Abstract
Background
Acetoin (AC) is a vital platform chemical widely used in food, pharmaceutical and chemical industries. With increasing concern over non-renewable resources and environmental issues, using low-cost biomass for acetoin production by microbial fermentation is undoubtedly a promising strategy.
Results
This work reduces the disadvantages of Bacillus subtilis during fermentation by regulating genes involved in spore formation and autolysis. Then, optimizing intracellular redox homeostasis through Rex protein mitigated the detrimental effects of NADH produced by the glycolytic metabolic pathway on the process of AC production. Subsequently, multiple pathways that compete with AC production are blocked to optimize carbon flux allocation. Finally, the population cell density-induced promoter was used to enhance the AC synthesis pathway. Fermentation was carried out in a 5-L bioreactor using bagasse lignocellulosic hydrolysate, resulting in a final titer of 64.3 g/L, which was 89.5% of the theoretical yield.
Conclusions
The recombinant strain BSMAY-4-PsrfA provides an economical and efficient strategy for large-scale industrial production of acetoin.
Similar content being viewed by others
Background
Acetoin (3-hydroxy-2-butanone) is widely used in food, pharmaceutical and chemical industries [1, 2]. Because of these important applications, in 2004, the US Department of Energy listed it as one of 30 bio-based platform chemicals that should be given higher priority for development and utilization [3]. The acetoin production methods include chemical and biological synthesis [4]. The raw materials for chemical synthesis methods are mainly derived from petroleum resources, such as diacetyl and 2,3-butanediol. Currently, the continuous reduction of petroleum resources and the environmental hazards of chemical production limit the large-scale production of acetoin by chemical synthesis methods. Furthermore, when acetoin is produced by chemical synthesis, the by-products often have certain toxicity, which limits its application in food, medicine and other industries [5,6,7]. Therefore, increased attention has been devoted to biological synthesis methods, which can be divided into enzymatic conversion and microbial fermentation. The enzymatic conversion approach is unsuitable for industrial acetoin production because the precursor substances are expensive and not readily available, and the production process is complex [8].
In contrast, the microbial fermentation method is less affected by natural environmental conditions, the production process is easy to control, green and environmentally friendly, and is conducive to maintaining the stability of product quality. In addition, microorganisms have an active metabolism and grow and multiply rapidly, allowing the production of a batch of fermented products in a short period. Therefore, fermentation is currently the most competitive approach for acetoin production. Microbial fermentation for acetoin production usually uses glucose as the primary carbon source, which is uneconomical for industrial production [9, 10]. Therefore, it would be more advantageous to use second-generation biofuel feedstocks, such as the hydrolysis products of lignocellulose, for the fermentation production of acetoin [11]. In the future, using low-cost and renewable plant biomass as a raw material for production is undoubtedly a promising production strategy that is more conducive to the industrial production of the product.
In nature, many bacteria can synthesize acetoin, including Klebsiella [12], Bacillus [13], Enterobacter [6], Lactococcus [14], and Serratia [15]. However, acetoin is generally present as a by-product of 2,3-butanediol and diacetyl in the metabolism of these strains that can synthesize acetoin naturally. Direct fermentation using these microorganisms results in a deficient synthesis of acetoin, making it challenging to meet the demands of industrial production and the pathogenicity of some strains. In order to solve the problem of the low yield of acetoin synthesized by microorganisms, the researchers obtained a batch of high-yielding strains through mutagenesis screening and fermentation optimization [16]. But the genetic stability of these strains has been altered through mutagenesis, and there are too many uncertainties and potential risks in product safety. Therefore, researchers gradually began to perform metabolic engineering of some model strains, aiming to change the carbon flow distribution of the strains through biotechnology and increase acetoin production [17]. B. subtilis is a typical industrial strain for food safety. It has been widely used in metabolic engineering due to its non-pathogenicity and lack of significant codon preference [18, 19], which is a very promising chassis cell with obvious advantages for the industrial mass production of acetoin.
