Abstract
Background
Low English language literacy is a common barrier to participation in clinical trials. Patient information leaflets (PILs) used in clinical trials are often lengthy, complex and have poor readability; this is a persistent and prevalent problem common to trials across the world. Simplifying the information provided in PILs can lead to improved understanding, comprehension and knowledge.
The aim of this project was to develop recommendations for developing accessible PILs for clinical trials through a literature review of published and grey literature and co-working with marginalised communities, patients, and health and social care charities.
Methods
A literature review of MEDLINE, Embase and online resources was conducted, and recommendations for developing accessible PILs were extracted from eligible published and grey literature. Grey literature which contained insights into more inclusive forms of communication was also identified and summarised. Meetings were held with two racially marginalised community groups, two groups involving autistic adults and/or adults with learning difficulties and a patient advisory group. Examples of accessible PILs were shared and discussions held about the content and format of the PILs and suggestions for changes/improvements. National Voices, a coalition of health and social care charities in England, held a national online workshop with charities and lived experience partners. Recommendations identified from the multiple sources were coded, collated and refined to develop an overarching framework of recommendations.
Results
The framework consists of 74 recommendations for developing accessible PILs for clinical trials. Recommendations cover the five topics of formatting, information presentation, writing style, content and accessibility.
Conclusions
This project has developed a comprehensive framework of recommendations to guide researchers in the development of accessible PILs for clinical trials. Findings from previous research and from co-working with marginalised communities, patients and health and social care charities were collated to ensure that a diverse range of voices and experiences informed the framework. These recommendations aim to support researchers to develop better study information to reduce English language literacy as a barrier to participation in clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
It is now widely acknowledged that action needs to be taken to improve diversity and inclusion in clinical trials and health research more broadly [1]. Trial sample populations need to reflect the communities that they serve to ensure equity, scientific integrity, a full understanding of differences in treatment responses, safety of new treatments, and the translation and applicability of findings into real-world application [2]. The imperative for more inclusive practices in clinical trials was highlighted during the COVID-19 pandemic, with a widespread lack of diversity in people participating in vaccine trials despite Black and Asian ethnic groups having a higher risk of death from COVID-19 [3]. There are a number of national- and government-level initiatives focussed on addressing the underrepresentation of diverse populations in clinical trials, such as the UK National Institute for Health Research (NIHR) Innovations in Clinical Trial Design and Delivery for the underserved (INCLUDE) project [4], Trial Forge [5] and the USA Clinical Trials Transformation Initiative [6]. However, the underrepresentation of marginalised groups in health research prevails due to multi-faceted barriers to research participation. The barriers experienced vary across marginalised groups and individuals but have broadly been identified as relating to language and communication, lack of trust, eligibility criteria, attitudes and beliefs, lack of knowledge around clinical trials and logistical and practical issues [7]. Specific to language and communication, low English language literacy levels are a well-known barrier to inclusion in clinical trials [7], relevant to different marginalised groups including people with a lower education level, those who do not read written English, have a learning disability, are living with dementia or who have had a stroke.
The National Literacy Trust estimates that 7.1 million people (16% of adults) living in England have very poor literacy [8]. Numerous studies have found that patient information leaflets (PILs) used in clinical research are often lengthy, inappropriately complex and have poor readability; this is a persistent and prevalent problem common to trials across the world [9,10,11,12]. For example, an evaluation of COVID-19 vaccine trials found the mean word count of PILs was 8333 words (average reading time of 35–48 min) and the language complexity was high [13]. There are substantial concerns about the increasing length and complexity of PILs for clinical trials and the potential impact on people’s comprehension of the information provided [14]. This also can pose challenges to translation of study information into different languages. Simplifying the information provided in PILs can lead to improved understanding, comprehension and knowledge [15,16,17]. ‘Easy read’ has been defined by as information which is written using simple words supported by images [18]. Information presented in an ‘Easy read’ format aims to be easier to understand than standard documents and can be beneficial for a range of audiences.
The aim of the MAPLE (Making trials more Accessible through better Patient information LEaflets) project was to develop recommendations for developing accessible PILs for clinical trials through a literature review of published and grey literature and co-working with marginalised communities, patients and health and social care charities.
Methods
The UK standards for Public Involvement in research defines it as ‘research being carried out ‘with’ or ‘by’ members of the public rather than ‘to,’ ‘about’ or ‘for’ them’ [19]. The UK National Institute for Health Research provides ‘commenting on and developing patient information leaflets or other research materials’ as an example of patient and public involvement in research [20]. This project involved working with members of the public to develop recommendations for developing accessible PILs for clinical trials, and therefore was conducted as public and community involvement and engagement (PCIE) activities, rather than research, and institutional ethics approval was not required.
This project was a partnership between academics at the University of Bristol and National Voices. National Voices (https://www.nationalvoices.org.uk/) is a leading coalition of health and social care charities in England. They have more than 200 members covering a diverse range of health conditions and communities, connecting them with the experiences of hundreds of thousands of people.
