Abstract
Introduction
Experimental studies in animals have yielded conflicting results on the role of Tumor Necrosis Factor (TNF) in sepsis and endotoxemia, with some reporting adaptive and others inappropriate effects. A meta-analysis of the available literature was performed to determine the factors explaining this discrepancy.
Methods
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered with PROSPERO (CRD42020167384) prior to data collection. PubMed and Embase were the databases queried. Risk of bias was evaluated using the SYRCLE Risk of Bias Tool. All animal studies investigating sepsis-related mortality and modified TNF signaling were considered eligible. The exclusion criteria were: lack of mortality data, 7-day mortality rates below 10% in both wild type and TNF-altered pathway animals, and absence of an English abstract. To determine the role of TNF according to the experimental protocol, three approaches were used: first an approach based on the statistical significance of each experiment, then the pooled mortality was calculated, and finally the weighted risk ratio for mortality was assessed.
Results
A total of 175 studies were included in the analysis, comprising a total of 760 experiments and involving 19,899 animals. The main species used were mice (77%) and rats (21%). The most common method of TNF pathway modulation was TNF pathway inactivation that was primarily associated with an inappropriate secretion of TNF. At the opposite, TNF injection was associated with an adaptive role of TNF. Lipopolysaccharide (LPS) injection was the most used stimulus to establish an infectious model (42%) and was strongly associated with an inappropriate role of TNF. Conversely, live bacterial models, especially the cecal ligation and puncture (CLP) model, pneumonia, meningitis, and gastrointestinal infection, were associated with an adaptive role. This was particularly evident for Listeria monocytogenes, Streptococcus pneumoniae.
Conclusion
The role of TNF during infection varies depending on the experimental model used. Models that mimic clinical conditions, based on virulent bacteria that cause high mortality even at low inocula, demonstrated an adaptive role of TNF. Conversely, models based on LPS or low-pathogenic live bacteria, administered at doses well above physiological thresholds and combined with early antibiotic therapy, were associated with an inappropriate role.
Graphical abstract

Similar content being viewed by others
Introduction
Ten years after its discovery in 1975 [1], early Tumor Necrosis Factor (TNF) secretion following injection of lipopolysaccharide (LPS) or live Escherichia coli was found to be associated with mortality in two studies conducted by Cerami’s team [2, 3]. These articles opened a new perspective on sepsis by demonstrating the crucial role of inflammation in sepsis-related pathophysiology. Thus, sepsis-related mortality may depend on host-related factors, which explain the persistence of high mortality rates in sepsis despite the use of antibiotics. The initial human studies tended to support this hypothesis. Waage et al. reported the presence of circulating TNF in patients with sepsis and found a correlation between circulating TNF levels and mortality in meningococcal infections [4]. In addition, the injection of low doses of LPS resulted in the production of TNF and physiological changes commonly observed in sepsis, such as fever, tachycardia, and changes in cardiac output [5]. Furthermore, in 1993, a voluntary self-injection of high doses of LPS rapidly led to septic shock with very high levels of TNF [6]. As a result, blocking TNF secretion was considered a new strategy to reduce sepsis mortality. However, subsequent human studies have shown that blocking TNF secretion did not reduce sepsis mortality [7,8,9,10], and one study even found excess mortality after blocking TNF biological activity in sepsis [11]. One explanation for these failures is that TNF may also play an adaptive role in sepsis. In fact, as early as 1987, other experimental studies found an adaptive role for TNF in cecal ligation and puncture (CLP) and in listeriosis [12, 13]. This led to the concept of cytokines as a double-edged sword in sepsis [14, 15].
Therefore, almost 50 years after its discovery, the precise role of TNF secretion in sepsis remains controversial: is it an adaptive or inappropriate factor? We conducted a systematic literature review to investigate the factors that may account for the varying outcomes of TNF action in experimental studies. These factors include the animal species, infectious model and pathogens used, and the type of modulation of the TNF pathway.
Methods
Recommendations for conducting a systematic review
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16] (Online Appendix 1) and the recommendation of Cochrane Handbooks for meta-analysis [17].
Data retrieval strategies
The study protocol was registered in 2020 with PROSPERO (CRD42020167384) before data collection began. We searched in 2020 PubMed and Embase databases for articles containing TNF-evoking terms in their titles, combined with terms related to infections (such as peritonitis, pneumonia), names of specific bacteria (e.g., E. coli), or references to endotoxin (LPS). The search equation in Embase was as follows: (‘tumor necrosis factor’:ti OR ‘TNF’:ti OR 'cachectin':ti OR ‘rTNF’:ti) and ('sepsis':ti OR 'septic shock':ti OR 'Gram-positive shock':ti OR 'Gram- negative shock':ti OR 'infection':ti OR 'infections':ti OR 'endotoxin':ti OR 'endotoxemia':ti OR 'pneumonitis':ti OR ‘peritonitis’:ti OR 'bacterial growth':ti OR ‘pneumoniae’:ti OR ‘pneumonia’:ti OR ‘aureus’:ti OR ‘coli’:ti OR ‘salmonella’:ti OR ‘pseudomonas’:ti OR 'caecal ligation and puncture':ti OR 'infectious':ti OR ‘listeria’:ti OR ‘listeriosis’:ti OR ‘pyogenes’:ti OR ‘lethality’:ti OR ‘endotoxic’:ti OR ‘pneumococcal’:ti OR ‘streptococcal’:ti OR ‘meningitis’:ti). The search equation for PubMed can be found in Online Appendix 2. To ensure completeness, studies known to the authors that did not match the search equation but met the inclusion criteria were also included. Study eligibility criteria were assessed using the Covidence online software (https://www.covidence.org/).
Two authors independently screened articles for inclusion based on their titles and abstracts. In case of disagreement, a third author intervened to resolve the issue. The third author was also responsible for including full-text articles. Data extraction was independently performed by the third and fourth authors.
Study eligibility criteria
All experimental studies were eligible, without time limitations. To be included in the analysis, studies had to fulfill all the inclusion criteria, and none of the exclusion criteria.
Inclusion criteria were: animal studies; investigation of mortality associated with sepsis, defined as an infection that causes mortality in less than 7 days; utilization of an infectious or infectious-like stimulus, including cecal ligation and puncture, injection of live bacteria or endotoxins, cutaneous infection, pneumopathy, peritonitis; modification of the TNF pathway in at least one group, encompassing any form of TNF inactivation (such as injection of anti-TNF antibodies, infusion of soluble TNF receptor, TNF knock-out animals, TNF receptor knock-out animals) or infusion of recombinant TNF.