In this study, we first blocked the spore synthesis pathway and knocked out essential genes that control cell autolysis, which improved the efficiency of substrate utilization by the strain. In addition, to address the problem of low acetoin production and high by-products during fermentation [20], we redistributed the carbon flux of the synthesis pathway through the intracellular redox-sensing regulator Rex [21, 22] and explored the relationship between changes in intracellular NADH/NAD+ levels and acetoin production during fermentation. The main by-product synthesis pathways competing with acetoin synthesis were combinatorially blocked, and their effects on acetoin production were analyzed. To further improve the efficiency of acetoin production while avoiding biomass reduction in the pre-fermentation period [23], we chose to use a population cell density-induced promoter to enhance the expression of α-acetolactate synthase (ALS, encoded by the gene alsS) and α-acetolactate decarboxylase (ALDC, encoded by the gene alsD), and increase the efficiency of the catalytic synthesis of acetoin from pyruvate (Fig. 1).
Biotechnological strategies for acetoin production by B. subtilis. This work blocked spore formation at different stages and mitigated autolysis in strains by regulating genes for peptidoglycan hydrolase and cell cannibalism. Subsequently, NADH/NAD+ was regulated by the redox global regulator Rex to optimize the allocation of carbon flux levels, followed by blocking the main pathway of lactate, succinate, and 2,3-butanediol production. Finally, the population cell density-induced promoter enhances the enhancement of α-acetolactate synthase and α-acetolactate decarboxylase. Bold arrows indicate overexpressed genes in this study, and red crosses indicate disrupted genes
Results and discussion
Knockout spore-formation genes to increase AC production by reducing nutrient wastage
Due to the presence of some organic compounds in the bagasse hydrolyzate, these substances accelerate spore formation in B. subtilis [24, 25]. The formation of spores consumes a lot of energy and substances, such as enzymes required for spore synthesis, various regulatory factors, lipids and dipicolinic acid [26], reducing the substrate utilization. In this study, we blocked spore formation at the initiation (encoded by spo0A) and asymmetric division stages (encoded by sigE and sigF). And we explored the effect of blocking the spore-formation pathway on acetoin synthesis in a fermentation experiment.
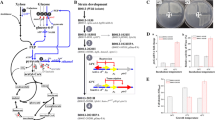
The control strain B. subtilis 168 (BS-1) and the recombinant strains B. subtilis 168Δspo0A (BSM-1) and B. subtilis 168ΔsigEΔsigF (BSM-2) were used in this study with bagasse hydrolysate as the substrate. The results indicated that the rate of sugar consumption was the same for all three strains, suggesting that the knockout of the spo0A, sigE, and sigF genes did not have a regulatory relationship with the related genes in the glycolytic pathway. Regarding acetoin production, the maximum titer for BSM-1 was only 23.7 g/L,13.8% lower than the 27.5 g/L observed for the control strain BS-1 (Fig. 2A). In contrast, the acetoin production by BSM-2 was improved compared with BS-1, with the titer reaching 30.8 g/L under the same conditions. Meanwhile, the biomass of the recombinant strain BSM-1 was significantly lower than that of the control strain BS-1, whereas there were no apparent variations between the control strain and the BSM-2 (Fig. 2B).
A Effect of blocking the formation of spores at different times and knocking out different types of autolysis-related genes on acetoin synthesis. B Trends in biomass of different recombinant strains over time. C and D Transmission electron micrographs (TEM) of the control strain BS-1 and recombinant strain BSMA-1. All assays were performed in triplicate, and the standard deviations of biological replicates are indicated by error bars
The exact reason for the knockout of the spo0A gene inhibiting bacterial growth is still unclear, and Takeko Kodama [27] speculated that the mutant with the gene spo0A deleted might have a higher cell wall lytic enzyme than the original strain, which may account for the reduced biomass of the mutant. Thus, it is more beneficial to block spore formation at the asymmetric division stage than at the initial stage to increase the biomass of the cells as well as acetoin production.
Construction of a robust chassis cell for AC production using lignocellulose hydrolysate
The B. subtilis occurs in autolysis in the middle and late stages of fermentation with increasing fermentation time, and this is particularly noticeable when lignocellulose is used as the fermentation substrate. The occurrence of cell lysis reduces the total biomass and decreases the fermentation yield [28]. Numerous factors mediate the lysis of B. subtilis, and we examined two more significant ones: intracellular cannibalism and peptidoglycan hydrolases. During fermentation, B. subtilis gradually produces spores as the nutrients decrease. The cells that prepare to form spores produce and release the toxic substances skf and sdp, which kill their non-spore-forming counterparts, and the nutrients released by the dead cells are used by the cells ready to form spores, a phenomenon we call cannibalism. The toxin production is mainly encoded by skfA and sdpC [29]. And the protein encoded by the lytC gene is considered the critical peptidoglycan hydrolase that mediates the autolysis of cells because the individual knockout of lytC effectively inhibits cell lysis [27, 30]. We took BSM-2 as the departure strain at this time and knocked out the relevant autolysis gene.
