Abstract
Background
Gastroscopy procedures are frequently performed under general sedation to minimize discomfort. Patients who refuse a sedative injection may experience more discomfort and adverse reactions such as pain and nausea. These instances reduce patient compliance and willingness to participate in future procedures. Acupuncture has been shown to have an anti-nausea and analgesic effect; however, there is limited data available that demonstrates the efficacy of acupuncture when applied before gastroscopy.
Methods
A total of 60 participants will be randomly assigned to the electroacupuncture (EA) group and the sham electroacupuncture (SEA) group at a ratio of 1:1. Acupuncture treatment will be performed before gastroscopy for a duration of 30 min. All patients will complete detailed questionnaires at 30 min and 7 days post-procedure to record the severity of their symptoms. The primary outcome will be the average of 4 standard visual analogue scale (VAS) scores in the categories of nausea, vomiting, throat discomfort, and agitation as reported by the patient. The secondary outcomes will be patient’s anxiety level as recorded by the 6-item short form of the State-Trait Anxiety Inventory (STAI-S6) and Amsterdam Pre-Operative Anxiety and Information Scale (APAIS), preference in a future endoscopy, pulse oxygen saturation (SpO2), heart rate (HR), and blood pressure (BP). Anxiety scales will be assessed before and after acupuncture; others will be completed at 30 min and 7 days post-procedure. The duration of the gastroscopy and the number of biopsies will be recorded after operation.
Discussion
This randomized controlled trial will explore the feasibility of the further clinical application of electroacupuncture for the improvement of patient discomfort during gastroscopy without systemic sedation.
Trial registration
ChiCTR2000040726. This trial has been approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (2020SHL-KY-11). Registration date 12 August 2020.
Similar content being viewed by others
Administrative information
Title {1} | Effect of Electroacupuncture on Discomfort during Gastroscopy: a study protocol for a randomized controlled trial |
Trial registration {2a and 2b} | This trial has been approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (2020SHL-KY-11) and is registered as ChiCTR2000040726. |
Protocol version {3} | version 1.0 |
Funding {4} | This research is supported by the Shanghai Hospital Development Center (grant SHDC2020CR3015A) |
Author details{5a} | 1.Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China |
Name and contact information for the trial sponsor {5b} | Contact name: Clinical Research Plan of SHDC Address: 2,Kangding Road, Shanghai, 200041, China Phone code: 021-96886 |
Role of sponsor {5c} | The sponsor played no part in study design; and will play no part in the collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. |
Introduction
Background and rationale {6a}
Upper gastrointestinal endoscopy (gastroscopy) is one of the most common diagnostic and therapeutic methods for assessing upper gastrointestinal diseases [1]. Gastroscopy is the first choice for clinical examination of upper gastrointestinal diseases because physicians can directly observe the inspection site and perform pathological biopsy and ligation on the diseased tissue in real time [2]. Due to the aging population and modern dietary changes, the average number of gastroscopies performed annually has sharply increased over the past few decades [3].
The strong stimulation from gastroscope insertion can often induce a gag reflex, which causes throat discomfort and nausea. Unmedicated patients also experience anxiety, increased blood pressure, and tachycardia during gastroscopy procedure. To curtail these symptoms, gastroscopy under general anesthesia is widely used. However, the use of anesthesia is costly and associated with inhibited cognitive function and mental decline. Moreover, the post procedural impairment affects a patients’ ability to drive and return to work immediately afterwards [4]. That is why approximately half of Chinese gastroscopy patients decline general anesthesia during gastroscopy [5, 6]. We therefore need to identify a safe and efficacy method to reduce adverse events during gastroscopy and offer patients a palatable alternative to sedation. Acupuncture analgesia has its unique advantages. Clinical trials have confirmed that preoperative acupuncture can reduce anxiety, decrease the use of anesthetic drugs, relieve nausea and vomiting, and shorten hospital stays [5,6,7,8]. Acupuncture during the perioperative period can help restore intestinal function and reduce postoperative pain [9, 10]. Studies have reported acupuncture analgesia as a successful alternative to pharmacological sedation during gastroscopy; however, the actual benefits still require further studies to corroborate [11, 12]. Therefore, high-quality randomized clinical trials are needed to re-validate previous findings and develop a clinical trial of electroacupuncture to reduce patients’ discomfort during standard gastroscopy.