Literature review of published and grey literature
As this was a literature review rather than a systematic review, the review protocol was not registered on PROSPERO.
Published literature
In designing a search strategy, we acknowledged that searching for studies relating to ‘patient information’ would be highly unspecific and identify a large quantity of irrelevant material and searches for ‘patient information leaflet’ would identify some relevant literature but may miss material addressing the issue with a broader consideration of the delivery of patient information. To address this, we applied both a search of online databases with a strategy based around patient information leaflets and a snowballing method with forward searching based on citations of key studies [21]. For a search of MEDLINE and Embase on the Ovid platform on 16th November 2023, we used a search based on textwords used in the review of Sustersic and colleagues [22] and a filter for randomised controlled trials and controlled clinical studies (see Supplementary Table 1). Risk of bias of included studies was not assessed.
To identify articles citing key publications, we used the citation tracking option in Web of Science. Initially, we focused on six key publications that we were aware of [22,23,24,25,26,27], and after screening of reference lists and forward citations, we tracked 22 studies [9, 11, 17, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Articles were included if they reported recommendations to inform the development of easy-read clinical trial PILs for adults. No limitations were placed on the study design. The scope of included articles was limited to recommendations focused on research; studies related to the development of PILs for clinical care were excluded. Article titles were screened in Endnote and clearly irrelevant articles were excluded. Abstracts and full text of potentially relevant articles were then screened to determine eligibility. Screening was performed by one reviewer.
Data extraction of recommendations from included articles was performed by one reviewer and comprised author, date, study design and recommendation. Recommendations were extracted verbatim, and extracted data were entered in Excel.
Grey literature
In November 2023, a search of grey literature of potential relevance was conducted through searches of online material published or catalogued by the King’s Fund, Care Quality Commission, Healthcare Quality Improvement Partnership and Health Research Authority. Opengrey and Google were also searched. Grey literature identified from eligible articles was also included.
To supplement the search of the grey literature, National Voices utilised knowledge and networks of equalities-focussed charities to identify grey literature which contained insights into more inclusive forms of communication. This included reflections on the innovations that could be used to ensure people with specific communication needs have an equal opportunity to participate in clinical research, including people with sensory impairments, those with learning disabilities, autistic people, those living with dementia, and people with low or no literacy or those who do not speak English fluently. This included literature specific to clinical trial participation as well as innovative work on how to improve and create accessible communications regardless of the subject matter.
Co-working with marginalised communities, patients and health and social care charities
Marginalised communities and patient groups
Following our co-produced guidance on inclusive involvement of community groups in health research [41], we co-worked with two racially marginalised community groups, two groups involving autistic adults and/or adults with learning disabilities and/or difficulties and one patient advisory group to generate recommendations for designing accessible PILs. An overview of the groups and meetings is provided in Table 1. Each meeting lasted 1–4 h and was held online or in the usual venue of the group and followed each group’s preferred format, with English interpretation provided for the researchers by the community leaders/facilitators as needed. Meetings were facilitated by group leaders, with researchers in attendance. Groups were reimbursed for their involvement by their preferred format [41]. All meetings were held for the purposes of this project, with the exception of the four meetings with The Adventurers. Three of these meetings focussed on co-developing an accessible PIL for a clinical trial and the fourth meeting involved a discussion about supporting research participation; with permission, notes and learning from those meetings were used in this project.
To inform the discussion during the meetings, a selection of example accessible PILs was obtained through the Bristol Trials Centre, and consent was gained from the trial teams to share the accessible PILs with community and patient groups. For each meeting, 2–3 accessible PILs were printed, and copies were shared with members to facilitate discussion. The agenda for the meetings were informal and adapted to the preferences for working of each group, to allow people the time and space to contribute their experiences to open discussion. Discussions focussed on whether the PILs were easy to understand, what people liked/disliked about them, what would make them better, and whether more/less information should be included. A researcher took notes of the discussion during each meeting, rather than audio-recording, to ensure that the group members felt comfortable to openly share their thoughts and views with the researchers.
Health and social care charities and lived experience partners
National Voices convened and facilitated a 1-h online workshop with 18 people, comprising a mixture of professionals working in health and social care charities and people with lived experience of long-term health conditions and/or disability. Health charities represented during the workshop were The Nerve of My Multiple Sclerosis, Macular Society, TransActual, Thomas Pocklington Trust, Roma Support Group, South Asian Health Action, BHA For Equality, Blood Cancer UK, British Heart Foundation, Age UK, and Rethink Mental Illness. A further five individuals were consulted individually in follow-up conversations. The workshop focused on reviewing barriers to participation and, asking participants to identify the key information researchers would need to include in an accessible format, and identifying solutions and approaches to ensure the proposed output meets the needs of people who are underrepresented in current research. A full report of the workshop is available on the National Voices website [42].