Analysis of the fermentation data showed that recombinant strain BSMA-1 with a double knockout of the skfA and sdpC genes did not significantly increase acetoin production, although it limited cell lysis to a certain extent. In contrast, knockout of the lytC gene in the recombinant strain BSMA-2 resulted in a significant increase in biomass by 18.3% compared to the original strain BS-1 in a medium with bagasse lignocellulose hydrolysate as the substrate, which ultimately led to a further increase in acetoin production, resulting in the titer of 33.7 g/L (Fig. 2A), an increase of 22.5% over the original strain (BS-1). This is because the knockout of the lytC gene increases the number of cells per unit volume in the fermentation broth and thus increases acetoin production, Wang et al. [29] also observed the same phenomenon when knocking out the gene lytC in B. subtilis. At the same time, we can see by transmission electron microscope that compared with the original strain BS-1, the recombinant strain BSMA-2 has no evident spores, but its cell morphology has changed significantly. The ends of the cells are darker in color, similar to the cellular areas of the forespores (Fig. 2C, D).
Compared to the original strain BS-1, the recombinant strain BSMA-2 optimized the intracellular carbon flux distribution, making the cells more conducive to acetoin production by fermentation. At the same time, the blocking of spores makes the recombinant strain easier to control during fermentation production and avoids pollution to the surrounding environment.
Enhancing AC production by regulating intracellular redox homeostasis through Rex protein
When microorganisms use glycolysis to produce large amounts of pyruvate, they produce large amounts of NADH. Acetoin produced in microorganisms can use the NADH to reduce itself to 2,3-butanediol when too much NADH is made, resulting in much less efficient acetoin production [31, 32]. Previous studies have shown that B. subtilis contains a factor that responds to intracellular redox levels, called the redox-sensing global regulator Rex (encoded by the gene ydiH) [22]. Rex regulates intracellular redox homeostasis by sensing changes in the NADH/NAD+ ratio [33] and binds to the promoter regions of many genes to repress gene expression. This process can be inhibited by NADH and derepressed by excess NAD+. Most of these Rex-binding genes are involved in carbon and energy metabolism, including NADH oxidation, hydrogen production, ATP synthesis, and lactate and succinate formation [34, 35].
The construction of strain BSMAY-1 by knocking out the gene ydiH from strain BSMA-2. In order to reduce the inconvenience to experimental analysis caused by increased carbon source variables due to the complex composition of lignocellulosic hydrolysates in the experiment. In the section exploring metabolic engineering modification strategies to improve the conversion of substrates in recombinant bacteria, glucose was used as the sole carbon source. Fermentation data showed that knockout of the gene ydiH intensified the flow of carbon flux towards acetoin synthesis, which directly led to a reduction in the associated by-products of the NADH-dependent pathway. We followed up on the intracellular redox coenzymes, and the coenzymes data supported this phenomenon. The coenzyme data showed that knockout of the gene ydiH altered the intracellular concentrations of NADH and NAD+, resulting in a significantly lower NADH/NAD+ ratio than the starting strain, as shown in Fig. 3. This reduced ratio was beneficial for optimizing the intracellular carbon flow distribution and promoting acetoin production, which is consistent with the Li et al. results [36]. However, the growth rate of the bacterium was reduced at this time, probably due to the disruption of the intracellular homeostatic environment by knocking out ydiH. Although the knockout of Rex affected the growth rate of B. subtilis to a certain extent, it also affected the distribution of carbon flow and enhanced the production of acetoin and substrate conversion. The final acetoin titer of the BSMAY-1 strain with glucose as the sole fermentation substrate was 34.1 g/L, while the acetoin titer of the original strain BS-1 was 30.5 g/L under the same conditions.
