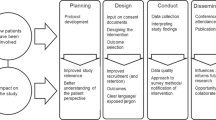
Abstract
Background
Sharing trial results with participants is a moral imperative, but too often does not happen in appropriate ways.
Methods
We carried out semi-structured interviews with patients (n = 13) and site staff (n = 11), and surveyed 180 patients and 68 site staff who were part of the Show RESPECT study, which tested approaches to sharing results with participants in the context of the ICON8 ovarian cancer trial (ISRCTN10356387). Qualitative and free-text data were analysed thematically, and findings used to develop the SHOW RESPECT adaptable framework of considerations for planning how to share trial results with participants. This paper presents the framework, with illustrations drawn from the Show RESPECT study.
Results
Our adaptable ‘SHOW RESPECT’ framework covers (1) Supporting and preparing trial participants to receive results, (2) HOw will the results reach participants?, (3) Who are the trial participants?, (4) REsults—what do they show?, (5) Special considerations, (6) Provider—who will share results with participants?, (7) Expertise and resources, (8) Communication tools and (9) Timing of sharing results. While the data upon which the framework is based come from a single trial, many of our findings are corroborated by findings from other studies in this area, supporting the transferability of our framework to trials beyond the UK ovarian cancer setting in which our work took place.
Conclusions
This adaptable ‘SHOW RESPECT’ framework can guide researchers as they plan how to share aggregate trial results with participants. While our data are drawn from a single trial context, the findings from Show RESPECT illustrate how approaches to communication in a specific trial can influence patient and staff experiences of feedback of trial results. The framework generated from these findings can be adapted to fit different trial contexts and used by other researchers to plan the sharing of results with their own participants.
Trial registration
ISRCTN96189403. Registered on February 26, 2019. Show RESPECT was supported by the Medical Research Council (MC_UU_12023/24 and MC_UU_00004/08) and the NIHR CRN.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Late-phase randomised controlled trials often require hundreds or thousands of participants to detect meaningful differences in outcomes. In order to successfully answer their research questions, trialists must recruit volunteers to take part, often asking participants to accept risk and/or inconvenience, with the aim of improving treatment, care or prevention of disease for future patients.
Sharing results with trial participants is an ethical imperative [1] and is recommended by authorities that govern the conduct of clinical trials. The World Medical Association’s Declaration of Helsinki, which outlines the principles for ethical conduct of medical research involving human participants, states that ‘all medical research subjects should be given the option of being informed about the general outcome and results of the study’ [2]. In the UK, the Health Research Authority (HRA) recently published guidance saying that participants have the right to know the findings of research in which they have taken part, and that sharing results directly with participants can help ‘build trust, show respect and helps participants feel valued’ [3].
There is evidence from a broad array of studies that most participants want to be offered the opportunity to receive trial results, ranging from 88 to 98% in studies conducted across a range of diseases (cancers, idiopathic scoliosis, internal derangement of the knee, HIV) and geographical settings (including USA, UK, Canada and Uganda) [4,5,6,7,8].
Despite the moral obligation and clear demand from most participants to receive results, in practice, sharing results often does not happen, or is not done well. The UK HRA research transparency report in 2021 states that ‘90% of clinical trials have not told participants about findings’ [9]. A survey conducted in 2016 of authors of clinical trial results papers published in 2014–2015 found that only 27% of respondents reported disseminating results to participants, with only a further 13% planning to do so [10]. Even when it does happen, it may not be done in a way that participants can understand. The survey found that 40% of authors who had shared results with participants had shared academic publications, which are not written in a way that is easy for participants to understand [10]. Previous studies have reported that many participants struggle to understand trial results which are shared with them. For example, a study within the context of a breast cancer trial found that only 56% of participants said the results letter was easy to understand [11], and a survey of cancer trial participants found fewer than half reported fully understanding the results [7].
Sharing results with participants is a complex issue, with trialists facing considerable challenges including practical [10, 12,13,14,15,16,17] and resource barriers [10, 12,13,14,15, 18, 19] and concerns about the emotional impact of sharing results [4, 7, 11, 12, 14, 19,20,21,22]. It is important that it is done well, as there is potential for harm [13, 23,24,25]. Little work has been done to compare approaches to sharing results with participants. Show RESPECT assessed approaches to sharing results in a cluster randomised factorial trial, comparing an enhanced versus basic webpage; mailed printed summary versus no printed summary; and email list invitation versus no email list invitation, within the context of an ovarian cancer trial (ICON8) [26]. A major finding was that the mailed printed summary significantly improved patient satisfaction with how results were shared compared to a webpage with or without an email list invitation, without the printed summary [26]. It also showed that these approaches were feasible for site staff to implement [27]. However, Show RESPECT was carried out within only a single trial, in a particular clinical and geographical setting, with a particular set of results to communicate, so the generalisability of these results is unclear. It is likely that there is no one-size-fits-all approach to sharing results, so it is important that trial teams draw on guidance that offers a sound structure that can be adapted to fit the specific context and requirements of their own trial and trial participants.