Objectives {7}
We designed a rigorous randomized controlled clinical trial using a single-center, patient- and assessor-blinded placebo-controlled research method to evaluate the impact of electroacupuncture (EA) on the discomfort of patients undergoing gastroscopy. We aim to observe whether EA can reduce the discomfort of patients during gastroscopy; the results will help provide evidence for whether EA is an effective therapy to reduce patient discomfort during general gastroscopy.
Trial design {8}
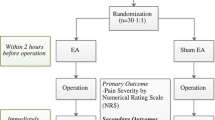
This RCT is a single-site randomized placebo-controlled trial. It will be implemented in Shanghai Municipal Hospital of Traditional Chinese Medicine. All eligible patients will be randomly assigned to the electroacupuncture group (EA) or the sham electroacupuncture (SEA) group in a 1:1 allocation ratio. All patients must sign the informed consent form before they are enrolled in the trial. The research process flow chart is detailed in Fig. 1. In this trial, the Consolidated Standards of Reporting Trials (CONSORT) [13] and acupuncture reporting guidelines, Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) [14], will be followed.
Methods: participants, interventions, and outcomes
Study setting {9}
All eligible participants will be recruited through the outpatient clinic or inpatient, hospital-based WeChat advertising, and posters in the Shanghai Municipal Hospital.
Eligibility criteria {10}
Inclusion criteria
Patients who meet the following conditions will be included:
-
18–70 years old, male or female
-
Patients willing to undergo gastroscopy with local anesthesia
-
Patients with clear consciousness and can answer the questions, understand the scales, and complete the assessment
-
Patients who agree to participate and sign a written informed consent
Exclusion criteria
Patients who meet any of the following conditions will be excluded:
-
Patients with mental disorders or severe cognitive impairments who cannot participate in cooperation
-
Patients with a history of bleeding disorders or who currently use warfarin or low-molecular-weight heparin
-
Patients who cannot receive acupuncture for reasons such as infections around the acupuncture points or allergy to acupuncture needles
-
Patients who have received acupuncture treatment in the past 6 months
-
Participants in other clinical trials that may interfere with the primary endpoint
Who will take informed consent? {26a}
All participants will be recruited by advertising through online and offline recruitment channels in Shanghai Municipal Hospital. People who need gastroscopy and are interested in participating can contact the researcher by calling or adding the WeChat. We will evaluate according to the inclusion and exclusion criteria. All participants will be clearly informed about the potential benefits and risks of the trial before they sign the informed consent.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable.
Interventions
Explanation for the choice of comparators {6b}
In this trial, all participants will receive the electroacupuncture or sham electroacupuncture before the gastroscopy. We will adopt the superficial acupuncture in the non-acupoints method. It has the advantages of good masking effect, not easily perceived by the patient, and a higher feasibility of operation.
Intervention description {11a}
All participants will receive the electroacupuncture or sham electroacupuncture before the gastroscopy. They will then undergo standard upper gastrointestinal endoscopy procedures. Patients in both groups will be discharged within 1 h after the endoscopy, which includes a 30-min observation period, a preference for future endoscopy assessment and blinding assessment. The acupuncture methods are described in Table 1. To maintain blinding, patients will be asked to wear an eye-patch and receive treatment in a supine position in an isolated space with limited communication with the acupuncturist. The acupuncturist will disinfect the patient’s skin with 75% alcohol cotton balls before treatment. Acupuncture treatment will last 30 min. After removing the needle, researchers will use a clean cotton ball to compress the acupoint to prevent bleeding. A total of 8 points will be used. Table 2 summarizes the acupoints and rationale.
The electroacupuncture group
Participants in the treatment group will receive EA treatment. The standard acupuncture method will be applied at 8 acupoints: bilateral Hegu (LI4), Neiguan (PC6), Zusanli (ST36), and Liangqiu (ST34). All acupoints were selected based on literature, clinical experience, and acupuncture textbooks. The acupuncture needles will be disposable sterile needles made of stainless steel (0.25 × 40 mm and 0.30 × 40 mm; Jia Jian, China). After insertion, needles will be manipulated using the lifting-thrusting and twirling technique. A pair of electroacupuncture apparatus (CMNS6-1, Wuxi Jiajian Medical Device CO, China) will be connected to 2 pairs of needles (LI4, ST36 bilaterally) to deliver a continuous wave, low frequency (2 Hz), and current of 2 mA.