Combined blockage of competitive pathways increases AC production
Although the Rex protein adjusted the intracellular redox levels, the ability of the recombinant strain to produce AC was improved to some extent. However, there was still some accumulation of by-products at the end of fermentation, including 2,3-butanediol, lactic acid and succinic acid at concentrations of 6.2 g/L, 2.3 g/L and 1.4 g/L, respectively. In B. subtilis, the formation of 2,3-butanediol, lactic acid and succinic acid is mainly catalyzed by bdhA, ldhA and mdh, respectively [13]. In order to obtain a higher AC yield, we knocked out these genes combinatorially in strain BSMAY-1. The recombinant strain was then incubated at 37 °C in 250-ml shake flasks with glucose being the only carbon source, and fermentation was completed when glucose was almost wholly consumed.
The bdhA gene of BSMAY-1 was knocked out, and strain BSMAY-2 was obtained. Analysis of the fermentation data of strain BSMAY-2 showed a significant decrease in the yield of 2,3-butanediol. However, this component did not disappear completely, indicating other production pathways of 2,3-butanediol in B. subtilis. Subsequently, the gene ldhA was knocked out from strain BSMAY-2 obtaining strain BSMAY-3. And the knockout of the gene ldhA almost blocked the synthesis of lactate and further promoted the accumulation of acetoin. Interestingly, the knockout of the mdh gene obtaining strain BSMAY-4 increased acetoin production and increased the growth rate of the recombinant strain compared with the starting strain BSMAY-3, resulting in an increased acetoin production efficiency.
Through a series of metabolic engineering modifications, we finally obtained the recombinant strain BSMAY-4. After 84 h of shake-flask fermentation, the AC titer of strain BSMAY-4 increased to 40.1 g/L, as shown in Table 1, which was 40.98% higher than the original strain BS-1. The by-products 2,3-butanediol, lactic acid and succinic acid presented significant decreases, ending at 1.9 g/L, 0.2 g/L, and 0.5 g/L, respectively. This result is consistent with the modification of Enterobacter cloacae through metabolic engineering by Zhang et al. [6]. At the same time, the substrate utilization was also greatly improved, with a theoretical yield of 82.0%.
Enhancement of AC metabolic pathway carbon fluxes in mid-to-late fermentation
In the metabolic pathway of acetoin synthesis in B. subtilis, ALS and ALDC are responsible for catalyzing acetoin production from pyruvate and are encoded by the genes alsS and alsD, respectively. Previous studies in our laboratory revealed that the overexpression of alsS and alsD in B. subtilis using strong constitutive promoters inhibited the growth of the strain and was detrimental to acetoin production [23]. Therefore, we tried to use different auto-induced promoters, such as Pylb, PaprE, Pcry3Aa, and PsrfA, to overexpress alsS and alsD in the middle and late stages of fermentation to study their effects on acetoin synthesis.
The recombinant expression plasmids pMA5-PHpaII-alsSD, pMA5-Pylb-alsSD, pMA5-PaprE-alsSD, pMA5-Pcry3Aa-alsSD, and pMA5-PsrfA-alsSD were first transformed into strain BSMAY-4, and the enzyme activities of ALS and ALDC and the acetoin yield were measured periodically during fermentation. We found that when alsS and alsD were expressed using the constitutive strong promoter HpaII in plasmid pMA5, the enzyme activities of ALS and ALDC in the strain were high throughout the fermentation process. In contrast, the growth of strain BSMAY-4-Pylb was severely inhibited in the fermentation medium, and the maximum value of OD600 was only 10. Therefore, the enzyme activities and fermentation data were no longer measured for this strain. The data comparison revealed that the enzyme activities of ALS and ALDC in the recombinant strains BSMAY-4-PaprE and BSMAY-4-Pcry3Aa were unchanged from the starting strain BSMAY-4 (Fig. 4A, B). The ALS and ALDC activities for the recombinant strain BSMAY-4-PsrfA were slightly higher than those for strain BSMAY-4 until 36 h. However, the activities of ALS and ALDC increased rapidly, reaching a maximum after about 60 h, which was almost twice those of the starting strain BSMAY-4. We subsequently performed shake-flask fermentation experiments for the strains BSMAY-4-PHpaII, BSMAY-4-PaprE, BSMAY-4-Pcry3Aa, BSMAY-4-PsrfA, and BSMAY-4. The fermentation data indicated that when alsS and alsD were expressed in strain BSMAY-4 using the constitutive promoter HpaII, the maximum biomass was reduced by 10.9% compared with the starting strain BSMAY-4 (Fig. 4C), while the acetoin titer reached only 32.5 g/L during the fermentation (Fig. 4D).