Analysis
Development of the framework of recommendations for the creation of accessible PILs was an iterative process. Extracted recommendations from articles and documents identified in the literature review were coded in Excel and grouped into topics by one researcher (VW). These preliminary codes were then reviewed by a second researcher (AB). Notes from the community and patient group meetings were then reviewed line-by-line by one researcher (VW) and coded in Excel, using the provisional framework developed from the literature review data. New codes were added as they arose, and existing codes refined during the coding process. This process was repeated for the National Voices report on inclusive communication and the report from the online workshop with health and social care charities and lived experience partners. The amalgamated matrix of coded recommendations, along with the supporting section of notes from the meetings, was then reviewed and refined by a second researcher (CJ). The overarching framework was then reviewed by all the co-authors to merge duplicate categories, review the topic categories and finalise the order of presentation of the recommendations.
Results
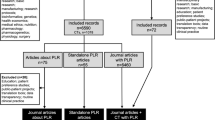
A flow diagram of the literature review is provided in Fig. 1. Database searches identified 5358 articles after duplicates were removed and another article was identified through direct discussion with the trial team. After initial screening, 4896 articles were removed as they were irrelevant and 462 were screened in-depth; of these 33 were included [7, 11, 15, 24, 28, 32, 34, 36, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. A summary of the characteristics of the included studies is provided in Supplementary Table 2 and the extracted recommendations from studies are presented in Supplementary Table 3. The grey literature search identified 32 online documents of which 9 were screened in depth and 3 were included [68,69,70]. Recommendations identified from the literature review of published and grey literature, review of grey literature by National Voices, community and patient group discussions, and the workshop with health and social care charities and lived experience partners were collated and brought together in an overarching framework of recommendations for developing accessible clinical trial PILs. This framework consists of 74 recommendations, grouped into five overarching topics of formatting, information presentation, writing style, content and accessibility. These are further divided into 31 subtopics to facilitate navigation of the framework. The recommendations are provided in full in Table 2 and summarised below.
Formatting
Text should be left-aligned, and bullet points, lists or sections used to break up the text. Use colour, considering readability in the selection of colours. Format text and images into two columns, with images in the left column. Headings should be easily distinguishable from the body of the text, short, and structured as questions. Print PILs on low-to-no gloss paper in an appropriate-sized booklet format and make it clear if readers need to turn overleaf. Select wider typefaces in a large font size (which can be increased if needed), avoid underlining, using text that is all in capitals and italics, and only use bold type face in the main text for emphasis. Include space without text (whitespace) to help with readability.
Information presentation
Using a layered/tiered approach can help structure the provision of accessible information. Information needs to be presented in a logical order, with key/important messages first. Focus on one message at a time with related information grouped together and consider including summaries. Keep the volume of information short and avoid repetition or unnecessary information; the focus should be on the provision of enough information for people to make an informed decision about participation. Use appropriate, familiar and inclusive images that are relevant to the trial, that explain the text, support the main messages of the PIL, and/or explain a difficult concept. Limit the use of statistics, and if they are used consider how best to convey these to readers, including the use of image and analogies to explain numbers and statistical concepts.
Writing style
Work in partnership with communities to co-produce accessible PILs and ensure a writing style that will be accessible to all readers. Use familiar, appropriate and inclusive phrasing, analogies and terminology. Use clear and familiar plain language, written in a conversational and narrative style, demonstrating respect and value for the readers. Ensure the PIL is written at an appropriate reading age and test readability with online readability tools (for example https://www.thefirstword.co.uk/readabilitytest/, https://goodcalculators.com/flesch-kincaid-calculator/ or https://www.thewriter.com/tools/readability) and/or user testing. Avoid jargon, assumptions and patronising language. Minimise the use of abbreviations and acronyms and where they are necessary explain them immediately and clearly. Write from the reader’s perspective; approach the information to be provided from the point of view of what the reader wants and needs to know, rather than what the researchers think they need to convey. Use an active voice and short words, sentences and paragraphs. Provide context for new information and ensure consistency throughout.
Content
If using a front page, provide a concise overview of the trial and avoid using too many logos. Explain the purpose of clinical trials and the purpose of the trial, emphasising that participation is voluntary and encourage readers to discuss with other people before deciding about participation. Describe the importance of research participation and clearly convey the existing uncertainty that underpins the need for the trial. Clearly describe eligibility criteria, treatment allocation, the treatment(s) and standard care, and any treatment side effects. Explain study processes, including how data will be collected, handled and stored. Describe the advantages and disadvantages of participation, any incentives for participation and withdrawal processes. Provide an ethics statement and contact information for the research team and an independent advisor/advocate.
Accessibility
Translate the accessible PIL into different languages and ensure communication and interpretation support is available for written and verbal information. Provide information in multiple formats e.g. braille, large print, plain text, audio, video format with voiceover/subtitles. Ensure the verbal information that is provided in any conversations with potential participants is clear, simple and culturally appropriate and offers wider support as well as information.