Exclusion criteria were: human studies; reviews and meta-analyses; lack of mortality data; 7-day mortality rate below 10% in both wild type and TNF-altered pathway animals; absence of an English abstract; articles in languages that are not easily translated.
The 10% mortality cut-off for defining sepsis was based on the Sepsis III definitions [18].
Data collection
The following data were collected:
-
Publication-related data: first author's name, journal title, and publication year.
-
Experimental protocol-related data: type of TNF pathway modulation (inactivation or recombinant TNF infusion), method of TNF pathway inactivation (monoclonal or polyclonal antibody injection, soluble receptor injection, TNF or its receptors, knock-out mice), and co-injection of antibiotics.
-
Animal-related data: species, age, and sex of the animals
-
Statistical data for each experiment: number of animals used, number of events by arm (control vs. TNF pathway modulation) and, if available, the p-value and the test used to assess the significance of the difference in mortality.
Statistics on included articles
If a study included as least one mortality experiment, all experiments were included in the analysis. The log-rank test was considered the gold standard for assessing mortality, and this test was preferred. If mortality statistics were missing, but mortality data were available, mortality statistics were performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, California, USA. A log-rank test was performed when available data allowed, otherwise a Fisher test was used. In addition, if a Fisher test was performed in the publication, but the data allowed for a log-rank test, the latter was performed and considered the primary statistical test to determine the role of TNF. A p-value of 0.05 was used to determine the statistical significance.
Definitions
Encapsulated bacteria were those known to possess an extracellular polysaccharide capsule, such as Klebsiella pneumoniae, Salmonella species, Streptococcus pneumoniae, and, when explicitly specified, Escherichia coli (strain 018: K1: H7) and Staphylococcus aureus (strain ATCC 25923, strain S 834). Parasitic infections were defined as those caused by eukaryotic organisms. Experiments (not studies) were classified into three groups according to the mortality observed and the p value of the statistic test used to determine the significance of the difference in mortality:
-
“Inappropriate" effect of TNF: if the mortality rate of control mice was statistically lower than that of mice in which the TNF pathway had been enhanced, or if the mortality rate of control mice was statistically higher than that of mice in which the TNF pathway had been decreased/blocked/inactivated/absent.
-
“non-significant" effect of TNF: if the mortality rate of control mice was not statistically different from that of mice in which the TNF pathway had been modified.
-
“Adaptive" effect of TNF: if the mortality rate of control mice was statistically higher than that of mice in which the TNF pathway had been enhanced, or if the mortality rate of control mice was statistically lower than that of mice in which the TNF pathway had been decreased/blocked.
A study was considered to show discordance in the role of TNF if it included experiments demonstrating both an inappropriate and an adaptive effect.
Assessing the risk of bias
Risk of bias was assessed using the SYRCLE Risk of Bias Tool [19]. This tool is an adaptation of the Cochrane Collaboration Risk of Bias Tool for use in animal studies. Of the ten possible biases, nine are well identified and the tenth corresponds to the other possible biases. In this study, this 10th bias included three items: lack of mortality statistics, the justification of the initial inoculum, and the presence of bacteriological and cytokine analyses. A bias was retained if it was present for at least one outcome of an experiment. Further details regarding the implementation of bias management strategies can be found in Online Appendix 3.
Deviations from the initial protocol
Two modifications were made to the initial protocol during the study to enhance its quality. Firstly, additional terms were integrated into the equation search to maximize the number of studies included. This was implemented three months after the study commenced, during the screening process (Online Appendix 4). Secondly, a random-effects meta-analysis was included in the statistical analysis to consider the heterogeneity of the included experiments more effectively.
Systematic review and meta-analysis statistics
To produce this systematic review statistics, all experiments were grouped together in a single database (no statistic was performed at the study level). Three distinct methodologies were employed to determine the role of TNF in accordance with the experimental protocol.
Firstly, an approach based on the statistical significance of each experiment was employed. Univariate analysis compared the three roles of TNF: inappropriate, non-significant and adaptive. Categorical variables were expressed as numbers and percentages and were compared using the Chi-square test. Quantitative variables were expressed as median and interquartile range [25–75] and were compared by the Mann–Whitney U test or Kruskal–Wallis test as appropriate. To identify the independent factor associated with the effect of TNF, the adaptive and inappropriate roles were evaluated in a multivariate analysis using a binary logistic regression model. For both models, variables with nominal two-tailed p values below 0.1 were included in the multivariate model. The final models were selected using the backward stepwise regression method using the AIC and by excluding all significant variables with clear collinearity or with a variance inflation factor (VIF) superior to 5 [20].
Two approaches were then employed based on the raw data. Firstly, overall mortality rates were calculated by summing the number of deceased and surviving animals in each experiment, categorized by infection type and pathogen, and compared using the Mann–Whitney U test. Finally, a meta-analysis was conducted, to calculate the weighted risk ratio of mortality. Considering the significant heterogeneity between studies, the Mantel–Haenszel approach in a random-effects model was used to determine the weighted risk ratio for mortality [17].
All statistical analyses were performed using R software (R Core Team, 2014). Figures were generated using the ‘ggplot2 package’, and the meta-analysis was performed using the ‘meta package’. A p value < 0.05 was considered statistically significant.
Results
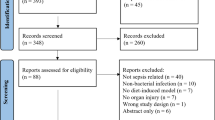
A total of 4190 distinct studies were identified through keyword searches of the Embase and PubMed databases and screened based on titles and abstracts. Of these, 261 studies were identified as potentially relevant and were further screened for eligibility based on their full text. Ultimately, 164 studies were included using this method. In addition, 11 studies that met the inclusion criteria but were not captured by the keyword searches were subsequently included. In total, 175 studies were included in the meta-analysis (Fig. 1 and Online Appendix 5). Ten eligible publications were not included, due to unavailable data, including three written in Chinese with no available translation. The publication dates of the articles span 34 years, from 1985 to 2019.
These publications represent a total of 760 experiments, with a median of 3 [2–7] experiments per article, and a total of 19,899 animals, with a median of 22 [16–37] animals per experiment. A non-significant secretion was the main effect observed in 310 (41%) experiments, while an inappropriate role was observed in 261 (34%) experiments and an adaptive role in 189 (25%) experiments.
Influence of the animal species and of the genetic background
Most experiments were performed in mice (583, 77%) and rats (157, 21%). Rarely, experiments were performed in non-human primates (0.9%), rabbits (0.8%) or pigs (0.9%) (Table 1). Overall, most experiments (41%) were inconclusive regarding a clear role of TNF, while the majority of conclusive experiments favored an inappropriate role. However, careful analysis revealed subtle differences between species. Indeed, experiments showing an adaptive effect of TNF were more frequently seen in mice (28% of mouse experiments) than in rats (16% of rat experiments). In rabbits, pigs, and non-human primates, TNF was associated with an inappropriate role in more than half of the experiments, without any adaptive role.