One of the aims of the Show RESPECT study was to develop guidance for trialists, based on our results. We realised that findings from Show RESPECT [26, 27] could be used to derive an adaptable framework of factors for trialists to consider when planning how to share results with trial participants. This paper presents the framework, based on and illustrated with qualitative insights from data collected from site staff and patients who took part in the Show RESPECT study.
Methods
Show RESPECT was a mixed methods study, comprised of a factorial cluster randomised controlled trial within a trial to assess multiple approaches to communicating trial results, and an embedded explanatory qualitative study. The full protocol for the study is available online [28]. Results from Show RESPECT with regard to participant satisfaction with how the results were shared, and the resources required from sites and the clinical trials unit (CTU) to implement these approaches, have been reported previously [26, 27] and the qualitative results reported in this paper have been published as part of a doctoral thesis [29]. Show RESPECT took place within the ICON8 ovarian cancer chemotherapy trial (ISRCTN10356387) [30]. As the methods have been reported previously, we do not duplicate this information in this article, but have included it as Additional File 1. This paper reports qualitative results from data collected from trial participants and site staff who had been involved in sharing or receiving results. The Standards for Reporting Qualitative Research checklist for this paper can be found in Additional File 2.
Information about patient and public involvement in this study and the context of the ICON8 trial is available in Additional File 1.
Qualitative data collection
The main source of qualitative data for Show RESPECT was semi-structured interviews with ICON8 patients and site staff who had been involved in sharing the ICON8 results with patients. In addition to the qualitative interviews, qualitative data were collected by free-text questions on the questionnaires that were completed by patients (after receiving results) and site staff (immediately after sharing results and 2–3 months later). The topic guide, questionnaires, details of how these were administered, and researcher characteristics and reflexivity can be found in our previous publications [26, 27, 29, 31]. Further information about our qualitative data collection is available in Additional File 1.
Sampling and participants
We used a purposive sampling approach for the semi-structured interviews with both participants and site staff, allowing us to collect data from respondents with a range of characteristics that may be related to their experiences and views on sharing results. For ICON8 patients, this included age, education level, frequency of internet use and reported satisfaction with how the ICON8 results were shared, while for site staff this included their role and number of ICON8 patients at the hospital at which they work. For both groups, we included which interventions their hospital had been randomised to. Further information about our sampling approach and participants is available in Additional File 1.
Qualitative analysis
We used a reflexive thematic analysis approach [32], with a critical realist stance (taking the ontological position that an external reality exists that is independent of our beliefs and understanding, but that our knowledge of that external reality is influenced by our historical, social and cultural situation), to analyse the data. The findings reported in this paper are further findings from the analysis carried out for our previous papers [26, 27], rather than a separate re-analysis of the Show RESPECT data. Further details of our analysis methods are available in Additional File 1.
Developing the framework
We shared our findings around what influenced the experience of patients and site staff around receiving/sharing trial results at a patient and public involvement meeting with women who were taking part in ovarian cancer treatment trials. We held meetings with site staff who had been involved in sharing the ICON8 results with participants, and met with the ICON8 trial team. We also held seminars at three clinical trials units and presented our findings at a clinical trials conference. At these meetings, we discussed with these key stakeholders the implications our results, how they might be transferred to trials in other settings, and recommendations they would make for future trials. Based on the themes, sub-themes and high-level codes from our data, and the stakeholder discussions, we developed a long list of considerations that we believe trial teams should take into account when planning how to share results with trial participants, either because it came from our qualitative data, or was raised as an additional consideration during our discussions with stakeholders. We grouped related considerations together into categories and organised the categories so the initials spell a memorable phrase (SHOW RESPECT).
