemoptysis (10%). Two patients presented with rapid DAH, and both died during follow-up. Compared to mixed cryoglobulinemia patients without pulmonary involvement, mixed patients with pulmonary involvement had higher frequencies of skin involvement, renal involvement, and peripheral nerve involvement. This could be due to that a significant proportion of mixed cryoglobulinemia patients (mainly type III patients) were asymptomatic.
The cryoglobulin in all patients with pulmonary involvement was IgM isotype. A previous study [10] showed that low C3 levels were associated with pulmonary involvement. However, comparing patients with pulmonary involvement and without pulmonary involvement, we found that patients with pulmonary involvement had a significantly higher RF titer, and no remarkable difference was found in C3 and C4 levels.
Few data are available on the radiographic presentation of cryoglobulinemia with pulmonary involvement. Generally, chest CT scans show various findings ranging from GGO to pulmonary fibrosis [21, 22]. An Italian study summarized 231 patients with mixed cryoglobulinemia [23]. Although mild dyspnea was observed in 26% of patients, only 2% (n = 4) of cases had abnormal chest CT scans. In another French study [24], pulmonary involvement consisted of intra-alveolar hemorrhage in four patients (1.7%) and bilateral interstitial infiltrates in one patient (0.4%) among 242 cases. In our cohort, bilateral and diffuse GGOs (1.7%) were the most common radiological findings, followed by pleural thickening (1.0%); diffuse, multiple, small nodules (1.0%); pleural effusions (0.6%); large solitary nodules (0.4%); reticulation (0.4%); and bilateral interlobular septa thickening (0.2%). Furthermore, the two patients with DAH presented with multiple patchy and diffuse GGOs.
Treatment should be designed according to the underlying diseases and the severity of clinical impairment. It is difficult to propose uniform management guidelines because of the heterogeneity in its manifestations and causations [25]. The Italian Study Group of Cryoglobulinemia (GISC) recommended rituximab as effective and safe for severe, not immediately life-threatening patients (LoE 1 A), and rituximab was more efficacious than conventional immunosuppressive treatments (1B) [26, 27]. All patients in our cohort received treatment initially, and the first-line treatment included corticosteroid-based regimens and rituximab-based regimens. Both patients with NR (#4, #8) received corticosteroid treatment alone, while all patients treated with rituximab-based regimens achieved good remission, which was consistent with GISC recommendations. Similar to previous findings [25, 28], the HCV-related patient in our study was treated with antiviral agents and DRC and achieved sustained virologic response and complete clinical remission. Furthermore, one essential patient did not achieve clinical remission by corticosteroid treatment alone but achieved clinical PR by DRC and clinical CR by BRD (bortezomib, rituximab, and dexamethasone) [19], which proved that bortezomib, given its direct effects on malignant plasma cells and antiangiogenic actions, is another promising biological agent.
Given the end-organ damage and life-threatening symptoms, patients with pulmonary involvement in cryoglobulinemia have poor survival. A French study of 242 cryoglobulinemia patients revealed that pulmonary involvement was an independent prognostic factor of poor outcomes [24]. In our cohort, patients with pulmonary involvement had an obviously poor prognosis compared with cryoglobuliemia patients without pulmonary involvement. Additionally, in our study, the two patients with DAH both died during follow-up because of underlying disease progression, consistent with previous studies showing that the mortality of rapid pulmonary hemorrhage in cryoglobulinemia patients was 80–100% [9, 10]. However, there were still no other survival data concerning the cohort of cryoglobulinemia patients with pulmonary involvement.
This study suffers from several limitations. First, this was a retrospective study and included a small number of cases. Patients with pulmonary involvement were only diagnosed with clinical pulmonary involvement in the absence of lung biopsy. However, all cases in our cohort were confirmed to have a cryoglobulinemia diagnosis and proved to have pulmonary abnormalities with no other explanation. Exploring the pathologic characteristics of cryoglobulinemia patients is needed in future studies. Second, we did not administer chest CT scans to all patients with cryoglobulinemia, and we may have missed some patients with moderate pulmonary involvement. Nevertheless, patients without chest CT scans did not have any pulmonary symptoms.