The genetic backgrounds of mice were mainly C57BL/6 (236 out of 583 experiments, 40%), BALB/C (112, 19%) and Swiss (54, 9.3%) (Supplemental Table 1). In the subgroup analyses, a significant proportion of the mice experiments (ranging from 50% for Swiss mice to 31% for C3H mice) demonstrated a non-significant effect of TNF. The analyses of the conclusive experiments showed clear differences between the mice strains. The ratio of experiments concluding to an inappropriate effect to those concluding to an adaptive effect was 1.19 for C57BL/6 mice, 5.54 for BALB/C and 0.28 for Swiss mice, showing that experiments in BALB/C mice were more often associated with an inappropriate role of TNF, whereas experiments in Swiss mice were mainly associated with an adaptive role. Furthermore, mice aged between 8 and 12 weeks were more often protected by TNF, whereas no clear difference was observed between the sexes.
Influence of the type of modulation of the TNF signaling pathway
Modulation of the TNF pathway was achieved more frequently by its inactivation (604 out of 760 experiments, 79%) than by its stimulation (156 experiments, 21%) (Table 2). Stimulation of the TNF pathway was mainly achieved by injection of recombinant TNF (140 out of 156 experiments, 90%). The type of modulation was strongly associated with the observed effect (Fig. 2). Stimulation of the TNF pathway, specifically by injection of recombinant TNF, was associated with an adaptive role in half of these studies (73 out of 140, 52%, p < 0.001). It should be noted that in the studies where recombinant TNF was injected, TNF was often found to be inappropriate when administered after infection (4 out of 7, 57%), whereas it was often found to be adaptive when administered before infection (60 out of 73, 82%). In contrast to the activation of the TNF pathway, the experiments with its inactivation led to the conclusion of an inappropriate role of TNF in 250 out of 604 experiments (41%), an undetermined role in 246 experiments (41%), and an adaptive role of TNF in 108 experiments (18%) (Table 2 and Fig. 2). However, a separate analysis of these experiments revealed a more complex picture. Experiments with knock-out (KO) mice for TNF pathways had a higher proportion of adaptive than inappropriate roles for TNF, 47 (32%) and 39 (26%) of 147 experiments, respectively (p = 0.029). Conversely, injection of anti-TNF antibodies or especially soluble TNF receptor, was strongly associated with an inappropriate role of TNF, especially when TNF inactivation occurred before or concomitantly with infection.
Influence of the sepsis model
Analysis of the conclusive experiments reveals that animal models of peritonitis (CLP), pneumonia, and meningitis were associated with an adaptive role of TNF, whereas intraperitoneal injection of feces or bacteria, and intravenous injection of pathogen (the most used model; 39% of experiments), were strongly associated with an inappropriate role (Table 3 and Fig. 3). Mice in models demonstrating an inappropriate role of TNF had a mortality rate ≥ 90% earlier than those showing an adaptive role, respectively 2 days [1–3] versus 4 days [3–5] (p < 0.001).
Animal mortality according to the model of sepsis, with TNF pathway inactivation. Box plot of mortality by experiments, compared using the Mann–Whitney U test. Number of animals: Intravenous injection: control = 3388, TNF inactivation = 2989; Intraperitoneal injection: control = 2424, TNF inactivation = 2224; Caecal ligation and puncture: control = 1030, TNF inactivation = 877; Pneumonia: control = 460, TNF inactivation = 444; Skin infection: control = 199, TNF inactivation = 199; Meningitis: control = 135, TNF inactivation = 144; Gastro-intestinal infection: control = 160, TNF inactivation = 166
Influence of the type of infectious stimuli
The role of TNF derived from the experiments was strongly associated with the type of infectious stimuli (Table 4 and Fig. 4). The results obtained in the main analyses were also found in the sensitivity analyses, in the subgroups of inactivated TNF experiments, intravenous pathogen injection experiments and intraperitoneal pathogen injection experiments (Supplemental Tables 2, 3, 4).
Animal mortality according to the type of infectious stimulus, with TNF pathway inactivation. Box plot of mortality by experiments, compared using the Mann–Whitney U test. Number of animals: LPS injection: control = 3657, TNF inactivation = 3154; Bacteria: control = 2629, TNF inactivation = 2604; Parasite: control = 232, TNF inactivation = 177; Virus: control = 137, TNF inactivation = 122. LPS: Lipopolysaccharide
LPS injection
LPS injection was the most frequently used model (319 experiments, 42%). LPS was mainly derived from E. coli (75%) and Salmonella species (21%) (Supplemental Table 5). LPS injection was associated with an inappropriate role for TNF in 173 experiments (56%) and with an adaptive role in only 16 experiments (5%). When LPS injection was sensitized by concomitant administration of d-galactosamine, 55 out of 81 experiments (68%) showed an inappropriate role of TNF, and none an adaptive role (Table 4).
In the subgroup of experiments with LPS, stimulation of the TNF pathway before LPS injection led to the conclusion of an adaptive role of TNF in 14 out of 24 experiments (58%), whereas inactivation of the TNF pathway led to the conclusion of an inappropriate role in 158 out of 280 experiments (56%) (Supplemental Table 6). This effect was mostly seen when the blocking agent was injected before LPS injection. In experiments involving LPS and TNF pathway inactivation, the relative risk of mortality was 0.66 (95% IC [0.61; 0.72], p < 0.001) (Supplemental Fig. 1), with significantly higher mortality in the control group than in the inactivated TNF group (94 vs. 33% respectively, p < 0.001) (Fig. 4).
Live bacterial inoculation
Infection models based on live bacterial inoculation were associated with an adaptive role of TNF in 136 out of 306 experiments (44%) and an inappropriate role in only 55 studies (18%). Mortality was higher in the inactivated TNF group than in the control group (75% vs. 50%, p < 0.001), with a mortality risk ratio of 1.21 (95% CI [1.00; 1.46], p = 0.005) (Supplemental Fig. 2).