Discussion
The MAPLE project has developed a comprehensive framework of recommendations to guide researchers in the development of accessible PILs for clinical trials. A previous literature review identified recommendations for accessible clinical research PILs and conducted work with stakeholders to support the development of patient-facing documents through expert consensus [24]. These were included in our work and extended further through working in partnership with marginalised community groups, patients and charities to ensure that a diverse range of voices and experiences informed the framework. These recommendations aim to support researchers to develop better study information to reduce English language literacy as a barrier to participation in clinical trials.
It is important to consider the strengths and limitations of this work within a broader context. A literature review was conducted rather than a systematic review, as the aim of the review was to gain an understanding of what is already known on the topic to inform the development of the recommendations framework rather than provide a definitive answer to a clinical question. While this approach was appropriate to the aim of this project, it may have led to relevant sources not being included as database searches were limited to MEDLINE and Embase. A key strength of this project was that it was conducted in partnership with diverse groups of people who may experience English language literacy as a barrier to research participation in different ways. Building trust and relationships and understanding preferred ways of working is an essential first stage to inclusive involvement in health research [41]. Based on the preferences of the groups and charities involved in this project, the discussions were not audio-recorded to ensure people felt comfortable and safe to contribute. Identifying and sharing example accessible PILs was a useful tool for promoting discussion, as many members of the community groups had not been approached about participating in a research project before and therefore had not seen a PIL. Discussions focussed on how the example accessible PILs could be improved and the reasons for the recommendations given were not explored due to time constraints. We acknowledged that the project likely did not encapsulate the views of all groups of people who may experience English language literacy as a barrier to research participation. We did not collect information on the protected characteristics of the people involved in the meetings and therefore are unable to comment on the full diversity of those involved; however, from working with community group members, they are likely at the intersection of multiple factors of marginalisation, for example, language, older age, digital exclusion, carers, multiple health conditions and disability. Also while some of our recommendations will apply across phase 1, phase 2 and phase 3 clinical trials, further work is needed to develop recommendations specifically focussed on developing accessible information for first-in-human clinical trials as the information requirements differ from later phase trials.
The MAPLE recommendations provide a preliminary framework to support the development of accessible PILs for clinical trials, however further work is needed to facilitate the use of the recommendations. Regulatory authorities are perceived by researchers as the largest barrier to the use of accessible PILs due to the need to meet regulatory and legal requirements [71]. However, regulatory authorities are often supportive of improving the accessibility of research, for example, the NHS Health Research Authority recommends a layered approach to information provision [72] and has developed principles and hallmarks of people-centred clinical research which includes ensuring that clinical research is accessible and communicated well to people [73]. There are also challenges in addressing all the recommendations regarding the content of an accessible PIL while ensuring readability and keeping the PIL short. Co-production work with patients and communities supports researchers to develop accessible PILs. However, this potentially poses a high and unsustainable burden to communities to be involved in creating new accessible PILs for each clinical trial, and further work is needed to support the creation of co-developed generic content that can then be tailored to individual clinical trials. Finally, investment from health research funders is needed to ensure that the additional funding required to implement measures to facilitate accessibility is available and prioritised within grant applications.
An additional important finding from this project was that a written PIL is only one method of providing information and needs to be supplemented with alternative formats to improve accessibility, for example, videos with subtitles provided in multiple languages and interpretation at research sites. The verbal information provided by research staff and clinicians about a trial is of paramount importance and needs to be culturally appropriate and clear, and support provided to enable people from marginalised backgrounds to participate in research. System-level change in approaches to recruitment is needed to improve the accessibility of clinical research, with researchers, patients, members of the public and regulatory authorities working in partnership to provide better information.
The development of the MAPLE recommendations can support researchers to develop accessible PILs for clinical trials and contribute towards addressing equity in health research participation. Our recommendations contribute to a growing body of work that aims to improve accessibility in clinical trials. However, providing more accessible and inclusive information is only one part of the complex array of barriers to research participation which need to be addressed. Historically, researchers have misconstrued that people from marginalised communities are unwilling to participate in research [1], when the reality is that people from these communities are not invited to participate, with barriers imposed by researchers [74]. Barriers to inclusive trials are surmountable and there is a need for investment to action systemic changes across health research to improve inclusivity and minimise the perpetuation of existing health inequalities [1, 6]. Evidence-based strategies and enablers to inclusive trials include cultural competency training, community partnerships, personalised approach, multilingual materials and staff, communication-specific strategies, increasing understanding and trust and tackling logistical barriers [5, 7, 75]. A multi-faceted approach, with investment from all stakeholders, is required to action and implement widespread changes to clinical trials and improve inclusion.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available as they comprise notes taken during community meetings and we do not have permission from community group members to share these notes.
Abbreviations
- INCLUDE:
-
Innovations in Clinical Trial Design and Delivery for the underserved
- MAPLE study:
-
Making trials more Accessible through better Patient information Leaflets
- NIHR:
-
National Institute for Health Research
- PCIE:
-
Public and community involvement and engagement
- PEP-R group:
-
Patient Experience Partnership in Research
- PIL:
-
Patient information leaflet
References
Bibbins-Domingo K, Helman A, Dzau VJ. The imperative for diversity and inclusion in clinical trials and health research participation. JAMA. 2022;327:2283–4. https://doi.org/10.1001/jama.2022.9083. 2022/05/18.