The sham electroacupuncture group
In the control group, we will use the same acupuncture needles as used in the EA group but repurposed as placebo needles. The acupuncturist will perform superficial acupuncture on the non-acupoints detailed in Table 3 and shown in Fig. 2. All of the needles will be inserted using a needle tube to a superficial depth (2–3 mm) without invoking the sensation of “De-qi.” The electroacupuncture apparatus will be connected to 2 pairs of needles (LI4, ST36 bilaterally) for 30 min, but all indicators will be set to “0” to ensure no electrical current is delivered.
Criteria for discontinuing or modifying allocated interventions {11b}
When acupuncture-related serious adverse reactions occur during the experiment or there is a failure to complete the study for any other reasons, we will record the details in a report for the early termination of the study.
Strategies to improve adherence to interventions {11c}
At the beginning of the trial, participants will be again informed of the experiment procedure. They can report their personal feelings at any time during the study and we will give corresponding feedback as appropriate to increase the interactivity and improve adherence to interventions.
Relevant concomitant care permitted or prohibited during the trial {11d}
Patients cannot eat 8 h before gastroscopy to ensure a fasting state.
Provisions for post-trial care {30}
At the end of the study, we will provide a subsidy to each subject.
Outcomes {12}
Primary outcome
A visual analogue scale (VAS) will be used to evaluate the patient’s level of discomfort, which is a line segment from 0 to 10, with 0 indicating low and 10 indicating high discomfort. All patients will complete 4 detailed VAS at 30 min. The questionnaire will evaluate 4 areas of discomfort including nausea and vomiting, throat discomfort, bucking, and agitation. The primary outcome is the average score of the 4 VAS.
Secondary outcomes
-
The patient’s level of discomfort at 7 days post-procedure by using VAS. The four VAS including nausea and vomiting, throat discomfort, bucking, and agitation will present separately as secondary outcomes at 30 min and 7 days post-procedure.
-
Cardiac-respiratory assessment
All patients’ pulse oxygen saturation (SpO2), heart rate (HR), and blood pressure (BP) measurements will be recorded prior to, during, and post-procedure. Then, each patient’s “double product” (DP) will be calculated at each point of the procedure, based on the systolic blood pressure (SBP) and heart rate (SBP×HR). DP is a useful prognostic tool for patients with poor cardiac function during exercise and rest, reflecting myocardial oxygen consumption and cardiac output during treadmill testing. A value of DP greater than 15,000 indicates cardiovascular stress. It is accepted that changes in cardiac output from baseline (preoperative) will reflect the level of stress experienced by patients undergoing the gastroscopy.
-
Anxiety level
A six-item short form of the State-Trait Anxiety Inventory (STAI-S6) and Amsterdam Pre-operative Anxiety and Information Scale (APAIS) will assess the degree of preoperative anxiety, postoperative anxiety, and 7 days post-procedure. These questionnaires are quick and simple for participants to complete, which is advantageous in time-restricted studies.
-
Preference in a future endoscopy
The questionnaire will ask patients whether they have undergone gastroscopy before, and then ask, “Do you have interest in taking acupuncture treatment for to avoid the post-operative discomfort before next gastroscopy?”
-
Safety assessment
Before participants sign the informed consent form, they will be informed that acupuncture clinical trials may have potential adverse events such as bleeding, hematomas, fainting, bruising, ecchymoma, and possible infection. All details of the adverse event will be recorded by the patients and doctors. Adverse events (AE) are rated as 1 (mild), 2 (moderate), or 3 (severe or medically significant). All details of AEs will be recorded in the case report form (CRF). At the end of the trial, we will analyze the impact of all events.
-
Blinding success assessment
After the gastroscopy procedure, researchers will evaluate the success of the blinding by asking participants the following question: “what kind of treatment do you think you have received?” The options are traditional acupuncture treatment, superficial acupuncture treatment, and uncertain. If participants do not choose “uncertain,” then the researcher will question the patient further for specific reasons.
-
Related to gastroscopy
The duration of the gastroscopy and the number of biopsies will be recorded after operation.
Participant timeline {13}
The timing of intervention and data collection is detailed in Table 4.