This adaptive role of TNF was particularly observed when Listeria monocytogenes (48/82, 58%) and encapsulated bacteria (61/100, 61%) were inoculated (Table 4). A inappropriate role of TNF was observed only in 2 experiments with Listeria monocytogenes, which investigated the role of TNF injection in reducing mortality 48 h after CLP [21], and was not observed in any experiment with Klebsiella pneumoniae, Salmonella sp, Legionella sp or Streptococcus pneumoniae. Moreover, the difference in mortality was particularly pronounced for these bacteria. When L. monocytogenes, Salmonella species or S. pneumoniae were inoculated, the inactivated TNF group had respectively an increased relative risk ratio of mortality of 4.43 [2.87; 6.83] (p < 0.001), 3.76 [1.25; 11.31] (p < 0.029) and 1.88 [1.25; 2.84] (p < 0.004), respectively (Supplemental Figs. 3, 4, 5, 6, 7).
No role for TNF could be determined in experiments with Pseudomonas aeruginosa, an organism commonly involved in nosocomial infections, except in pneumonia, the most common clinical infection with this pathogen, where an adaptive role of TNF was observed in 55% of experiments (5/9) (Supplemental Table 7).
Importantly, in all these experiments, inoculum analysis showed that high initial bacterial loads were strongly associated with an inappropriate role of TNF, whereas low loads were associated with an adaptive role: 9 × 109 colony forming units per kg (CFU/Kg) [4 × 104-4 × 1010], vs. 106 CFU/kg [2 × 105–4 × 108], respectively. Antibiotic therapy, which was mainly used in experiments with high initial inoculum (9 × 109 CFU/Kg [2x109-3x1010]), was also associated with an inappropriate role of TNF. In the meta-analysis approach, the risk ratio of mortality when both TNF was inactivated and antibiotic were administrated was 0,69 [74] (p < 0,001) (Supplemental Fig. 8).
Parasite & fungus inoculation
Candida species was the most common pathogen of this group. The other two pathogens used were Histoplasma spp. and Plasmodium berghei. As with live bacteria, in the conclusive experiments, inoculation of parasites and fungi was strongly associated with an adaptive role of TNF (Table 4). However, no significant difference in mortality was observed (Fig. 4 and Supplemental Fig. 9).
Virus injection
The main viruses studied were influenza [22,23,24,25] and dengue [26,27,28] using mice with altered interferon pathways. Viral infections were associated with an inappropriate role of TNF in 54% of experiments, with no significant difference (p = 0.097) (Table 4). While a difference in mortality was initially observed (Fig. 4), this difference disappeared when the weighted pooled risk ratio of mortality analysis was performed (Supplemental Fig. 10). A study investigating the role of TNF in cytomegalovirus (CMV) meningoencephalitis [29] reported 13 experiments showing an adaptive role of TNF when injected before infection and an inappropriate role of TNF when injected after infection.
Multivariate analysis
To more objectively define the main factors associated with the role of TNF, two multivariate analyses were performed: the first to determine the adaptive role of TNF and the second to determine its inappropriate role (Fig. 5 and Supplemental tables 8 and 9). Both analyses yielded comparable results: pneumonia, CLP, and live bacterial inoculation (especially encapsulated bacteria and Listeria monocytogenes), were strongly associated with an adaptive role of TNF, whereas concomitant LPS (especially LPS and galactosamine) and antibiotic injection were strongly associated with an inappropriate role of TNF.
Forest plot of multivariate analysis determining role of TNF. Odds ratios and confidence intervals were calculated using a binary regression model. The interest variable was the adaptive role of TNF (A) and the inappropriate effect of TNF (B). In model A, the variable ‘adaptive role of TNF’ was coded as 1 for experiments that found an adaptive role of TNF and 0 for experiments that found an inappropriate or a non-significant role. In model B, the variable ‘inappropriate role of TNF’ was coded as 1 for experiments that found an inappropriate role of TNF and 0 for experiments that found an adaptive or a non-significant role. Number of experiments included in both analyses: 760. R2 Tjur model A: 0.310. R2 Tjur model B: 0.293. The details of the Odds ratio are given in Supplemental tables 8 and 9. In model A, LPS (Lipopolysaccharides) referred to a variable that comprised all experiments that used LPS (LPS alone or with adjuvant like galactosamine). List of variables included in both initial models: Female, Mice, Rat, Monkey, Intraperitoneal injection, Cecal ligature and puncture, Pneumonia, LPS, LPS alone, LPS and galactosamine, Alive Bacteria, Encapsulated bacteria, Listeria monocytogenes, Streptococcus pneumonia, Parasite & fungus, Virus, Antibiotherapy, TNF pathway stimulation, TNF pathway inactivation, Anti-TNF antibody, TNF soluble receptor, Blocking TNF before infection, Blocking TNF simultaneous
Contradictory roles of TNF.
Sixteen studies reported a paradoxical role of TNF depending on the experimental model (Table 5). Ten studies showed a different role of TNF when LPS injection was compared with infections induced by live bacteria [30,31,32], most commonly L. monocytogenes [21, 33,34,35,36,37,38]. As early as 1989, a study showed: an adaptive role of TNF when an encapsulated E. coli (resistant to phagocytosis) was injected, no role when the same microorganism without capsule was injected, and finally an inappropriate role when LPS derived from the same E. coli was injected [30].
Risk of bias results
Risk of bias assessment revealed biases in all studies (Online Appendix 6). Randomization was used in 20 out of the 141 studies (16%) that did no used KO animals. Blinded analysis was conducted in 9 (5%) studies. The number of animals used was unclear in 16 (9%) studies and missing in 31 (18%) studies. Mortality statistics were missing or inadequate in 62 (35%) studies and Fisher’s test was used instead of log-rank test in 42 (24%) studies. Information on the initial bacterial load of the pathogen and its justification was missing in 45 (26%) studies. Additionally, 88 (50%) studies did not simultaneously measure cytokine and bacterial loads, thus only investigating one aspect of infection. Finally, some models are difficult to interpret, such as that of P. aeruginosa enteric infection in neutropenic animals, in which the normal microbiota was profoundly altered after several days of antibiotic therapy. P. aeruginosa, an aerobic microorganism, is rarely associated with digestive infections but is typically found in pneumonia [39]. For this organism, models of digestive translocations show an inappropriate role of TNF, whereas models of pneumonia show an adaptive role.
Discussion
The objective of this meta-analysis was to identify the factors that influence the effect of TNF in endotoxemia and sepsis animal models. Its primary finding is the significant association between the effect of TNF and experimental parameters. These parameters include the animal species, their genetic background, the method used for experimental modulation of the TNF pathway, the sequence of injection of modulation agent of the TNF pathway, the type of pathogen, and the infection model used. The variety of these parameters, which may interact in divergent ways, may explain why most experiments (41%) did not provide a clear conclusion regarding the role of TNF.