Treweek S, Banister K, Bower P, et al. Developing the INCLUDE Ethnicity Framework-a tool to help trialists design trials that better reflect the communities they serve. Trials. 2021;22:337. https://doi.org/10.1186/s13063-021-05276-8. 2021/05/12.
Herieka H, Babalis D, Tzala E, et al. How inclusive were UK-based randomised controlled trials of COVID-19 vaccines? A systematic review investigating enrolment of Black adults and adult ethnic minorities. Trials. 2024;25:255. https://doi.org/10.1186/s13063-024-08054-4. 2024/04/12.
Witham MD, Anderson E, Carroll C, et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials. 2020;21:694. https://doi.org/10.1186/s13063-020-04613-7. 2020/08/03.
Dawson S, Banister K, Biggs K, et al. Trial Forge Guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups-practical guidance to support better practice. Trials. 2022;23:672. https://doi.org/10.1186/s13063-022-06553-w. 2022/08/18.
Corneli A, Hanlen-Rosado E, McKenna K, et al. Enhancing Diversity and Inclusion in Clinical Trials. Clin Pharmacol Ther. 2023;113:489–99. https://doi.org/10.1002/cpt.2819. 2023/01/12.
Bodicoat DH, Routen AC, Willis A, et al. Promoting inclusion in clinical trials-a rapid review of the literature and recommendations for action. Trials. 2021;22:880. https://doi.org/10.1186/s13063-021-05849-7. 2021/12/06.
National Literacy Trust. Adult literacy rates in the UK. https://literacytrust.org.uk/parents-and-families/adult-literacy/, (accessed 11/04/2024).
O’Sullivan L, Sukumar P, Crowley R, et al. Readability and understandability of clinical research patient information leaflets and consent forms in Ireland and the UK: a retrospective quantitative analysis. BMJ Open. 2020;10:e037994. https://doi.org/10.1136/bmjopen-2020-037994. Article 2020/09/05.
Symons T, Davis JS. Creating concise and readable patient information sheets for interventional studies in Australia: are we there yet? Trials. 2022;23:8. https://doi.org/10.1186/s13063-022-06712-z. Article.
Foe G, Larson EL. Reading Level and Comprehension of Research Consent Forms: An Integrative Review. J Empir Res Hum Res Ethics. 2016;11:31–46. https://doi.org/10.1177/1556264616637483. Review 2016/04/24.
Santel F, Bah I, Kim K, et al. Assessing readability and comprehension of informed consent materials for medical device research: a survey of informed consents from FDA’s center for devices and radiological health. Contemp Clin Trials. 2019;85:8. https://doi.org/10.1016/j.cct.2019.105831. Article.
Emanuel EJ, Boyle CW. Assessment of length and readability of informed consent documents for COVID-19 vaccine trials. JAMA Netw Open. 2021;4:5. https://doi.org/10.1001/jamanetworkopen.2021.10843. Article.
Bull S, Cheah PY, Lwin KM, et al. Consent and community engagement in diverse research contexts: reviewing and developing research and practice. J Empir Res Hum Res Ethics. 2013;8:1–18. https://doi.org/10.1525/jer.2013.8.4.1. Article.
Benatar JR, Mortimer J, Stretton M, Stewart RAH. A booklet on participants’ rights to improve consent for clinical research: a randomized trial. PLoS One. 2012;7:7. https://doi.org/10.1371/journal.pone.0047023. Article.
Nishimura A, Carey J, Erwin PJ, et al. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics. 2013;14:15. https://doi.org/10.1186/1472-6939-14-28. Review.
Bader M, Zheng L, Rao D, et al. Towards a more patient-centered clinical trial process: A systematic review of interventions incorporating health literacy best practices. Contemp Clin Trials. 2022;116:106733. https://doi.org/10.1016/j.cct.2022.106733. Review 2022/03/19.
NHS England. Guide to making information accessible for people with a learning disability. LearningDisabilityAccessCommsGuidance.pdf (england.nhs.uk. 2018. https://www.england.nhs.uk/wp-content/uploads/2018/06/LearningDisabilityAccessCommsGuidance.pdf.
UK Standards for Public Involvement. Definitions used in the Standards.https://sites.google.com/nihr.ac.uk/pi-standards/standards/definitions. Accessed 16th August 2024.
National Institute for Health research. Briefing notes for researchers - public involvement in NHS, health and social care research.https://www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371. Accessed 16th August 2024.
Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Metzendorf M-I, Noel-Storr A, Paynter R, Rader T, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane. 2023. Available from www.training.cochrane.org/handbook.