To explore how useful the framework was for a trial that was very different to the ICON8 trial in which we carried out Show RESPECT, we applied it to CHAPAS-4 (ISRCTN 22964075) [33]. This was done by a single researcher (AS) who worked with the study teams and (for CHAPAS-4) community representatives to consider the factors identified in the framework, and how they affect the communication of results to participants for these trials. AS had been involved in CHAPAS-4 from the proposal development stage, so was familiar with the study.
Results
A description of the patient and site staff participants in Show RESPECT is reported in our previous papers [26, 27]. A table showing a summary of their characteristics can be found as Additional File 3.
The adaptable framework of factors trialists should consider when planning how to share results with trial participants is shown in Table 1, with illustrative quotes from the Show RESPECT data. An editable template with the adaptable framework can be found online [34]. The framework covers supporting and preparing participants to receive results; how the communications tools will reach participants; who the trial participants are; what the results show; special considerations; who will provide results to participants; the expertise and resources the trial team have access to for sharing results; which communication tools will be used; and timing of results communication. Additional File 4 shows how the framework items relate to the qualitative themes, sub-themes and high-level codes from Show RESPECT. Additional File 5 illustrates these factors with findings from the qualitative interviews conducted during Show RESPECT.
Consideration needs to be given to supporting and preparing participants to receive trial results. This includes what participants are told when they join the trial, and immediately before receiving the trial results. It also includes how participants will be able to access support around dealing with the emotional aspects of processing the trial results, and finding answers to questions they have about the results and their implications. Patients in ICON8 differed in the extent to which they felt comfortable asking site staff for more information or clarification, and their confidence in searching for health information from other sources, such as online. Some patients were part of local support groups for people with cancer, whereas others felt they received sufficient support from family and friends. Still other patients were neither linked to support groups, nor had family or friends they could talk to about their cancer. In this context, both patients and site staff felt that links to further information and support might be useful for some patients (even if not themselves), particularly those with less access to support with processing the results.
Thought also needs to be given to how the communication tool(s) will reach participants, and the accessibility needs of your patient population. Alongside the question of how the results will reach participants is the question of where. Receiving results in the clinic may make support and clarification more easily available but provides less privacy and time for processing the results than patients receiving them at home.
Participant characteristics may affect the appropriateness of different communication approaches. The people taking part in the ICON8 trial were women with an average age of 67 by the time results were available. Four in ten of them used the internet and email less than daily [26]. In this context, printed summaries were viewed as being easy to access for all participants (including older participants and those who are not confident computer users). Other patient characteristics that may affect results communication include education level and health literacy. Non-written forms of communication (such as videos) may be useful for those who do not like to read. Consideration should also be given to what participants are likely to want to do with the results. Many patient interviewees kept folders with all the information they received about the ICON8 trial, to allow them to refer to it for future reference. Printed summaries of the results facilitated this, while email or webpages required printing. Printed summaries also made it easier to share results with others, such as family and friends.
The nature of the trial and its results also affects how results should be shared. ICON8 found no difference between the different chemotherapy schedules tested. In some ways, this made it easier for some patients to receive the results, as although they were disappointed that the trial did not find an improvement in treatment of ovarian cancer, no one was allocated to an inferior arm. The approaches used to share results in Show RESPECT were felt to be appropriate in this context. If the results had been different, with a clear difference between the arms, some patients and site staff felt that there may have been a need to communicate results to people in the group that had done less well overall in a more personal way. This may be less important in trials for less severe conditions than ovarian cancer, where participants have less riding on the results. Similarly, if the results are complex, they may need personal discussion to help patients to understand. One item in the framework that came from engaging with stakeholders rather than directly from the Show RESPECT data was that of ‘special considerations’ that need to be taken into account, such as if the trial had closed early, or experienced adverse media coverage. In ICON8, some patients wanted explanations on why the ICON8 results differed from those of previous similar trials in different settings. Patient and public involvement in the design of tools and processes is essential.
Communication of results takes place within the context of relationships that have developed over the course of the trial between patients and site staff. Participants in ICON8 have been in follow-up for 5 to 8 years, with regular clinic visits during that time. At sites where participants were seen by the same site staff each time, many developed close relationships, almost friendships. Where this was the case, site staff felt uncomfortable sharing the results without some degree of personalisation, so some added personalised cover notes, or called participants to let them know the study results were about to be disseminated. Communication of trial results should consider the strength of relationships developed between site staff and patients, for example allowing a degree of personalisation of how the results are shared where these relationships are close. Some staff at the largest sites did not know participants so well and felt uncomfortable telephoning patients out of the blue. There may be less need for personalisation in trials with shorter follow-up, or with follow-up that does not involve face-to-face visits with consistent staff over time, or when results come from staff other than those who had developed relationships with participants.