However, when considering the conclusive experiments, an apparent pattern emerges suggesting that TNF typically exerts an adaptive role unless the immune system is experimentally overwhelmed by a sudden and intense infectious stimulus (e.g., LPS as a superantigen), in which case TNF appears to be detrimental. Consequently, TNF tends to play an inappropriate role in models that induce rapid and high mortality rates, such as infectious models that use remarkably high pathogen loads or direct injections of LPS. These models are rarely representative of the situations encountered in clinical practice, which should serve as the basis for understanding the pathophysiological role of key players. In contrast, TNF appears to play an adaptive role in models that more closely mimic sepsis scenarios encountered in clinical practice, such as pneumonia or CLP. However, these findings must be interpreted with caution due to their important level of bias and their limited relevance to the standards of good practice expected in clinical trials, such as randomization and blinding.
The results of this systematic review are illustrated by the 1987 study by Tracey et al. [40], which was the first to suggest an inappropriate role for TNF using live bacteria (E. coli). In this study, a laboratory strain of E. coli was administered at a dose greater than 1.2 × 10 [12] bacteria inoculated intravenously over 30 min, in conjunction with early administration of antibiotics. However, this initial inoculum does not appear to be physiological. In fact, feces, the natural element containing the highest concentration of E. coli, typically has concentrations around 108 Enterobacterales per gram [41]. Consequently, the bacterial load used in this study would be equivalent to 10 kg of feces. The authors did not provide any justification for the choice of such a dose, and it is difficult to imagine such a scenario occurring in nature, especially via intravenous administration. In addition, the rapid use of antibiotics makes these models more akin to acute inflammation models, similar to LPS injection, with extremely high TNF levels, exceeding those found in more physiological models such as CLP [42]. For instance, in the 1985 article by Beutler et al. [2], which was the first to suggest an inappropriate role of TNF in infections, LPS was progressively injected until lethality was induced. However, the lethal dose was not compared with the circulating levels of LPS found in clinical situations of infection. Other studies have also investigated this subject and found relatively low circulating levels. In their 2003 study, Echtenacher et al. found that 48 h after a CLP, the concentration of LPS in serum was 0.358 ng/ml in mice [21]. However, after the injection of just 1 µg of LPS, the concentration increased to 1004 ng/ml, which is 3000 times higher. Remarkably, this dose of LPS did not induce any mortality, with the lethal dose being at least 100 µg/ml, i.e., 300,000 times higher than the concentrations observed in physiological CLP situations. This is in line with studies that evaluate circulating LPS levels in humans. In the first study to measure circulating endotoxins in human sepsis [43], only 17% of patients had endotoxemia, and at relatively low concentrations (5 to 0.5 ng/ml). This result has been confirmed by more recent studies [44, 45].
Additionally, 25% of LPS experiments involve the injection of galactosamine along with LPS. Galactosamine is used to sensitize mice to LPS and reduce the amount needed to cause mortality. Unlike other species such as rabbits, mice are highly resistant to LPS and require large doses to be lethal [46, 47]. However, galactosamine can induce fulminant hepatitis [48] by sensitizing hepatocytes to LPS, resulting in significant apoptotic cell death of nearly all hepatocytes [49]. This mechanism differs from the mortality induced by LPS. Indeed, mice resistant to the association LPS-galactosamine [50] present the same mortality rate than wild-type mice to LPS injection [51]. As LPS accounts for 40% of the experiments and is the main infectious stimulus assessing the role of TNF, this could explain why this cytokine is often considered to be inappropriate in sepsis [52, 53]. Nowadays these limitations of LPS are known, and LPS is no longer recommended for studying sepsis [54,55,56]. However, sepsis definitions are still partially based on these results. Sepsis is defined as a dysregulated host response to infection [18], whereas experimental data that focused on particularly virulent bacteria found, on the contrary, an adaptive role of TNF.
In our review, experiments with virulent bacteria, which used much lower initial inocula, appear to more closely reflect the situations observed in clinical contexts. For instance, L. monocytogenes, a pathogen that is well-known for inducing meningitis and maternal–fetal infections, was the primary organism used and was strongly associated with an adaptive role of TNF. The initial intravenous dose varied between 102 and 105 CFU (104–107 CFU/Kg). However, the threshold for food contamination with L. monocytogenes is set at 100 CFU/g of food [57]. The doses used in these articles corresponded to quantities of contaminated food ranging from 1 g to 1 kg, which are more realistic scenarios than the bacterial load used with E.coli as previously mentioned [40]. The use of encapsulated bacteria was associated with even lower doses of pathogens. Cross et al. [30] found that less than a dozen encapsulated E. coli bacteria were sufficient to kill all the TNF KO mice, while the control mice died with a bacterial load 105 times higher (5 × 105 CFU/kg) CFU. In contrast, the same E. coli lacking the capsule required an inoculum of about 107 CFU (5 × 108 CFU/kg) to induce mortality in all the mice, with no difference observed between the TNF KO and control mice. The capsule appears to cause mortality variations that are equally significant as the absence of TNF. Capsules are commonly present in E. coli bacteria and act as virulence factors that are prevalent in clinical settings, particularly in neonatal meningitis and urinary tract infections [58, 59]. Due to their capsule, these bacteria are resistant to phagocytosis and the complement pathway, particularly in children [60, 61]. These findings are consistent with experiments demonstrating increased invasiveness of encapsulated E. coli compared to non-encapsulated E. coli [59, 62]. Additionally, E. coli encountered in clinical settings can produce toxins such as Shiga toxin, contributing to their high virulence [63, 64]. Overall, these results underscore that laboratory bacterial strains are unable to induce the same level of sepsis as the virulent bacteria encountered in clinical settings. A prime example of this is S. pneumoniae, which is the microorganism most commonly found in young and elderly patients. Despite the use of antibiotic therapy and the development of pneumococcal vaccination, pneumococcal infection remains a leading cause of bacterial pneumonia and community-acquired meningitis, and almost 800,000 children under the age of five die each year worldwide from this infection [65]. In our review, no study found an inappropriate role of TNF in relation to this pathogen. For example, in two studies, a dose of 100 pneumococci injected intraperitoneally was sufficient to kill more than 80% of Tnfr1- KO mice, whereas 107 CFU were required to induce similar mortality in control mice [66, 67]. These results support the notion that TNF plays an adaptive role in pneumococcal infections, which is consistent with other experimental studies involving animals lacking genes encoding molecules such as IL-1B [68], TLR-4 [69] and TLR-2 [70], TLR-9 [71] and MYD88 [72, 73].