A and B Effects of various promoters on the activities of ALS (encoded by alsS) and ALDC (encoded by alsD), respectively. C Effects of different promoters on the growth of B. subtilis after overexpression of the alsS and alsD genes. D Effects of various promoters on acetoin production by B. subtilis after overexpression of the alsS and alsD genes. All assays were performed in triplicate, and the standard deviations of biological replicates are indicated by error bars
The lack of increase in acetoin yield may result from the synthesis of ALS and ALDC in large quantities during the early stage of fermentation competing with cell growth for substrates and coenzymes, thus limiting the cell growth and acetoin yield. When the population cell density-induced promoters PaprE and Pcry3Aa were used to express alsS and alsD, bacterial growth was inhibited, with little overall change and only a limited increase in acetoin production. However, when alsS and alsD were expressed using the population cell density-induced promoter PsrfA, which has weak activity in the early stage of fermentation, the alsS and alsD expression remained low and had little effect on bacterial growth. After 36 h of fermentation, the activation of PsrfA led to rapid increases in the ALS and ALDC activities, which promoted acetoin accumulation. The final acetoin titer for this strain reached 44.4 g/L, an increase of 55.8% compared to 28.5 g/L for the original strain BS-1, at which point the molar conversion reached 90.8% of the theoretical maximum yield.
The above results indicate that the enhanced expression of alsS and alsD in the mid-to-late fermentation stage using the population cell density-induced promoter solved the problem of inhibited growth when using strong promoters to express alsS and alsD. This significantly increased the acetoin yield while avoiding the use of inducers and reducing production costs. In addition, the improved expression of alsS and alsD enhanced acetoin anabolic flow and increased acetoin yield while lowering the production of other metabolic by-products to a certain extent.
Fed-batch fermentation strain BSMAY-4-PsrfA
The fermentation was carried out in batches using bagasse lignocellulosic hydrolysate as the carbon source for strain BSMAY-4-PsrfA and the original strain BS-1. As shown in Fig. 5A, the lignocellulosic hydrolysate was added to the fermentation broth to maintain the glucose concentration at no less than 10.0 g/L. After 84 h of fermentation, 64.3 g/L AC was obtained, and the yield of AC reached 89.5% of the maximum theoretical yield. In addition, the main by-products were 2,3-butanediol, lactic acid and succinic acid, which were obtained at concentrations of 3.4 g/L, 0.9 g/L and 0.7 g/L, respectively (Table 2).
The environmental and climate impacts of fossil fuels are of great concern. The fermentable sugars in lignocellulosic biomass are a potential source of fermentable sugars that can be converted into biochemical products and fuels. Therefore, the development of suitable fermentation strategies using cheap, non-food and renewable resources has great economic use.
Conclusions
In this study, with sugarcane bagasse lignocellulose as the only fermentation substrate, the final acetoin titer for the recombinant strain BSMAY-4-PsrfA reached 64.3 g/L with the productivity of 0.765 g L−1 h−1 in a replenishment fractionated fermentation. As far as I know, this is the highest yield available for AC production by modified B. subtilis using bagasse lignocellulosic hydrolysate as a carbon source (Table 3). Key features of these strains include (1) knockouts of spore and autolysis genes that increase biomass and avoid waste of carbon sources; (2) Rex to regulate intracellular redox balance through redistribution of carbon flux; (3) blocking of competing pathways to increase acetoin yield; (4) enhancement of the acetoin pathway using the population cell density-induced promoter. In conclusion, compared to previous single-strategy modifications of model strains for acetoin production, this study uses a combination of optimized chassis cells and modular multi-strategy to redistribute intracellular carbon fluxes, providing a cost-effective and efficient approach for the industrial production of acetoin.