When considering the expertise and resources needed for sharing results with participants, thought needs to be given to the skills, staff time and budget needed for the development of the information product (e.g. writing the content in plain language, patient and public involvement, scientific review to ensure the summary is accurate, and technical skills required for the chosen communications tools); distribution of the results (e.g. site staff time for posting information, costs of distribution [e.g. printing, postage]) and supporting participants and dealing with queries. Our previous report describes the resources required from sites and the clinical trials unit for sharing the results in the ways tested in Show RESPECT [27]. Budget or staff time limitations may rule out certain approaches to sharing results, if they have not been included as part of the initial budget for and funding of the trial.
Choice of communication tools will be influenced by the factors described above. Patient and public involvement is important in helping to make these decisions. In addition to deciding what type of communication tool(s) to use, consideration needs to be given to the content of that tool. It should include the language(s) used and the appropriate reading level for the target audience (if a written tool is used). It should also include consideration of how to make the information attractive and easy to use (which may require input from design specialists). It may be appropriate to offer participants a choice of communication tools, possibly with different levels and forms of information.
The final factor for consideration is timing—when should the results be communicated to participants? This will depend on the point at which the research team are confident that the messages for participants are unlikely to change, and whether (and when) the results are likely to receive media or social media attention, to avoid participants finding out the results from others before hearing from the trial team. It should take into account when results will be released to other audiences (e.g. via conferences, peer-reviewed publications and public trial databases and registries), and associated embargoes and deadlines (such as the European Medicines Authority requirement to post a summary of results within a certain time period from the end of the trial).
Practical examples of the application of the framework to two very different trials can be found in Table 2: the ICON8 ovarian cancer trial in which Show RESPECT was conducted, and the CHAPAS-4 paediatric HIV treatment trial, which was conducted in Uganda, Zambia and Zimbabwe.
Discussion
Summary of key findings
We propose several considerations when planning how to share results with participants in clinical trials. This includes how participants will be prepared and supported when receiving results and how the communication tool(s) will reach participants. Participant-related factors, such as demographics, education levels and computer literacy, alongside their health and expectations around receiving results, must also be considered. The trial results themselves (whether they will be considered as good, bad or neutral news by some or all participants, and their complexity) also need to be taken into account. Trials with more complex or potentially upsetting results (e.g. where the participants allocated to an arm did less well than participants allocated to other arms, or where one sub-group did less well than others) may need to offer participants additional support, for example through sharing results face-to-face or in individual video calls, or offering follow-up appointments or phone calls with doctors or research nurses if results are shared via written summaries. Trial results communication must also consider whether participants have developed relationships with site staff over the course of their participation, and how and from whom they are used to receiving communication about the trial. It may be appropriate to reflect this in some way, for example through allowing personalisation or one-to-one communication. The expertise and resources available to trial teams to communicate trial results is an important factor when deciding how this is done. Any communication tools used must reflect what the participants are likely to want to know and be understandable (using appropriate language(s) and reading levels) and accessible to the intended audience. It may be appropriate to provide a choice of tools, as different participants are likely to have different preferences and needs. The timing of when results are shared also needs to be carefully considered, avoiding participants finding out results from other sources prior to being informed by the trial team, if possible. Considering these factors, and involving patients and the public, can help develop communication tools and processes that are appropriate to the trial context, population and messages.
Strengths of this study
A key strength of this study is its integration of qualitative data from both site staff and ICON8 trial participants, giving us insight into the views of those who are responsible for sharing results, alongside those who have experienced receiving trial results. Many of the site staff who took part in Show RESPECT worked across many trials and were able to draw on their experience from other studies in addition to Show RESPECT. The qualitative data provide a rich understanding of the perspectives of ICON8 trial participants and site staff on the experience of receiving or sharing trial results. Applying an established theoretical model (the Information Seeking and Communication Model [35, 36]) increased our ‘information power’ [37], through synthesising existing knowledge, extending the sources of knowledge beyond our empirical data and explaining relations between different aspects of the empirical data in a coherent way [37]. Applying the model helped us to ground our conclusions in the context of existing knowledge about the process of information seeking and communication.