Sustersic M, Gauchet A, Foote A, Bosson JL. How best to use and evaluate Patient Information Leaflets given during a consultation: a systematic review of literature reviews. Health Exp Int J Public Particip Health Care Health Pol. 2017;20:531–42. https://doi.org/10.1111/hex.12487. 2016/09/28.
Gillies K, Huang W, Skea Z, et al. Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation. Trials. 2014;15:62. https://doi.org/10.1186/1745-6215-15-62. 2014/02/20.
Coleman E, O’Sullivan L, Crowley R, et al. Preparing accessible and understandable clinical research participant information leaflets and consent forms: a set of guidelines from an expert consensus conference. Res Involv Engagem. 2021;7:31. https://doi.org/10.1186/s40900-021-00265-2. 2021/05/20.
Medina-Cordoba M, Cadavid S, Perez-Acosta AM, Amaya-Giraldo V. Factors that facilitate and hinder the comprehension of Patient Information Leaflets (PILs): a brief scoping review. Front Pharmacol. 2021;12:740334. https://doi.org/10.3389/fphar.2021.740334. Mini Review 2021/12/04.
Brierley G, Richardson R, Torgerson DJ. Using short information leaflets as recruitment tools did not improve recruitment: a randomized controlled trial. J Clin Epidemiol. 2012;65:147–54. https://doi.org/10.1016/j.jclinepi.2011.06.005. 2011/09/06.
Hilton P, Buckley BS, McColl E, et al. Understanding variations in patient screening and recruitment in a multicentre pilot randomised controlled trial: a vignette-based study. Trials. 2016;17:522. https://doi.org/10.1186/s13063-016-1652-2. 2016/10/27.
Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–601. https://doi.org/10.1001/jama.292.13.1593. Review 2004/10/07.
Breese PE, Burman WJ, Goldberg S, Weis SE. Education level, primary language, and comprehension of the informed consent process. J Empir Res Hum Res Ethics. 2007;2:69–79. https://doi.org/10.1525/jer.2007.2.4.69. Article 2007/12/01.
Muzanyi G, Sekitoleko I, Johnson JL, et al. Level of education and preferred language of informed consent for clinical research in a multi-lingual community. Afr Health Sci. 2020;20:955–9. https://doi.org/10.4314/ahs.v20i2.51. Article 2020/11/10.
Baiden F, Akazili J, Chatio S, et al. Should consent forms used in clinical trials be translated into the local dialects? A survey among past participants in rural Ghana. Clin Trials. 2016;13:234–9. https://doi.org/10.1177/1740774515609290. Article 2015/10/11.
Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. https://doi.org/10.1186/1471-2288-14-42. Article 2014/03/29.
Busisiwe N, Seeley J, Strode A, Parker M. Beyond translations, perspectives for researchers to consider to enhance comprehension during consent processes for health research in sub-saharan Africa: a scoping review. BMC Med Ethics. 2023;24:43. https://doi.org/10.1186/s12910-023-00920-1. Review 2023/06/22.
Cohn E, Larson E. Improving participant comprehension in the informed consent process. J Nurs Scholarsh. 2007;39:273–80. https://doi.org/10.1111/j.1547-5069.2007.00180.x. Article 2007/09/01.
Cortes DE, Drainoni ML, Henault LE, Paasche-Orlow MK. How to achieve informed consent for research from Spanish-speaking individuals with low literacy: a qualitative report. J Health Commun. 2010;15(Suppl 2):172–82. https://doi.org/10.1080/10810730.2010.499990. Article 2010/09/29.
Hughson JA, Woodward-Kron R, Parker A, et al. A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials. 2016;17:263. https://doi.org/10.1186/s13063-016-1384-3. Review 2016/05/28.
Woodward-Kron R, Fraser C, Rashid H, et al. Perspectives of junior doctor intercultural clinical communication: Lessons for medical education. Focus Health Prof Educ. 2016;17:82–95. https://doi.org/10.11157/fohpe.v17i3.179. Article.
Shiely F, Daly A. Trial lay summaries were not fit for purpose. J Clin Epidemiol. 2023;156:105–12. https://doi.org/10.1016/j.jclinepi.2023.02.023. Article 2023/03/04.
Sudore RL, Landefeld CS, Williams BA, et al. Use of a modified informed consent process among vulnerable patients: a descriptive study. J Gen Intern Med. 2006;21:867–73. https://doi.org/10.1111/j.1525-1497.2006.00535.x. Article 2006/08/03.
Yu Z, Kowalkowski J, Roll AE, Lor M. Engaging underrepresented communities in health research: lessons learned. West J Nurs Res. 2021;43:915–23. https://doi.org/10.1177/0193945920987999. Article 2021/01/16.
Jameson C, Haq Z, Musse S, et al. Inclusive approaches to involvement of community groups in health research: the co-produced CHICO guidance. Res Involv Engagem. 2023;9:76. https://doi.org/10.1186/s40900-023-00492-9. 13063_2024_8471.
National Voices. English literacy as a barrier to participation in clinical trials.https://s42139.pcdn.co/wp-content/uploads/English-literacy-as-a-barrier-to-participation-in-clinical-trials-MAPLE-REND-Project.pdf. 2024.