Conclusions
Pulmonary involvement is rare in cryoglobulinemia. Pulmonary presentations vary from mild dyspnea to DAH, which is associated with high mortality. The chest CT scans mainly show diffuse GGOs. Patients of cryoglobulinemia with pulmonary involvement have a remarkably poor prognosis compared with non-pulmonary involvement patients with cryoglobulinemia. Rituximab-based treatment may be more effective.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATS:
-
American Thoracic Society
- B-NHL:
-
B-cell non-Hodgkin’s lymphomas
- BRD:
-
Bortezomib, rituximab, and dexamethasone
- C3:
-
Complement 3
- C4:
-
Complement 4
- CNS:
-
Central nervous system
- CR:
-
Complete remission
- CS:
-
Corticosteroids
- CT:
-
Computed tomography
- CTX:
-
Cyclophosphamide
- DAH:
-
Diffuse alveolar hemorrhage
- DLBCL:
-
Diffuse large B-cell lymphoma
- DLCO:
-
Diffuse lung capacity for carbon monoxide
- DRC:
-
Rituximab, cyclophosphamide and dexamethasone
- EBV:
-
Epstein‒Barr virus
- ERS:
-
European Respiratory Society
- EULAR:
-
European League Against Rheumatism
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GGO:
-
Ground-glass opacity
- GI:
-
Gastrointestinal
- GISC:
-
Italian Study Group of Cryoglobulinemia
- HCV:
-
Hepatitis C virus
- IFE:
-
Immunofixation electrophoresis
- MMF:
-
Mycophenolate mofetil
- NR:
-
No remission
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PFTs:
-
Pulmonary function tests
- PR:
-
Partial remission
- R-CHOP:
-
Rituximab, cyclophosphamide, vincristine, and prednisone
- RTX:
-
Rituximab
- SLE:
-
Systematic lupus erythematosus
- RF:
-
Rheumatoid factor
- SLL/CLL:
-
Small lymphocytic lymphoma/chronic lymphocytic leukemia
- SPE:
-
Serum protein electrophoresis
- TLC:
-
Total lung capacity
References
Damoiseaux J, Cohen Tervaert JW. Diagnostics and treatment of cryoglobulinaemia: it takes two to tango. Clin Rev Allergy Immunol. 2014;47(3):299–310.
Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57(5):775–88.
Sidana S, Rajkumar SV, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Hayman SR, Dingli D, Kapoor P, Gonsalves WI, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92(7):668–73.
Harel S, Mohr M, Jahn I, Aucouturier F, Galicier L, Asli B, Malphettes M, Szalat R, Brouet JC, Lipsker D, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168(5):671–8.
Ramos-Casals M, Forns X, Brito-Zerón P, Vargas A, Ruiz M, Laguno M, Yagüe J, Sánchez-Tapias JM, Gatell JM, Font J. Cryoglobulinaemia associated with hepatitis C virus: influence of HCV genotypes, HCV-RNA viraemia and HIV coinfection. J Viral Hepat. 2007;14(10):736–42.
Trejo O, Ramos-Casals M, García-Carrasco M, Yagüe J, Jiménez S, de la Red G, Cervera R, Font J, Ingelmo M. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Med (Baltim). 2001;80(4):252–62.
Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379(9813):348–60.
Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86(6):707–13.
Retamozo S, Díaz-Lagares C, Bosch X, Bové A, Brito-Zerón P, Gómez ME, Yagüe J, Forns X, Cid MC, Ramos-Casals M. Life-threatening cryoglobulinemic patients with Hepatitis C: clinical description and outcome of 279 patients. Med (Baltim). 2013;92(5):273–84.
Ramos-Casals M, Robles A, Brito-Zerón P, Nardi N, Nicolás JM, Forns X, Plaza J, Yagüe J, Sánchez-Tapias JM, Font J. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthritis Rheum. 2006;36(3):189–96.
Motyckova G, Murali M. Laboratory testing for cryoglobulins. Am J Hematol. 2011;86(6):500–2.
Vermeersch P, Gijbels K, Knockaert D, Blockmans D, Westhovens R, Mariën G, Bossuyt X. Establishment of reference values for immunoglobulins in the cryoprecipitate. Clin Immunol. 2008;129(2):360–4.