Discussion of our findings with key stakeholders working on a wide variety of trials allowed us to ensure the framework is applicable beyond the ovarian cancer setting. The applicability of our framework to very different trials is illustrated by the example of the CHAPAS-4 trial, shown in Table 2. Applying the framework was helpful for thinking through how to share results with participants in CHAPAS-4. The answers to the individual questions were very different from those for ICON8, and very different communication approaches were selected, but the considerations were all relevant. While the framework does not directly prescribe how to share results, having a structured framework to follow gave confidence that nothing important had been overlooked. We envisage the framework being most useful as the basis of discussion of ideas and plans between members of the trial team and patient representatives.
Limitations of this study
Show RESPECT was carried out within the context of a single trial, a limitation of this study, raising questions about the transferability of the findings to trials with different patient populations, diseases, results scenarios and settings. We acknowledge this possibility and emphasise that in this paper we focus on exploring factors that trialists should consider when preparing to share results with participants, rather than recommending that the approach that worked best within Show RESPECT should be used in trials with very different contexts or patient populations. We further acknowledge that we were not able to take account of ethnicity of respondents, nor on factors such as socio-economic background, as these data were not collected for this study. Very few of our patient participants reported having a first language other than English, but patient participants did report widely varying education levels.
The development of the framework was not a formal deliberative process—the framework is an output from our research that we believe will be of value to other researchers. However, we acknowledge that, given it is largely informed by evidence from a single trial, there may be considerations that we have missed that might be important for other trial contexts. We see this as being a starting point for improving practice in this area, but recognise that further refinement of the framework may be needed after it has been applied in a wide variety of trials. We invite readers to send us feedback around their experience of using the framework and will consider revising it in the future if further important considerations are identified or improvements need to be made.
Our results in the context of what was already known
Our adaptable framework of factors to consider when planning how to share results is similar to guidance released by the UK Health Research Authority in 2023 [3], after the Show RESPECT patient results had been published. These similarities are unsurprising, given that the results of Show RESPECT helped inform this guidance, and several authors of this paper were involved in developing them. The HRA guidance on what to consider covers:
-
a)
Who will receive the findings
-
b)
How you will communicate the findings
-
c)
Giving participants a choice
-
d)
Responsibility for communicating findings
-
e)
Exceptions
-
f)
When to communicate findings
-
g)
Evaluating your communication [3]
Our findings around giving participants a choice over whether to receive the results or not reinforces previous recommendations that a two-stage approach should be used, offering results and then providing them, rather than simply distributing results to all participants [38]. Choosing not to access results was, for some patients, a way of protecting themselves from potentially finding out that they missed out on the better treatment. This concept of people choosing what information to engage with or not as a protective mechanism is similar to findings from the BRACELET study, where some bereaved parents of babies who died while participating in a trial for very high-risk neonates advised that communication from the trial should be managed in a way that would suit any parents who felt that they might be upsetting for themselves or their partner [39].
Only by providing information in a way that is understandable to the intended audience can we meet the objectives of sharing research results. Care needs to be taken when preparing results summaries, to ensure they are comprehensible for participants. Previous research has found that much written information about clinical trials exceeds the average reading age [40]. The UK National Health Service Digital Service Manual style guide states that they aim for a reading age of 9–11 years old where possible [41]. Artificial intelligence can be used to help researchers produce plain language summaries, but these will still require review from both investigators and patient representatives to ensure the content is correct and appropriate for the intended audience.
Many of our findings align with findings from the RECAP study [42, 43], supporting the transferability of our framework to trials beyond the UK ovarian cancer setting in which our work took place.
Further research
Further research involving participants and site staff receiving and sharing trial results in trials with different patient populations, trial characteristics and results scenarios would be valuable for exploring the transferability of our findings to other contexts. Research is also needed to address how demographic factors such as geographical location, socio-economic status, ethnicity and different levels of language proficiency influence how results should be shared with participants.
Conclusion
To ensure that trials meet their moral obligations to participants to share trial results, trialists must consider how results should be shared with participants from the planning stage of trials, to ensure that adequate resources are budgeted for and included in agreements with sites. Relevant information about how results will be shared should be included in the Patient Information Sheet. When deciding how to share results with participants, trialists should consider the following factors: how to support and prepare participants to receive results, including whether to use an opt-in or opt-out approach and who will be available to answer participant questions; how the results will reach participants; the characteristics and expectations of participants in relation to the results; what the results show and how they are likely to be perceived by participants; special considerations; who will provide the results to participants; the expertise and resources available for sharing results; the communication tool(s) to be used; and the timing of results communication. Patient and public involvement is essential for planning how to share results with participants, identifying the outcomes and study results that are important and relevant to participants, and developing the content of results summaries to ensure they are written in a clear and sensitive manner.