Materials and methods
Strains, plasmids, media, and materials
The primers used in the study and details of the strains are given in the Additional file 1. The gene-editing method of B. subtilis uses Cre/loxP site-specific gene operating system [37] and the competence of strains was prepared by reference [38]. The gene sequences of the promoters Pylb [39], PaprE [40], Pcry3Aa [41] and PsrfA [42] were obtained according to the reference and GenBank and gene synthesis. And primer designs were performed according to the gene sequences (see Additional file 1). The corresponding gene fragments were obtained by PCR reaction amplification. The plasmid pMA5 and the target gene fragment were double digested with endonucleases EcoR I and EcoR V (the enzymes EcoR V and Kpn I for the Pylb fragment), and the purified gene fragment and pMA5 fragment were ligated to transform E. coli JM109. After the plasmid is successfully chemically transferred, extraction and sequencing are performed. Finally, recombinant vectors pMA5-PsrfA-alsSD, pMA5-PaprE-alsSD, pMA5-Pcry3Aa-alsSD and pMA5-Pylb-alsSD were obtained. The recombinant plasmid pMA5-PHpaII-alsSD was obtained by the same method. The constructed expression vector was transferred to B. subtilis, and finally a recombinant strain that could correctly express the target gene was obtained.
All recombinant plasmids and recombinant strains were constructed in lysogeny broth (LB) medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl), with appropriate antibiotics (100 μg/mL ampicillin, 50 μg/mL kanamycin, or 30 μg/mL bleomycin) added if necessary.
The pretreated lignocellulosic hydrolysis product of bagasse contains 307.0 g/L glucose, 71.8 g/L xylose, small amounts of arabinose, mannose, and galactose, 6.2 g/L furfural, 3.3 g/L acetic acids, and small amounts of 5-hydroxymethylfurfural and formic acid (Shanghai Ting Pu Industrial Co kindly supplied them). The chemicals used in the experiments were purchased from Sangon Biotech (Shanghai, China). DNA gel purification kits, plasmid extraction kits, and bacterial genome rapid extraction kits were purchased from Generay Biotech (Shanghai, China). The 2 × Phanta Max Master Mix high-fidelity DNA polymerase for fragment amplification and colony Taq DNA polymerase for polymerase chain reaction were purchased from Vazyme Biotech (Nanjing, China). Restriction endonucleases were purchased from Takara Biotech (Beijing, China).
Cultivation in shake flasks
First, single colonies were picked from LB solid medium plates and inoculated into 10 mL of LB liquid medium, then incubated in a shaker for 10 h at 37 °C. Next, 1 mL of this culture was seeded into a 50 mL LB liquid medium and incubated for 10 h at 37 °C. Finally, 3 mL of this culture was inoculated into 250-mL conical flasks containing 50 mL of acetoin fermentation medium (5 g/L yeast extract, 2 g/L urea, 15 g/L corn syrup, 100 g/L glucose solution, or lignocellulose sugarcane bagasse hydrolysate), which were fermented at 37 °C in a shaker at 180 rpm. The acetoin yield, OD600, and fermentation by-product yields were measured after sampling at regular intervals.
Fed-batch fermentation conditions
The conditions for batch fermentation were as follows: fermenter volume of 5 L, inoculum volume of 6% of volume, fill volume of 2 L, aeration of 2 vvm, pH always controlled at 6.8, automatic addition of H2SO4 and NH3·H2O by using a computer-coupled peristaltic pump, fermentation temperature of 37 °C and dissolved oxygen concentration controlled at no less than 30% during the fermentation process. When the residual glucose in the fermentation broth dropped to approximately 10 g/L, the fermentation was split by adding lignocellulosic hydrolysate for replenishment.
Basal medium for batch fermentation (/L): 5 g yeast extract, 2 g urea, 15 g corn syrup, bagasse lignocellulosic hydrolysate at an initial glucose concentration of 100 g,2 g KH2PO4, 0.2 g CaCl2. Supplementary media (/L): 60 g corn syrup, bagasse lignocellulosic hydrolysate at an initial glucose concentration of 300 g.
Analysis methods
The OD600 values and concentrations of glucose, acetoin, 2,3-butanediol, and each organic acid were determined as described in a previous study [13]. The ALS and ALDC enzyme activity assays were conducted according to a previous report [43]. Due to the CCR effect [44], B. subtilis preferentially uses glucose as the only carbon source, therefore, only the amount of glucose was calculated for the carbon source consumption in this experiment. The redox coenzymes NAD+ and NADH concentrations were measured using microbial NADH and NAD+ kits from Enzyme Link Biotechnology (Shanghai, China).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
References
Zhang Y, Li S, Liu L, Jing W. Acetoin production enhanced by manipulating carbon flux in a newly isolated Bacillus amyloliquefaciens. Biores Technol. 2013;130:256–60.
Zhu C, Shen T, Liu D, Wu J, Chen Y, Wang L, Guo K, Ying H, Ouyang P. Production of liquid hydrocarbon fuels with acetoin and platform molecules derived from lignocellulose. Green Chem. 2016;18:2165–74.