Zhang LL, Cao XX, Shen KN, Han HX, Zhang CL, Qiu Y, Zhao H, Gao XM, Feng J, Zhang L, et al. Clinical characteristics and treatment outcome of type I cryoglobulinemia in Chinese patients: a single-center study of 45 patients. Ann Hematol. 2020;99(8):1735–40.
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022, 60(1).
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
Aguiar MF, Faria-Janes AL, Garcia-Brandes GI, Takemi-Emori C, Ferraz MLG, Andrade LEC, de Souza AWS. Prevalence of cryoglobulinemia and cryoglobulinemic vasculitis in chronically HCV-infected Brazilian patients. Ann Hepatol. 2019;18(5):685–92.
Fiorini GF, Sinico RA, Winearls C, Custode P, De Giuli-Morghen C, D’Amico G. Persistent Epstein-Barr virus infection in patients with type II essential mixed cryoglobulinemia. Clin Immunol Immunopathol. 1988;47(3):262–9.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–9.
Han HX, Qiu Y, Tian XL, Su W, Li J, Cao XX. A 47-Year-old woman with progressive exertional dyspnea and fatigue. Chest. 2022;161(2):e81–4.
Saccardo E, Novati P, Sironi D, Castelnovo L, Rinaldi G, Monti G. Causes of death in symptomatic cryoglobulinemia: 30 years of observation in a Department of Internal Medicine. Dig Liver Dis. 2007;39(Suppl 1):S52–54.
De Vita S, Soldano F, Isola M, Monti G, Gabrielli A, Tzioufas A, Ferri C, Ferraccioli GF, Quartuccio L, Corazza L, et al. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis. 2011;70(7):1183–90.
Bombardieri S, Paoletti P, Ferri C, Di Munno O, Fornal E, Giuntini C. Lung involvement in essential mixed cryoglobulinemia. Am J Med. 1979;66(5):748–56.
Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–74.
Terrier B, Carrat F, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint Martin L, Quemeneur T, Huart A, et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: data from 242 cases included in the CryoVas survey. Ann Rheum Dis. 2013;72(3):374–80.
Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129(3):289–98.
Galli M, Monti G, Marson P, Scaini P, Pietrogrande M, Candela M, Castelnovo L, Faggioli P, Novati P, Zani R, et al. Recommendations for managing the manifestations of severe and life-threatening mixed cryoglobulinemia syndrome. Autoimmun Rev. 2019;18(8):778–85.
Quartuccio L, Bortoluzzi A, Scirè CA, Marangoni A, Del Frate G, Treppo E, Castelnovo L, Saccardo F, Zani R, Candela M, et al. Management of mixed cryoglobulinemia with rituximab: evidence and consensus-based recommendations from the Italian Study Group of Cryoglobulinemia (GISC). Clin Rheumatol. 2023;42(2):359–70.
Kondili LA, Monti M, Quaranta MG, Gragnani L, Panetta V, Brancaccio G, Mazzaro C, Persico M, Masarone M, Gentile I, et al. A prospective study of direct-acting antiviral effectiveness and relapse risk in HCV cryoglobulinemic vasculitis by the Italian PITER cohort. Hepatology. 2022;76(1):220–32.
Acknowledgements
The authors thank the patients and their families for their trust, respect and support. The authors also thank all clinicians for their help and advices in accomplishing this work.
Funding
This study was funded by Beijing Natural Science Haidian Frontier Foundation (Grant No. L222081 to CXX) and the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-193).
Author information
Authors and Affiliations
Contributions
XC contributed to study conception and design; HH led the data management and statistical analyses and draft the manuscript. WS contributed to cryoglobulin detection. XT reviewed all chest CT scans and manuscript. JL and DZ contributed to patient enrollment. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Written informed consent was obtained from the patient included in the study.
Ethics approval and consent to participate
Informed consent was obtained from all patients, and the protocol was approved by the Peking Union Medical College Hospital Ethics Committee.
Competing interests
The authors declare that they have no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, Hx., Su, W., Tian, X. et al. Clinical characteristics, radiological features and outcomes in pulmonary involvement of cryoglobulinemia. Orphanet J Rare Dis 19, 185 (2024). https://doi.org/10.1186/s13023-024-03159-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-024-03159-0