Availability of data and materials
The protocol is available on the MRC CTU website https://www.mrcctu.ucl.ac.uk/media/1980/show-respect_protocol_v30_20aug2018_clean.pdf. The data that underlie the results reported in this article, after de-identification, will be available beginning 12 months after publication following the CTU’s standard moderated access approach (details of which are available https://www.mrcctu.ucl.ac.uk/our-research/other-research-policy/data-sharing/). Applicants will need to state the aims of any analyses and provide a methodologically sound proposal. Applications should be directed to mrcctu.datareleaserequest@ucl.ac.uk. Data requestors will need to sign a data access agreement at an institutional level.
Abbreviations
- BRACELET:
-
Bereavement and Randomised Controlled Trials
- CHAPAS-4:
-
Children with HIV in Africa—Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens
- CTU:
-
Clinical trials unit
- HIV:
-
Human immunodeficiency virus
- HRA:
-
Health Research Authority
- ICON8:
-
An international phase III randomised trial of dose fractionated chemotherapy compared to standard three weekly chemotherapy, following immediate primary surgery or as part of delayed primary surgery, for women with newly diagnosed epithelial ovarian, fallopian tube or primary peritoneal cancer
- ISCM:
-
Information Seeking and Communication Model
- PPI:
-
Patient and public involvement
- RECAP:
-
REporting Clinical trial results Appropriately to Participants
- Show RESPECT:
-
Show Results to Participants Engaged in Clinical Trials
- UK:
-
United Kingdom
- USA:
-
United States of America
References
Taylor J. Reporting research findings to participants is an ethical imperative. BMJ. 2019;367:l6324.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Health Research Authority. Communicating study findings to participants: guidance: Health Research Authority. 2023 [Available from: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/research-transparency/informing-participants/communicating-study-findings-participants-guidance/.
Partridge AH, Wong JS, Knudsen K, Gelman R, Sampson E, Gadd M, et al. Offering participants results of a clinical trial: sharing results of a negative study. Lancet. 2005;365(9463):963–4.
Donaldson S, Khetani N, Maniatis G, Stephens D, Wright JG. Sharing clinical trial results with adolescent idiopathic scoliosis patients. J Pediatr Orthop. 2009;29(5):467–75.
Brealey S, Andronis L, Dennis L, Atwell C, Bryan S, Coulton S, et al. Participants’ preference for type of leaflet used to feed back the results of a randomised trial: a survey. Trials. 2010;11:116.
Elzinga KE, Khan OF, Tang AR, Fernandez CV, Elzinga CL, Heng DY, et al. Adult patient perspectives on clinical trial result reporting: a survey of cancer patients. Clin Trials. 2016;13(6):574–81.
Baylor A, Muzoora C, Bwana M, Kembabazi A, Haberer JE, Matthews LT, et al. Dissemination of research findings to research participants living with HIV in rural Uganda: challenges and rewards. PLoS Med. 2013;10(3):e1001397.
Health Research Authority. Make it public: research transparency annual report 2021. London: Health Research Authority; 2021.
Schroter S, Price A, Malički M, Richards T, Clarke M. Frequency and format of clinical trial results dissemination to patients: a survey of authors of trials indexed in PubMed. BMJ Open. 2019;9(10):e032701.
Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, et al. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Res Treat. 2009;115(1):123–9.
MacNeil SD, Fernandez CV. Attitudes of research ethics board chairs towards disclosure of research results to participants: results of a national survey. J Med Ethics. 2007;33(9):549–53.
Dixon-Woods M, Tarrant C, Jackson CJ, Jones DR, Kenyon S. Providing the results of research to participants: a mixed-method study of the benefits and challenges of a consultative approach. Clin Trials. 2011;8(3):330–41.
Fernandez CV, Kodish E, Shurin S, Weijer C. Offering to return results to research participants: attitudes and needs of principal investigators in the Children’s Oncology Group. J Pediatr Hematol Oncol. 2003;25(9):704–8.
Aldinger C, B B, Collyar D, Li R, Myers L. MRCT Return of Results Guidance Document: The Multi-regional Clinical Trials Center of Brigham and Women's Hospital and Harvard; 2017. Version 3.1. Available from: https://mrctcenter.org/wp-content/uploads/2023/04/2017-12-07-MRCT-Return-of-Aggregate-Results-Guidance-Document-3.1.pdf.