When interpreting experiments that report lethal injections of TNF, the dose must also be considered. In the 1987 study by Tracey et al., the administered dose of TNF required to induce mortality in 50% of the rats was 0.6 mg/kg, resulting in a peak plasma TNF concentration of 600 ng/ml. In the study by Rothe et al., the dose required to induce mortality in 50% of the mice was 1 µg per mouse, equivalent to 0.05 mg/kg, resulting in an estimated peak plasma TNF concentration of 66 ng/ml [33]. These levels of circulating TNF are significantly higher than those found in septic patients, which typically do not exceed 0.1 ng/ml [4, 11, 57, 58]. Furthermore, Feuerstein et al. found that injection of recombinant TNF at concentrations 10–100 times higher (107 UI/ml) than those found in rats succumbing to LPS injection did not result in lethality [76]. Some experimental models based on E. faecalis [77, 78], with mortality rates exceeding 50%, have failed to detect measurable levels of TNF. Furthermore, in the 1989 study by Sheppard et al., the injection of 50 µg/ml TNF in rats, resulting in a peak concentration of approximately 50 ng/ml, was not inappropriate but rather adaptive, shielding the animals from a lethal injection of LPS administered 24 h later [79]. In the same year, Hershman et al. conducted a study wherein the administration of 0.1 µg of recombinant TNF, resulting in an estimated peak concentration of 6 ng/ml, exhibited an adaptive effect by increasing the survival rate of mice with K. pneumoniae skin infection [80]. These experimental findings suggest that remarkably high levels of TNF, well above those typically observed in septic patients, may not only be compatible with life but may also confer an adaptive effect during infection. This phenomenon may be related to increased secretion of soluble TNF receptors, which may attenuate the increase of TNF, except during the very early stages of infection [81]. This may help elucidate why certain studies in the mid-1990s showed that circulating TNF levels were not correlated with mortality and could even be adaptive in human sepsis [82, 83].
It is important to acknowledge that our meta-analysis is constrained by certain limitations inherent to the nature of the included studies. Primarily, most of the studies were not randomized (84%) or blinded (95%), which introduces the possibility of bias in the analysis. These percentages are consistent with those reported in a 2011 meta-analysis on bias in experimental studies that included 277 experimental studies conducted between 1999 and 2005, which found rates of 87% and 86%, respectively [84]. However, a more recent meta-analysis focusing on animal studies in cardiology from 2006 to 2016 found better rates (78% and 67%), suggesting some improvement over time [85]. Furthermore, no study included a calculation for sample size. Therefore, the non-significant effect that was the main effect observed in our meta-analysis may be attributed to the small number of animals included in the experiments. However, the number of animals per experiment was not associated with the type of TNF effect (Table 1) thus the lack of statistical significance may be linked to a non-optimized experimental protocol. Of course, the lack of rigorous methodology can result in erroneous conclusions, as illustrated by Perrin and colleagues in their study on amyotrophic lateral sclerosis [86]. Nonetheless, this is likely to have minimal impact on the overall findings of our study, as there seems to be a general consistency across the studies. None of them indicated that injecting low doses of LPS or virulent microorganisms, such as encapsulated bacteria, was associated with an inappropriate of TNF. On the other hand, ten of the studies included highlighted the dual role of TNF. It was found to be adaptive in the case of infection, but inappropriate when LPS was administered (Table 5). The contrasting role of TNF in response to LPS and E. coli injection compared to more virulent pathogens such as S. pneumonia and Listeria spp was also highlighted in a systematic review conducted in 2005 [87].
A further crucial aspect of this study is the low rate of antibiotic utilization, which was observed in 10% of all experiments and in 20% of experiments involving live bacteria and was associated with an inappropriate effect of TNF in our meta-analysis. In clinical practice, antibiotics are the primary treatment for sepsis in humans. However, it is essential to acknowledge that antibiotics can disrupt the immune response by triggering the release of LPS, leading to elevated levels of TNF and IL-6. Furthermore, the bactericidal properties of antibiotics limit the beneficial effects of TNF release and its adaptive impact, thus emphasizing the visualization of the inappropriate effects.
Finally, our meta-analysis has limitations associated with its conception. Although we aimed to be as inclusive as possible, our literature search strategy may not have been able to include all articles where both the TNF signaling pathway was altered and mortality was assessed. Moreover, almost 6% of the eligible publications could not be analyzed because the articles were not locatable. However, half of these articles were not written in English and might have minimal impact on the current opinion. Nonetheless, this investigation, comprising 175 studies, represents the largest meta-analysis on this topic to date.
Our statistical approach, particularly our multivariate analyses, can also be discussed. Firstly, observations must be independent to be included in the logistic regression, but this assumption may be invalid, as several experiments within each study were included. However, it is currently impossible to evaluate the dependence of observation, and several studies have found different effects of TNF based on the experimental protocol. Therefore, this potential bias may have minimal impact on our multivariate analyses.
Moreover, achieving a completely objective selection of variables for the multivariate analysis is challenging. To address the subjectivity in the variable selection process, we conducted two separate analyses: one focusing on the adaptive effect and another on the inappropriate effect, both yielding comparable results. Furthermore, the predictive values of these multivariate analyses were reasonable (R2 Tjur around 0.3), but this indicates that many experiments are not well classified by our model. The lack of discrimination in our model may be due to the high proportion of non-significant experiments (41%). However, our three statistical approaches consistently point in the same direction. Hence, we believe the risk of error in our main results, as described in the conclusion and visual abstract, is extremely low.
Conclusion
The role of TNF during infection, whether adaptive or inappropriate, varies depending on the experimental model used. Models based on virulent bacteria, which result in high mortality even at low inocula, have demonstrated the adaptive role of TNF. Conversely, models based on LPS or low-pathogenic live bacteria, administered at doses well above physiological thresholds and combined with early antibiotic therapy, have been associated with an inappropriate role of TNF on survival. As the first mentioned models are closest to the conditions encountered in clinical practice, this meta-analysis suggests that TNF may play an adaptive role in infections. This observation may explain the lack of efficacy of anti-TNF treatments in sepsis. Indeed, the rationale behind the administration of such drugs is based on a poor pathophysiological understanding of sepsis, as experimental models with low-pathogenic bacteria do not allow to elucidate the mortality of virulent bacteria.
Data availability
Files with data extraction and R statistical analysis code are available on request.
References
Carswell EA, Old LJ, Kassel R, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci. 1975;72(9):3666–70.
Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229(4716):869–71.
Tracey KJ, Lowry SF, Fahey TJ, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164(5):415–22.
Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. The Lancet. 1987;329(8529):355–7.
Suffredini AF, Fromm RE, Parker MM, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321(5):280–7.
da Silva AMT, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL. Shock and multiple-organ dysfunction after self-administration of salmonella endotoxin. N Engl J Med. 1993;328(20):1457–60.