Head KJ, Hartsock JA, Bakas T, et al. Development of written materials for participants in an alzheimer’s disease and related dementias screening trial. J Patient Exp. 2022;9:7. https://doi.org/10.1177/23743735221092573. Article.
Atwere P, McIntyre L, Carroll K, et al. Informed consent documents used in critical care trials often do not implement recommendations. Crit Care Med. 2018;46:E111–7. https://doi.org/10.1097/ccm.0000000000002815. Article.
Dellson P, Nilbert M, Carlsson C. Patient representatives’ views on patient information in clinical cancer trials. BMC Health Serv Res. 2016;16:5. https://doi.org/10.1186/s12913-016-1272-2. Article.
Addissie A, Abay S, Feleke Y, et al. Cluster randomized trial assessing the effects of rapid ethical assessment on informed consent comprehension in a low-resource setting. BMC Med Ethics. 2016;17:12. https://doi.org/10.1186/s12910-016-0127-z. Article.
Eeckhout D, Aelbrecht K, Van der Straeten C. Informed consent: research staff’s perspectives and practical recommendations to improve research staff-participant communication. J Empir Res Hum Res Ethics. 2023;18:3–12. https://doi.org/10.1177/15562646221146043. Article.
Spellecy R, Tarima S, Denzen E, et al. Easy-to-read informed consent form for hematopoietic cell transplantation clinical trials: results from the blood and marrow transplant clinical trials network 1205 study. Biol Blood Marrow Transpl. 2018;24:2145–51. https://doi.org/10.1016/j.bbmt.2018.04.014. Article.
Simonds VW, Garroutte EM, Buchwald D. Health literacy and informed consent materials: designed for documentation, not comprehension of health research. J Health Commun. 2017;22:682–91. https://doi.org/10.1080/10810730.2017.1341565. Article.
Lentz J, Kennett M, Perlmutter J, Forrest A. Paving the way to a more effective informed consent process: Recommendations from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2016;49:65–9. https://doi.org/10.1016/j.cct.2016.06.005. Article.
Lorell BH, Mikita JS, Anderson A, et al. Informed consent in clinical research: Consensus recommendations for reform identified by an expert interview panel. Clin Trials. 2015;12:692–5. https://doi.org/10.1177/1740774515594362. Article.
Kass NE, Taylor HA, Ali J, et al. A pilot study of simple interventions to improve informed consent in clinical research: Feasibility, approach, and results. Clin Trials. 2015;12:54–66. https://doi.org/10.1177/1740774514560831. Article.
Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44–55. https://doi.org/10.1525/jer.2012.7.5.44. Article.
Denzen EM, Santibáñez MEB, Moore H, et al. Easy-to-read informed consent forms for hematopoietic cell transplantation clinical trials. Biol Blood Marrow Transplant. 2012;18:183–9. https://doi.org/10.1016/j.bbmt.2011.07.022. Review.
Dellson P, Nilbert M, Bendahl PO, et al. Towards optimised information about clinical trials; identification and validation of key issues in collaboration with cancer patient advocates. Eur J Cancer Care. 2011;20:445–54. https://doi.org/10.1111/j.1365-2354.2010.01207.x. Article.
Jefford M, Moore R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 2008;9:485–93. https://doi.org/10.1016/s1470-2045(08)70128-1. Article.
Adams V, Miller S, Craig S, et al. Informed consent in cross-cultural perspective: Clinical research in the Tibetan Autonomous Region. PRC Cult Med Psychiatr. 2007;31:445–72. https://doi.org/10.1007/s11013-007-9070-2. Article.
Corneli AL, Bentley ME, Sorenson JR, et al. Using formative research to develop a context-specific approach to informed consent for clinical trials. J Empir Res Hum Res Ethics. 2006;1:45–60. https://doi.org/10.1525/jer.2006.1.4.45. Article.
Simonds VW, Buchwald D. Too Dense and Too Detailed: Evaluation of the Health Literacy Attributes of an Informed Consent Document. J Racial Ethn Health Disparities. 2020;7:327–35. https://doi.org/10.1007/s40615-019-00661-1. Article.
Brockhoven F, Raphael M, Currier J, et al. REPRESENT recommendations: improving inclusion and trust in cancer early detection research. Br J Cancer. 2023;129:1195–208. https://doi.org/10.1038/s41416-023-02414-8. Article.
Cunningham-Erves J, Kusnoor SV, Villalta-Gil V, et al. Development and pilot implementation of guidelines for culturally tailored research recruitment materials for African Americans and Latinos. BMC Med Res Methodol. 2022;22:14. https://doi.org/10.1186/s12874-022-01724-4. Article.
Jilka S, Hudson G, Jansli SM, et al. How to make study documents clear and relevant: the impact of patient involvement. BJPsych Open. 2021;7:8. https://doi.org/10.1192/bjo.2021.1040. Article.