Sample size {14}
Hypotheses: We hope to provide conclusive evidence to test the hypothesis that acupuncture is superior than sham acupuncture in reducing the discomfort of patients during gastroscopy. In our pilot study, the discomfort VAS assessment at 30 min post-procedure was 4.8 (SD, 1.76) in the EA group and 6.3 (SD, 1.53) in the SEA group. We used PASS software to calculate that 50 patients would be needed to provide a 90% power to detect a difference of discomfort VAS in 2 groups at a 2-sided significance level of 5%. If we assume that 20% of patients would be lost to follow-up, 60 would need to be enrolled.
Recruitment {15}
Participants of the study will be recruited through the outpatient clinic or inpatient, hospital-based WeChat advertising, and posters in the Shanghai Municipal Hospital. Any patients who are willing to participate will be screened by telephone or on site and consented for the study. There is one specialist who will answer the phone to answer questions from these kinds of disposed patients.
Research assistants will screen the patients, obtain written informed consent, and assign them to either the EA or SEA group, to receive a traditional acupuncture or an acupuncture-like simulation treatment.
Assignment of interventions: allocation
Sequence generation {16a}
In this trial, we plan to use SPSS24.0 software to generate a random number table, which divides eligible participants into the EA group or SEA group with a 1:1 ratio.
Concealment mechanism {16b}
A random distribution card will be made and sealed with an opaque envelope. Participants will be informed that they have an equal chance of being assigned to the EA or SEA group before the trial.
Implementation {16c}
An independent researcher, blinded to the study protocol, will generate the allocation sequence. Another researcher will give the opaque envelope to the acupuncturist according to the timing sequence of participant registration for the trial. Then, the acupuncturist will open the envelope to perform the corresponding acupuncture operation.
Assignment of interventions: blinding
Who will be blinded {17a}
This is a patient-assessor-blinded study. To ensure that participants are blind, they will be treated in an isolated space and be required to wear blindfolds. Only the acupuncturist who performs the treatment will know the group assignment at the time of treatment. Gastroenterologists, data analysts, and statisticians will remain blinded.
Procedure for unblinding if needed {17b}
Unmasking is not needed.
Data collection and management
Plans for assessment and collection of outcomes {18a}
We will record the evaluation results in the CRFs in a timely fashion.
Plans to promote participant retention and complete follow-up {18b}
Patients will come to the hospital 1 week after gastroscopy to complete the final questionnaires. We will follow up and debrief patients at this time point. At the end, patients will receive a transportation subsidy.
Data management {19}
Patient characteristics will be recorded in the CRFs and stored in the researcher’s office. The clinical trial management platform ResMan will be used to manage the raw data. The raw data will be collected by assessors who are blinded to the group assignment and repeated input methods will be used to ensure that the entered data is correct. Before the platform is officially launched, it will be tested and the users will be trained. After the platform is officially launched, the database will be locked with a password, which will only be known by relevant personnel. The clinical director will oversee the work of the clinical trial center at least once a month.
Confidentiality {27}
All the documents and materials related to this will be kept strictly confidential. Only once the trial is finished will the principal investigator distribute the data to the third parties. We will protect the privacy of participants’ personal medical information as required by law. The staff in this study is also bound by this agreement.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable. This trial does not have biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
The independent statistician is responsible for the statistical analysis with spss24.0 software. Patient data that falls off will not be included in the analysis. In the statistical analysis, measurement data between the two groups will be analyzed with the t-test and rank-sum test, while categorical data will be analyzed with χ2 test. Data will be recorded as mean ± standard deviation or median (first quartile, third quartile). The significance level used for statistical analysis will be two-sided with confidence intervals at the 95% level.
Interim analyses {21b}
Not applicable.
Methods for additional analyses (e.g., subgroup analyses) {20b}
Not applicable.
Methods in analysis to handle protocol non-adherence and any statistical methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
We perform statistical analysis on complete case.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
All data sharing will be conducted in accordance with the regulatory requirements.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
In order to control the quality of the clinical trial, the whole process of the trial will be conducted under the supervision of a qualified clinical trial expert in Shanghai Municipal Hospital of Traditional Chinese Medicine. The Clinical Research Center for Drugs of Shanghai University of Traditional Chinese Medicine will conduct the data monitoring. When problems occur in the trial, the center has the right to make the final decision to terminate the trial if necessary. Any changes will be notified in writing to all participants in the trial after approval by the ethics committee. The principle investigator will be fully responsible for conducting the trial and will make any final decisions.
Adverse event reporting and harms {22}
We will record any adverse events such as bleeding, hematomas, fainting, bruising, ecchymoma, and possible infection.