Abraham E. p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock: a randomized controlled multicenter trial. JAMA. 1997;277(19):1531.
Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group Lancet. 1998;351(9107):929–33.
Reinhart K, Menges T, Gardlund B, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study. Crit Care Med. 2001;29(4):765–9.
Gallagher J, Fisher C, Sherman B, et al. A multicenter, open-label, prospective, randomized, dose-ranging pharmacokinetic study of the anti-TNF-α antibody afelimomab in patients with sepsis syndrome. Intensiv Care Med. 2001;27(7):1169–78.
Fisher CJ, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. N Engl J Med. 1996;334(26):1697–702.
Urbaschek R, Urbaschek B. Tumor necrosis factor and interleukin 1 as mediators of endotoxin-induced beneficial effects. Clin Infect Dis. 1987;9(5):S607–15.
Havell EA. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987;139(12):4225–31.
Chaudhry H, Zhou J, Zhong Y, Ali MM, Nagarkatti PS, Nagarkatti M. Role of Cytokines as a Double-edged Sword in Sepsis. 2015;28.
Cavaillon JM. Pro-versus anti-inflammatory cytokines: myth or reality. Cell Mol Bio. 2001;47(4):695–702.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(1):g7647–g7647.
Deeks J, Higgins J, Altman D (editors). Chapter 10: Analysing data and undertaking meta-analyses [Internet]. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4. 2023. Available from: https://training.cochrane.org/handbook/current/chapter-10
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801.
Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–69.
Echtenacher B, Urbaschek R, Weigl K, Freudenberg MA, Männel DN. Treatment of experimental sepsis-induced immunoparalysis with TNF. Immunobiology. 2003;208(4):381–9.
Perrone LA, Szretter KJ, Katz JM, Mizgerd JP, Tumpey TM. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J Infect Dis. 2010;202(8):1161–70.
Belisle SE, Tisoncik JR, Korth MJ, et al. Genomic profiling of tumor necrosis factor alpha (TNF-α) receptor and interleukin-1 receptor knockout mice reveals a link between TNF-α signaling and increased severity of 1918 pandemic influenza virus infection. J Virol. 2010;84(24):12576–88.
Peper RL, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19(3):175–83.
Shi X, Zhou W, Huang H, et al. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit Care. 2013;17(6):R301.
Branche E., Tang W.W., Viramontes K.M., et al. Synergism between the tyrosine kinase inhibitor sunitinib and Anti-TNF antibody protects against lethal dengue infection. Antiviral Research 2018;158((Shresta S., sujan@lji.org) Department of Medicine, School of Medicine, University of California, La Jolla, San Diego, CA, United States):1–7.
Phanthanawiboon S, Limkittikul K, Sakai Y, Takakura N, Saijo M, Kurosu T. Acute systemic infection with dengue virus leads to vascular leakage and death through tumor necrosis factor-α and Tie2/angiopoietin signaling in mice lacking type I and II interferon receptors. PLoS ONE. 2016;11(2): e0148564.
Atrasheuskaya A, Petzelbauer P, Fredeking TM, Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol. 2003;35(1):33–42.
Doherty PC, Allan JE, Clark IA. Tumor necrosis factor inhibits the development of viral meningitis or induces rapid death depending on the severity of inflammation at time of administration. J Immunol. 1989;142(10):3576–80.
Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989;169(6):2021–7.
Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145(11):3762–6.
Dharmana E, Keuter M, Netea MG, Verschueren ICMM, Kullberg BJ. Divergent effects of tumor necrosis factor-α and lymphotoxin-α on lethal endotoxemia and infection with live Salmonella typhimurium in mice. Eur Cytokine Netw. 2002;13(1):104–9.
Rothe J, Lesslauer W, Lötscher H, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to IMF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802.
Pfeffer K, Matsuyama T, Kündig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–67.
Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160(2):943–52.
Garcia I, Miyazaki Y, Araki K, et al. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive toListeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25(8):2401–7.
Xanthoulea S, Pasparakis M, Kousteni S, et al. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med. 2004;200(3):367–76.
Wroblewski R, Armaka M, Kondylis V, et al. Opposing role of tumor necrosis factor receptor 1 signaling in T cell-mediated hepatitis and bacterial infection in mice: autoimmune. Cholest Biliary Dis Hepatol. 2016;64(2):508–21.
Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Clin Infect Dis. 1983;5(2):279–313.
Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–4.
Edberg SC, Rice EW, Karlin RJ, Allen MJ. Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol. 2000;88(S1):106S-116S.
Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepis: lipopolysaccharide vs cecal ligation and punctre. Shock. 2000;13(2):110–6.
Levin J, Poore TE, Zauber NP, Oser RS. Detection of endotoxin in the blood of patients with sepsis due to gram-negative bacteria. N Engl J Med. 1970;283(24):1313–6.
Bottiroli M, Monti G, Pinciroli R, et al. Prevalence and clinical significance of early high endotoxin activity in septic shock: an observational study. J Crit Care. 2017;41:124–9.
Adamik B, Zielinski S, Smiechowicz J, Kübler A. Endotoxin elimination in patients with septic shock: an observation study. Arch Immunol Ther Exp. 2015;63(6):475–83.
Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69(11):7169–72.
Munford RS. Murine responses to endotoxin: another dirty little secret? J Infect Dis. 2010;201(2):175–7.
Apte U. Galactosamine [Internet]. In: Encyclopedia of Toxicology. Elsevier; 2014 [cited 2023 Sep 22]. p. 689–690.Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123864543003158
Pritchard MT, Apte U. Models to study liver regeneration [Internet]. In: Liver Regeneration. Kansas city: Elsevier; 2015 [cited 2023 Sep 22]. p. 15–40.Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780124201286000026
Amiot F, Boussadia O, Cases S, et al. Mice heterozygous for a deletion of the tumor necrosis factor-α and lymphotoxin-α genes: biological importance of a nonlinear response of tumor necrosis factor-α to gene dosage. Eur J Immunol. 1997;27(4):1035–42.
Amiot F, Fitting C, Cavaillon J-M. Lipopolysaccharide-induced cytokine cascade and lethality in LTa/TNFa-deficient mice. Mol Med. 1997;3:864–75.
van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–20.
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. The Lancet. 2018;392(10141):75–87.
Cavaillon J, Singer M, Skirecki T (2020) Sepsis therapies learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med https://doi.org/10.15252/emmm.201810128
Translational Research Committee of the French Intensive Care Society (Société de Réanimation de Langue Française), Guillon A, Preau S, et al. Preclinical septic shock research: why we need an animal ICU. Ann Intensive Care 2019;9(1):66.