Werpy T, Petersen G. Top value added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas. National Renewable Energy Lab., Golden, CO (US); 2004.
Yan P, Wu Y, Yang L, Wang Z, Chen T. Engineering genome-reduced Bacillus subtilis for acetoin production from xylose. Biotech Lett. 2018;40:393–8.
Gu L, Lu T, Li X, Zhang Y. A highly efficient thiazolylidene catalyzed acetoin formation: reaction, tolerance and catalyst recycling. Chem Commun. 2014;50:12308–10.
Zhang L, Liu Q, Ge Y, Li L, Gao C, Xu P, Ma C. Biotechnological production of acetoin, a bio-based platform chemical, from a lignocellulosic resource by metabolically engineered Enterobacter cloacae. Green Chem. 2016;18:1560–70.
Lu L, Mao Y, Kou M, Cui Z, Jin B, Chang Z, Wang Z, Ma H, Chen T. Engineering central pathways for industrial-level (3 R)-acetoin biosynthesis in Corynebacterium glutamicum. Microb Cell Fact. 2020;19:1–16.
Hui X, Jia SR, Liu JJ. Present research situation of acetoin production by biological method. Food Ferment Ind. 2010;36:137–44.
Fan Y, Tian Y, Zhao X, Zhang J, Liu J. Isolation of acetoin-producing Bacillus strains from Japanese traditional food—natto. Prep Biochem Biotechnol. 2013;43:551–64.
Xu H, Jia S, Liu J. Development of a mutant strain of Bacillus subtilis showing enhanced production of acetoin. Afr J Biotech. 2011;10:779–99.
Li D, Dai J-Y, Xiu Z-L. A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2,3-butanediol by Klebsiella pneumoniae. Biores Technol. 2010;101:8342–7.
Guo XW, Zhang YH, Cao CH, Shen T, Wu MY, Chen YF, Zhang CY, Xiao DG. Enhanced production of 2,3-butanediol by overexpressing acetolactate synthase and acetoin reductase in Klebsiella pneumoniae. Biotechnol Appl Biochem. 2015;61:707–15.
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S. The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng. 2014;23:34–41.
Nadal I, Rico J, Pérez-Martínez G, Yebra MJ, Monedero V. Diacetyl and acetoin production from whey permeate using engineered Lactobacillus casei. J Ind Microbiol Biotechnol. 2009;36:1233–7.
Zhang L, Yang Y, Sun J, Shen Y, Wei D, Zhu J, Chu J. Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Biores Technol. 2010;101:1961–7.
Luo Q, Jing W, Wu M. Enhanced acetoin production by Bacillus amyloliquefaciens through improved acetoin tolerance. Process Biochem. 2014;49:1223–30.
Sun JA, Zhang LY, Rao B, Shen YL, Wei DZ. Enhanced acetoin production by Serratia marcescens H32 with expression of a water-forming NADH oxidase. Bioresour Technol. 2012. https://doi.org/10.1016/j.biortech.2012.05.108.
Chen T, Liu WX, Fu J, Zhang B, Tang YJ. Engineering Bacillus subtilis for acetoin production from glucose and xylose mixtures. J Biotechnol. 2013;168:499–505.
Yang TW, Rao ZM, Xian Z, Xu MJ, Xu ZH, Yang ST. Effects of corn steep liquor on production of 2,3-butanediol and acetoin by Bacillus subtilis. Process Biochem. 2013;48:1610–7.
Nakano MM, Dailly YP, Zuber P, Clark DP. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J Bacteriol. 1997;179:6749–55.
Bae SJ, Kim S, Hahn JS. Efficient production of acetoin in Saccharomyces cerevisiae by disruption of 2,3-butanediol dehydrogenase and expression of NADH oxidase. Sci Rep. 2016;6:27667.
Pagels M, Fuchs S, Pané-Farré J, Kohler C, Menschner L, Hecker M, McNamarra PJ, Bauer MC, Von Wachenfeldt C, Liebeke M. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Mol Microbiol. 2010;76:1142–61.
Zhang X, Zhang R, Bao T, Yang T, Xu M, Li H, Xu Z, Rao Z. Moderate expression of the transcriptional regulator ALsR enhances acetoin production by Bacillus subtilis. J Ind Microbiol Biotechnol. 2013;40:1067–76.