Frequency and plans for auditing trial conduct {23}
We will audit every 3 months; any modification that may have an impact on the study and potential benefit to patients or affect patient safety, including changes of the study objective, study design, patient population, sample size, study procedure, or serious adverse events, will be reported to the Committee. This will be decided by jointly with the monitoring committee, the Clinical Research Center of Drugs, and approved by the Ethics Committee.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
When the trial protocol is modified during the trial, we will conduct a discussion group composed of statisticians, acupuncturists, gastroenterologists, etc. After collective discussion, the next step of the trial plan will be drawn up and the clinical plan updated on the clinical trial registration website.
Dissemination plans {31a}
The publication of the outcomes of this study will provide baseline data. The outcomes may be presented at conferences, symposiums, university classes, etc., if applicable.
Discussion
Acupuncture therapy has been effectively used in clinical practice for relieving symptoms caused by clinical surgery. For example, Wang et al. [15] ADDIN show that acupuncture may reduce the side effect of vomiting during gastroscopy by adjusting the function of vegetative nerves, regulating gastrointestinal tract function, and reducing the concentration of blood 5-HT. Taras I U [16] found that auricular acupuncture (AA) is a promising alternative treatment for situational anxiety. Andrzejowski JC [17] found that acupuncture at the EX-HN3 point reduces preoperative anxiety levels in patients awaiting neurosurgery. However, these existing literatures have inevitable deficits: differing acupuncture intervention method such as using ear acupuncture or intra-dermal needles and a lack of a uniform standard for the setting of intervention time. Some papers have reported that when applied before gastroscopy acupuncture has no apparent differences compared to sham acupuncture [18, 19]. So, in this trial, we used the study by Liu et al. [20], which has been widely used in clinical practice as a successful implementation of sham acupuncture, to rule out the placebo effect of acupuncture in a rigorous way. Studies have shown that superficial acupuncture in non-acupoints method has the advantages of providing a good masking effect and being not easily perceived by the patient and has a higher feasibility of operation. One clinical trial of acupuncture treatment for pain found that the superficial acupuncture at non-acupoints method is an effective way to control for placebo [21]. By designing this RCT, we aim to contribute better evidence for the efficacy of electroacupuncture in reducing discomfort during gastroscopy through an improved design and methods.
For patients, surgical procedures performed in hospitals are a strong stressor and can lead to an anxiety response. Excessive anxiety can affect immune mechanisms through the release of corticosteroids and may be associated with abnormal hemodynamics as a consequence of endocrine changes and activation of the central nervous system [22, 23]. A high level of preoperative anxiety will also reduce patients’ satisfaction with the procedure and increase the risk of postoperative nausea and vomiting [24, 25]. So, in this trial, we will focus on preoperative anxiety. The STAI-S6 and APAIS are reliable means of assessing patient anxiety [26, 27]. By using these questionnaires, we can quickly and accurately assess patients’ anxiety level in a few minutes.
Previous findings have shown that gastroscopy may bring a series of complications to patients with hypertension, such as myocardial infarction, cardiac arrest, and other complications [28]. Some research has shown that in patients older than 45 years, the risk of cardiovascular disease doubles with an increase in systolic blood pressure of 20 mmHg or a diastolic blood pressure increase of 10 mmHg [29, 30]. So, in our study, we will monitor BP before, during, and after gastroscopy, then analyze the correlation between DP and the primary outcome of VAS score to determine whether EA can reduce stress placed on the heart, and thus benefit elderly people with cardiovascular related diseases who need gastroscopy.
There are several limitations in this study. First of all, we did not limit any comorbidities that patients may have. Patients who meet our inclusion criteria and need upper gastrointestinal endoscopy according to the assessment of a gastroenterologist can be included in this trial. Some comorbidities like pharyngitis might increase sensitivity and discomfort when the gastroscope passes through the pharynx. Therefore, we cannot precisely determine the impact of comorbid diseases on the level of anxiety during gastroscopy. Secondly, based on the previous pilot research, the sample size of this RCT include 60 patients, which is still a small-scale study involving a small sample, but the data obtained in this study will support the sample size calculation of future multi-center, large-scale clinical trials. We hope, in the future, the data will have guiding significance for subsequent clinical trials related to acupuncture intervention in gastroscopy. Finally, it is inevitable for the acupuncturist to know the grouping of patients. We hope that this limitation will be offset by the fact that the acupuncturist does not participate in the evaluation of the questionnaire and that the patients are subjected to rigorous blinding practices.