Osuchowski MF, Ayala A, Bahrami S, et al. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. ICMx. 2018;6(1):26.
Réglement (CE) No 2073/2005 de la comission du 15 novembre 2005 concernant les critères microbiologiques applicables aux denrées alimentaires [Internet]. Journal officiel. 2005;Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R2073:20060101:fr:PDF
Cross AS, Gemski P, Sadoff JC, Ørskov F, Ørskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984;149(2):184–93.
Kim KS, Itabashi H. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the Rat. J Clin Invest. 1992;90:897.
Lassiter HA, Tanner JE, Miller RD. Inefficient bacteriolysis of Escherichia coli by serum from human neonates. J Infect Dis. 1992;165(2):290–8.
Opal S, Cross A, Gemski P. K antigen and serum sensitivity of rough Escherichia coli. Infect Immun. 1982;37(3):956–60.
Bortolussi R, Ferrieri P, Björkstén B, Quie PG. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979;25(1):293–8.
Crowe SJ, Bottichio L, Shade LN, et al. Shiga toxin-producing E. coli infections associated with flour. N Engl J Med. 2017;377(21):2036–43.
Grimaldi D, Bonacorsi S, Roussel H, et al. Unusual “Flesh-eating” strain of Escherichia coli. J Clin Microbiol. 2010;48(10):3794–6.
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. The Lancet. 2009;374(9693):893–902.
Wellmer A, Gerber J, Ragheb J, et al. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect Immun. 2001;69(11):6881–6.
O’Brien DP, Briles DE, Szalai AJ, Tu A-H, Sanz I, Nahm MH. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect Immun. 1999;67(2):595–601.
Kafka D, Ling E, Feldman G, et al. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int Immunol. 2008;20(9):1139–46.
Branger J, Knapp S, Weijer S, et al. Role of toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004;72(2):788–94.
Dessing MC, Florquin S, Paton JC, van der Poll T. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol 2007;0(0):070817225835002
Albiger B, Dahlberg S, Sandgren A, et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9(3):633–44.
Koedel U, Rupprecht T, Angele B, et al. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 2004;127(6):1437–45.
Albiger B, Sandgren A, Katsuragi H, et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice: role of MyD88 in murine pneumococcal infection. Cell Microbiol. 2005;7(11):1603–15.
Monneret G, Finck M-E, Venet F, et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95(2):193–8.
Debets JM, Kampmeijer RE, Marielle P, Buurman WA, Van Der Linden CJ. Plasma tumor necrosis factor and mortality in critically ill septic patients. Crit Care Med. 1989;17(6):489–94.
Feuerstein G, Hallenbeck JM, Vanatta B, Rabinovici R, Perera PY, Vogel SN. Effect of gram-negative endotoxin on levels of serum corticosterone, TNF alpha, circulating blood cells, and the survival of rats. Circ Shock. 1990;30(3):265–78.
Papasian CJ, Silverstein R, Gao JJ, Bamberger DM, Morrison DC. Anomalous role of tumor necrosis factor alpha in experimental enterococcal infection. Infect Immun. 2002;70(12):6628–37.
Steinshamn S, Waage A. Tumor necrosis factor and interleukin-6 in Candida albicans infection in normal and granulocytopenic mice. Infect Immun. 1992;60(10):4003–8.
Sheppard BC, Fraker DL, Norton JA. Prevention and treatment of endotoxin and sepsis lethality with recombinant human tumor necrosis factor. Surgery. 1989;106(2):156–61.
Hershman MJ, Pietsch JD, Trachtenberg L, Mooney THR, Shields RE, Sonnenfeld G. Protective effects of recombinant human tumour necrosis factor α and interferon γ against surgically simulated wound infection in mice. Br J Surg. 1989;76(12):1282–6.
Girardin E, Roux-Lombard P, Grau GE. Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. Immunology. 1992;76:20–3.
Riché F, Panis Y, Laisné M-J, et al. High tumor necrosis factor serum level is associated with increased survival in patients with abdominal septic shock: a prospective study in 59 patients. Surgery. 1996;120(5):801–7.
Rigato US, Castelo A, Salomo R. Tumor necrosis factor alpha (TNF-a) and sepsis: evidence for a role in host defense. Infection. 1996;24:5.
Kilkenny C, Parsons N, Kadyszewski E, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4(11): e7824.
Ramirez FD, Motazedian P, Jung RG, et al. Methodological rigor in preclinical cardiovascular studies: targets to enhance reproducibility and promote research translation. Circ Res. 2017;120(12):1916–26.
Perrin S. Preclinical research: make mouse studies work. Nature. 2014;507(7493):423–5.
Lorente A, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24(Supplement 1):107–19.
Acton RD. Differential sensitivity to Escherichia coli infection in mice lacking tumor necrosis factor p55 or interleukin-1 p80 receptors. Arch Surg. 1996;131(11):1216.
Kasner L, Chan CC, Whitcup SM, Gery I. The paradoxical effect of tumor necrosis factor alpha (TNF-alpha) in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1993;34(10):2911–7.
Vogels MT, Hermsen CC, Huys HL, Eling WM, van der Meer JW. Roles of tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, platelet-activating factor, and arachidonic acid metabolites in interleukin-1-induced resistance to infection in neutropenic mice. Infect Immun. 1994;62(5):2065–70.
D’Souza M, Oettinger CW, Milton GV. Microspheres containing neutralizing antibodies to tumor necrosis factor-α and interleukin-1β protect rats from Staphylococcus aureus-induced peritonitis. J Interferon Cytokine Res. 2000;20(10):907–13.
Kinoshita M, Uchida T, Nakashima H, Ono S, Seki S, Hiraide H. Opposite effects of enhanced tumor necrosis factor-alpha production from Kupffer cells by gadolinium chloride on liver injury/mortality in endotoxemia of normal and partially hepatectomized mice. Shock (Augusta, Ga). 2005;23(1):65–72.
Funding
No funding
Author information
Authors and Affiliations
Contributions
Conceptualization, CDT. Screened articles for inclusion: CM, CK, Resolution of discrepancies for inclusion: CDT. Article retrieval: IC, CDT, MB; Data extraction: CDT, LIT; Resolution of discrepancies for data extraction: CK. Statistical analysis: CDT; Writing—original draft, CDT, CK; Writing—review and editing, CDT, CK, DL, LDC, PM. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kassasseya, C., Torsin, L.I., Musset, C. et al. Divergent effects of tumor necrosis factor (TNF) in sepsis: a meta-analysis of experimental studies. Crit Care 28, 293 (2024). https://doi.org/10.1186/s13054-024-05057-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-05057-0