Burks AC, Keim-Malpass J. Health literacy and informed consent for clinical trials: a systematic review and implications for nurses. Nursing. 2019;9:31–40. https://doi.org/10.2147/nrr.S207497. Review.
Mayers SA, Cook SK, Rantala C, et al. The RIC Recruitment & Retention Materials Toolkit - a resource for developing community-informed study materials. J Clin Transl Sci. 2023;7:e182.https://doi.org/10.1017/cts.2023.607
Tait AR, Voepel-Lewis T, Malviya S, Philipson SJ. Improving the readability and processability of a pediatric informed consent document: effects on parents’ understanding. Arch Pediatr Adolesc Med. 2005;159:347–52. https://doi.org/10.1001/archpedi.159.4.347. 2005/04/06.
Beasant L, Realpe A, Douglas S, et al. Autistic adults' views on the design and processes within randomised controlled trials: The APRiCoT study. Autism 2023: 13623613231202432. https://doi.org/10.1177/13623613231202432.
Tait AR, Voepel-Lewis T, Nair VN, et al. Informing the uninformed: optimizing the consent message using a fractional factorial design. JAMA Pediatr. 2013;167:640–6. https://doi.org/10.1001/jamapediatrics.2013.1385. 2013/05/24.
US Department of Health and Human Services Centres for Disease Control and Prevention. Simply Put: A guide for creating easy-to-understand materials. https://stacks.cdc.gov/view/cdc/11938. July 2010, Third Edition.
Health Research Authority. Consent and Participant Information Guidance. https://www.hra-decisiontools.org.uk/consent/style.html, (accessed 5th January 2024).
Dunman M. Producing patient information : how to research, develop and produce effective information sources London: King's Fund; 2003 [Available from: https://archive.kingsfund.org.uk/concern/published_works/000030954?locale=en#?cv=8&xywh=191,168,1219,696.
Solomon ED, Mozersky J, Wroblewski MP, et al. Understanding the use of optimal formatting and plain language when presenting key information in clinical trials. J Empir Res Hum Res Ethics. 2022;17:177–92. https://doi.org/10.1177/15562646211037546. 2021/08/20.
Health Research Authority. Applying a proportionate approach to the process of seeking consent. https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/Proportionate_approach_to_seeking_consent_HRA_Guidance.pdf. 2019.
NHS Health Research Authority. People-Centred Clinical Research. https://www.hra.nhs.uk/planning-and-improving-research/best-practice/people-centred-clinical-research/, (2024, accessed 20th May 2024).
Brijnath B, Muoio R, Feldman P, et al. “We are not invited”: Australian focus group results on how to improve ethnic diversity in trials. J Clin Epidemiol. 2024;170:111366. https://doi.org/10.1016/j.jclinepi.2024.111366 2024/04/18.
Goodwin VA, Low MSA, Quinn TJ, et al. Including older people in health and social care research: best practice recommendations based on the INCLUDE framework. Age Ageing. 2023;52:afad082. https://doi.org/10.1093/ageing/afad082. 2023/06/01.
Acknowledgements
We would like to thank Dhek Bhal, My Friday Coffee Morning, Lawnmowers, PEP-R, The Adventurers, The Nerve of My Multiple Sclerosis, Macular Society, TransActual, Thomas Pocklington Trust, Roma Support Group, South Asian Health Action, BHA For Equality, Blood Cancer UK, British Heart Foundation, Age UK, and Rethink Mental Illness for being involved in this project and providing their valuable time to help improve research participation and make it more accessible for everyone. We would like to thank the following Clinical Trials Units for providing examples of their PILs and/or Easy Read documentation for this project: Bristol Trials Centre, Leeds Clinical Trials Research Unit, Cardiff Centre for Trials Research and Sheffield Clinical Trials Research Unit.
Funding
This activity was funded through the ICS Research Engagement Network (REN) development programme. This study is also supported by the National Institute for Health and Care Research Bristol Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the NHS England or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
All authors were involved in the acquisition of funding. VW and AB performed the literature review. KR led on engagement with Trials Centres to identify examples of accessible patient information leaflets. CJ, EJ and VW were involved in the meetings with patients and community groups. SB led the work conducted by National Voices. VW led on the analysis of the data and all authors were involved in the development of the final recommendations. VW drafted the manuscript, and all authors revised the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work included a literature review and public and community involvement and engagement (PCIE) activities and therefore research ethics and participant consent were not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13063_2024_8471_MOESM1_ESM.docx
Supplementary Material 1: Supplementary Table 1: Scoping review’ search strategy as applied to Embase. Supplementary Table 2: Summary of included articles identified in the literature view. Supplementary Table 3: Extracted recommendations from included sources, grouped by topic.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wylde, V., Brennan, S., Johnson, E. et al. Recommendations for developing accessible patient information leaflets for clinical trials to address English language literacy as a barrier to research participation. Trials 25, 624 (2024). https://doi.org/10.1186/s13063-024-08471-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-024-08471-5