We aim to design a standard and rigorous RCT to reduce discomfort during gastroscopy by acupuncture treatment. We believe acupuncture will increase patient compliance and ease the gastroscopy-related burden on public health services. With positive results, our research will serve as a new standard of care for patients hoping to forego systemic sedation during gastroscopy procedures.
Trial status
We started recruiting participants in February 2021 and the recruitment is expected to end late 2021.
Abbreviations
- VAS:
-
Visual analogue scales
- STAI-S6:
-
The 6-item short form of the State-Trait Anxiety Inventory
- APAIS:
-
Amsterdam Pre-Operative Anxiety and Information Scale
- SpO2:
-
Pulse oxygen saturation
- HR:
-
Heart rate
- BP:
-
Blood pressure
- STRICTA:
-
Standards for Reporting Interventions in Clinical Trials of Acupuncture
References
Januszewicz W, Kaminski MF. Quality indicators in diagnostic upper gastrointestinal endoscopy. Therap Adv Gastroenterol. 2020;13:1756284820916693.
Nishizawa T, Suzuki H. Propofol for gastrointestinal endoscopy. United European Gastroenterol J. 2018;6(6):801–5.
Zhang XL, Lu ZS, Tang P, Kong JY, Yang YS. Current application situation of gastrointestinal endoscopy in China. World J Gastroenterol. 2013;19(19):2950–5.
Wu L, Zhao H, Weng H, Ma D. Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. J Anesth. 2019;33(2):321–35.
Zheng HR, Zhang XQ, Li LZ, Wang YL, Wei Y, Chen YM, et al. Multicentre prospective cohort study evaluating gastroscopy without sedation in China. Br J Anaesth. 2018;121(2):508–11.
Wang HL, Ye F, Liao WF, Xia B, Zheng GR. Unsedated versus sedated gastrointestinal endoscopy: a questionnaire investigation in Wuhan, central China. J Huazhong Univ Sci Technolog Med Sci. 2013;33(6):857–61.
Yuan W, Wang Q. Perioperative acupuncture medicine: a novel concept instead of acupuncture anesthesia. Chin Med J (Engl). 2019;132(6):707–15.
Acar HV. Acupuncture and related techniques during perioperative period: a literature review. Complement Ther Med. 2016;29:48–55.
Yang J, Jiang Y, Chen Y, Sun M, Chen J, Zheng Q, et al. Acupressure the PC6 point for alleviating postoperative nausea and vomiting: a systematic review protocol. Medicine (Baltimore). 2019;98(33):e16857.
Wu MS, Chen KH, Chen IF, Huang SK, Tzeng PC, Yeh ML, et al. The efficacy of acupuncture in post-operative pain management: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150367.
Chen JM, Li DD, Chen YS, et al. The effectiveness of electro-acupuncture combined with dyclonine hydrochloride in relieving the side effects of gastroscopy: a controlled trial[J]. Ann Palliat Med. 2021;10(3):2958.
Dong Y, Liang Z, Xu Z, et al. Effect of wrist-ankle acupuncture on propofol dosage in painless gastroscopy of elderly patients: a randomized controlled trial[J]. Am J Ther. 2020. Online ahead of print.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55.
Wen W, Yang L, Liu S, Zhong Y, Hu X, Huang X, et al. Reporting quality and effect size comparison in randomized controlled trials of bo's abdominal acupuncture using CONSORT statement and STRICTA. J Tradit Chin Med. 2016;36(3):382–91.
Wang SM, Peloquin C, Kain ZN. The use of auricular acupuncture to reduce preoperative anxiety. Anesth Analg. 2001;93(5):1178–80 table of contents.
Wunsch JK, Klausenitz C, Janner H, Hesse T, Mustea A, Hahnenkamp K, et al. Auricular acupuncture for treatment of preoperative anxiety in patients scheduled for ambulatory gynaecological surgery: a prospective controlled investigation with a non-randomised arm. Acupunct Med. 2018;36(4):222–7.
Wiles MD, Mamdani J, Pullman M, Andrzejowski JC. A randomised controlled trial examining the effect of acupuncture at the EX-HN3 (Yintang) point on pre-operative anxiety levels in neurosurgical patients. Anaesthesia. 2017;72(3):335–42.