Rigby H, Fernandez CV. Providing research results to study participants: support versus practice of researchers presenting at the American Society of Hematology annual meeting. Blood. 2005;106(4):1199–202.
Hinds PS. Sharing our research findings with study participants. Cancer Nurs. 2008;31(3):173–4.
Macneil SD, Fernandez CV. Informing research participants of research results: analysis of Canadian university based research ethics board policies. J Med Ethics. 2006;32(1):49–54.
Partridge AH, Winer EP. Informing clinical trial participants about study results. JAMA, J Am Med Assoc. 2002;288(3):363–5.
Partridge AH, Hackett N, Blood E, Gelman R, Joffe S, Bauer-Wu S, et al. Oncology physician and nurse practices and attitudes regarding offering clinical trial results to study participants. J Natl Cancer Inst. 2004;96(8):629–32.
Di Blasi Z, Kaptchuk TJ, Weinman J, Kleijnen J. Informing participants of allocation to placebo at trial closure: postal survey. BMJ. 2002;325(7376):1329–31.
Cox K, Moghaddam N, Bird L, Elkan R. Feedback of trial results to participants: a survey of clinicians’ and patients’ attitudes and experiences. Eur J Oncol Nurs. 2011;15(2):124–9.
Dalal H, Wingham J, Pritchard C, Northey S, Evans P, Taylor RS, Campbell J. Communicating the results of research: how do participants of a cardiac rehabilitation RCT prefer to be informed? Health Expect. 2010;13(3):323–30.
Dixon-Woods M, Jackson C, Windridge KC, Kenyon S. Receiving a summary of the results of a trial: qualitative study of participants’ views. BMJ. 2006;332(7535):206–10.
Snowdon C, Garcia J, Elbourne D. Reactions of participants to the results of a randomised controlled trial: exploratory study. BMJ. 1998;317(7150):21–6.
South A, Joharatnam-Hogan N, Purvis C, James EC, Diaz-Montana C, Cragg WJ, et al. Testing approaches to sharing trial results with participants: the Show RESPECT cluster randomised, factorial, mixed methods trial. PLoS Medicine. 2021;18(10). https://doi.org/10.1371/journal.pmed.1003798.
South A, Bailey J, Bierer BE, Burnett E, Cragg WJ, Diaz-Montana C, et al. Site staff perspectives on communicating trial results to participants: cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial. Clinical Trials. 2023. https://doi.org/10.1177/17407745231186088.
Show RESPECT team. Show RESPECT: Show RESults to Participants Engaged in Clinical Trials: a cluster randomised factorial trial of different modes of communicating results to participants of the ICON8 phase III ovarian cancer trial London. 2018. Available from: https://www.mrcctu.ucl.ac.uk/media/1980/show-respect_protocol_v30_20aug2018_clean.pdf. Cited 2021.
South A. Showing RESPECT: a mixed methods study into communicating the results of a phase III clinical trial to trial participants. London: UCL; 2023.
Clamp AR, James EC, McNeish IA, Dean A, Kim JW, O’Donnell DM, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019;394(10214):2084–95.
South A. Show RESPECT: OSF. 2023 [Available from: https://osf.io/6tpf4/?view_only=10add9b2f1b34814a400e329988d993d.
Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exerc Health. 2019;11(4):589–97.
Registry I. ISRCTN22964075: children with HIV in Africa – pharmacokinetics and acceptability of simple second-line antiretroviral regimens. London: BioMed Central. 2018 [Available from: https://www.isrctn.com/ISRCTN22964075.
South A, Snowdon C, Burnett E, Bierer BE, Gillies K, Isaacs T, Sydes MR. The SHOW RESPECT Adaptable Framework of considerations for planning how to share trial results with participants: template for trialists version 1.0: OSF; 2024. Available from: https://osf.io/9hwmx/?view_only=1fed4585370e4f0a9d43a79d4940cf97. Cited 2024 01/02/2024.
Robson A, Robinson L. The Information Seeking and Communication Model. J Doc. 2015;71(5):1043–69.
Robson A, Robinson L. Building on models of information behaviour: linking information seeking and communication. J Doc. 2013;69(2):169–93.
Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2015;26(13):1753–60.
Partridge AH, Winer EP. Sharing study results with trial participants: time for action. J Clin Oncol. 2009;27(6):838–9.
Snowdon C, Brocklehurst P, Tasker R, Ward Platt M, Harvey S, Elbourne D. Death, bereavement and randomised controlled trials (BRACELET): a methodological study of policy and practice in neonatal and paediatric intensive care trials. Health Technol Assess. 2014;18(42):1–410.
Isaacs T, Murdoch J, Demjén Z, Stevenson F. Examining the language demands of informed consent documents in patient recruitment to cancer trials using tools from corpus and computational linguistics. Health. 2022;26(4):431–56.
National Health Service. Content style guide: how we write. UK: National Health Service. 2019. Available from: https://service-manual.nhs.uk/content/how-we-write. Cited 2021 22/12/2021.
Bruhn H, Campbell M, Entwistle V, Humphreys R, Jayacodi S, Knapp P, et al. What, how, when and who of trial results summaries for trial participants: stakeholder-informed guidance from the RECAP project. BMJ Open. 2022;12(3):e057019.
Bruhn H, Cowan EJ, Campbell MK, Constable L, Cotton S, Entwistle V, et al. Providing trial results to participants in phase III pragmatic effectiveness RCTs: a scoping review. Trials. 2021;22(1). https://doi.org/10.1186/s13063-021-05300-x.
Acknowledgements
We are very thankful to the people who participated in this study and the women who contributed to our patient and public involvement activities. We are very grateful for the work of the Show RESPECT site teams in carrying out this study. We thank the members of the Show RESPECT study management group and steering group who are not authors on this manuscript: Andrew Copas, Cara Purvis, Nalinie Joharatnam-Hogan, Carlos Diaz-Montana, Archie Macnair, William J. Cragg and Conor Tweed. We thank the ICON8 trial team for their support of this project, particularly Andrew Clamp, Babasola Popoola, Francesca Schiavone, Jonathan Badrock and Rick Kaplan. We also thank the CHAPAS-4 trial team for allowing us to use CHAPAS-4 as an illustration of the application of our framework.
Funding
The Show RESPECT study was funded by the Medical Research Council through core grants to MRS at the MRC CTU at UCL for Trial Conduct Methodology (MC_UU12023/24 and MC_UU_00004/08) https://mrc.ukri.org/. The funder had no role in the study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the article for publication. All authors had full access to the study data, including statistical reports and tables, and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
AS was responsible for the conception of the study, the acquisition and analysis of the data, and the drafting of the manuscript. MS was responsible for the acquisition of funding. All authors contributed to the design of the study, the interpretation of the data and substantively revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study obtained ethics approval from the London-Chelsea Research Ethics Committee, MREC number 18/LO/1011 (Additional File 6). Participants who were interviewed gave written informed consent, while questionnaires contained an embedded informed consent element, with completion and return of the questionnaire taken to indicate consent to use the data has been given. Patient interviewees received a £20 voucher to thank them for their participation. Site staff received no financial incentive to take part in the interviews. Consent to participate in the interviews included consent for use of the data collected to be used for other research in the future.
Consent for publication
Not applicable.
Competing interests
We have read the journal’s policy and the authors of this manuscript have the following competing interests: AS, CS, EB, KG, TI and BEB have nothing to declare. BEB reports receiving consulting fees from Lilly outside the submitted work. MRS reports grants from Clovis; grants and non-financial support from Astellas, Janssen, Novartis, Pfizer and Sanofi; and personal fees from AstraZeneca, Lilly Oncology and Janssen, outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13063_2024_8291_MOESM1_ESM.docx
Additional file 1: Description of methods. Text file describing the methods for this work (duplicated from our previous publications).
13063_2024_8291_MOESM3_ESM.docx
Additional file 3: Characteristics of qualitative interviewees. Table showing the characteristics of qualitative interviews (this data has been previously published).
13063_2024_8291_MOESM4_ESM.docx
Additional file 4: The SHOW RESPECT adaptable framework concepts, and related themes, sub-themes and high-level codes from the Show RESPECT qualitative data. Table showing the concepts from the adaptable framework, and the themes, sub-themes and high-level codes that relate to those concepts.
13063_2024_8291_MOESM5_ESM.docx
Additional file 5: Illustration of the framework with findings from Show RESPECT. Qualitative findings from the Show RESPECT study that illustrate concepts from the SHOW RESPECT framework.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
South, A., Snowdon, C., Burnett, E. et al. The SHOW RESPECT adaptable framework of considerations for planning how to share trial results with participants, based on qualitative findings from trial participants and site staff. Trials 25, 467 (2024). https://doi.org/10.1186/s13063-024-08291-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-024-08291-7