Melchart D, et al. Integrating patient preferences in clinical trials: a pilot study of acupuncture versus midazolam for gastroscopy. J Altern Complement Med. 2002;8(3):265–74.
Tarcin O, et al. Acustimulation of the Neiguan point during gastroscopy: its effects on nausea and retching. Turk J Gastroenterol. 2004;15(4):258–62.
Liu Z, Liu J, Zhao Y, Cai Y, He L, Xu H, et al. The efficacy and safety study of electro-acupuncture for severe chronic functional constipation: study protocol for a multicenter, randomized, controlled trial. Trials. 2013;14:176.
Mao WC, Liu BY, He LY, Liu ZS, Liu J, Zhao Y. Discussion on existing problems of placebo acupuncture design based on acupuncture analgesia. Zhen Ci Yan Jiu. 2013;38(2):163–7.
Markland D, Hardy L. Anxiety, relaxation and anaesthesia for day-case surgery. Br J Clin Psychol. 1993;32(4):493–504.
Basak F, Hasbahceci M, Guner S, Sisik A, Acar A, Yucel M, et al. Prediction of anxiety and depression in general surgery inpatients: a prospective cohort study of 200 consecutive patients. Int J Surg. 2015;23(Pt A):18–22.
Acar HV, Cuvas O, Ceyhan A, Dikmen B. Acupuncture on Yintang point decreases preoperative anxiety. J Altern Complement Med. 2013;19(5):420–4.
Bae H, Bae H, Min BI, Cho S. Efficacy of acupuncture in reducing preoperative anxiety: a meta-analysis. Evid Based Complement Alternat Med. 2014;2014:850367.
Moerman N, van Dam FS, Muller MJ, Oosting H. The Amsterdam Preoperative Anxiety and Information Scale (APAIS). Anesth Analg. 1996;82(3):445–51.
Mohd Fahmi Z, Lai LL, Loh PS. Validation of the Malay version of the Amsterdam Preoperative Anxiety and Information Scale (APAIS). Med J Malaysia. 2015;70(4):243–8.
Murray AW, Morran CG, Kenny GN, Macfarlane P, Anderson JR. Examination of cardiorespiratory changes during upper gastrointestinal endoscopy. Comparison of monitoring of arterial oxygen saturation, arterial pressure and the electrocardiogram. Anaesthesia. 1991;46(3):181–4.
Amornyotin S, Lertakayamanee N, Wongyingsinn M, Pimukmanuskit P, Chalayonnavin V. The efficacy of intravenous sedation in diagnostic upper gastrointestinal endoscopy. J Med Assoc Thai. 2007;90(2):301–6.
Zhao P, Xiao SM, Tang LC, Ding Z, Zhou X, Chen XD. Proximal gastrectomy with jejunal interposition and TGRY anastomosis for proximal gastric cancer. World J Gastroenterol. 2014;20(25):8268–73.
Acknowledgements
We would like to thank Dr. Lao for guiding experimental design. Shifen Xu provided general support as the head of the Acupuncture Department in Shanghai Municipal Hospital of Traditional Chinese Medicine and was responsible for the design of this RCT. Thanks for Gastroscope Center of Shanghai Municipal Hospital of Traditional Chinese Medicine.
Authors’ contributions {31b}
Shifen Xu and Binyu Yu conceived the design of the trial and Lixing Lao revised it. Philippa Jemma Hazlewood and Xuan Yin participated in the design of the statistical analysis. Shanshan Li, Hongyu Yue, Kun Xu, and Yiqun Mi helped in the recruitment of patients. Binyu Yu, Philippa Jemma Hazlewood, and Xuan Yin helped in drafting the paper and quality control. All authors read and approved the final draft of the paper.
Funding {4}
This research is supported by the Shanghai Hospital Development Center (grant SHDC2020CR3015A).
Availability of data and materials {29}
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate {24}
This trial has been approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (2020SHL-KY-11) with the Helsinki Declaration. Each participant will be clearly aware of the purpose, procedure, and the potential risk of this trial before enrollment, then give us their written informed consent.
Consent for publication {32}
Not applicable
Competing interests {28}
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, B., Hazlewood, P.J., Yin, X. et al. Effect of electroacupuncture on discomfort during gastroscopy: a study protocol for a randomized controlled trial. Trials 23, 364 (2022). https://doi.org/10.1186/s13063-022-06165-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06165-4