Bressuire-Isoard C, Broussolle V, Carlin F. Sporulation environment influences spore properties in Bacillus: evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol Rev. 2018;42:614–26.
Nakagawa M, Kawano Y, Akasaka Y, Takabayashi T, Miyazawa N. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Dig Endosc. 2003;16:84–7.
Fujita M, Gonzalez-Pastor JE, Losick R. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–68.
Kodama T, Endo K, Ara K, Ozaki K, Kakeshita H, Yamane K, Sekiguchi J. Effect of Bacillus subtilis spo0A mutation on cell wall lytic enzymes and extracellular proteases, and prevention of cell lysis. J Biosci Bioeng. 2007;103:13–21.
Foster SJ, Smith TJ, Blackman SA. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146(Pt 2):249–62.
Wang Y, Chen Z, Zhao R, Jin T, Zhang X, Chen X. Deleting multiple lytic genes enhances biomass yield and production of recombinant proteins by Bacillus subtilis. Microb Cell Fact. 2014;13:1–11.
Blackman SA, Smith TJ, Foster SJ. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology. 1998;144(Pt 1):73–82.
Zhang Y, Li S, Liu L, Wu J. Acetoin production enhanced by manipulating carbon flux in a newly isolated Bacillus amyloliquefaciens. Biores Technol. 2013;130:256–60.
Teixeira RM, Cavalheiro D, Ninow J, Furigo A Jr. Optimization of acetoin production by Hanseniaspora guilliermondii using experimental design. Braz J Chem Eng. 2002;19:181–6.
Wang E, Bauer MC, Rogstam A, Linse S, Wachenfeldt CV. Structure and functional properties of the Bacillus subtilis transcriptional repressor Rex. Mol Microbiol. 2010;69:466–78.
Wietzke M, Bahl H. The redox-sensing protein Rex, a transcriptional regulator of solventogenesis in Clostridium acetobutylicum. Appl Microbiol Biotechnol. 2012;96:749–61.
Hu L, Huang H, Yuan H, Tao F, Xie H, Wang S. Rex in Clostridium kluyveri is a global redox-sensing transcriptional regulator. J Biotechnol. 2016. https://doi.org/10.1016/j.jbiotec.2016.06.024.
Li S, Gao X, Xu N, Liu L, Chen J. Enhancement of acetoin production in Candida glabrata by in silico-aided metabolic engineering. Microb Cell Fact. 2014;13(1):55.
Yan X, Yu HJ, Hong Q, Li SP. cre/lox system and pcr-based genome engineering in Bacillus subtilis. Appl Environ Microbiol. 2018; 74.
You J, Yang C, Pan X, Mengkai H, Yuxuan D, Osire T, Yang T, Rao Z. Metabolic engineering of Bacillus subtilis for enhancing riboflavin production by alleviating dissolved oxygen limitation. Bioresour Technol. 2021;333:125228. https://doi.org/10.1016/j.biortech.2021.125228.
Yu X, Xu J, Liu X, Chu X, Wang P, Tian J, Wu N, Fan Y. Identification of a highly efficient stationary phase promoter in Bacillus subtilis. Sci Rep. 2015;5:1–9.
Jan J, Valle F, Bolivar F, Merino E. Construction of protein overproducer strains in Bacillus subtilis by an integrative approach. Appl Microbiol Biotechnol. 2001;55:69–75.
Lee S-J, Pan J-G, Park S-H, Choi S-K. Development of a stationary phase-specific autoinducible expression system in Bacillus subtilis. J Biotechnol. 2010;149:16–20.
Guan C, Cui W, Cheng J, Zhou L, Guo J, Hu X, Xiao G, Zhou Z. Construction and development of an auto-regulatory gene expression system in Bacillus subtilis. Microb Cell Fact. 2015;14:1–15.
Zhang X, Zhang R, Yang T, Zhang J, Xu M, Li H, Xu Z, Rao Z. Mutation breeding of acetoin high producing Bacillus subtilis blocked in 2, 3-butanediol dehydrogenase. World J Microbiol Biotechnol. 2013;29:1783–9.
Singh KD, Schmalisch MH, Stulke J, Gorke B. Carbon catabolite repression in Bacillus subtilis: quantitative analysis of repression exerted by different carbon sources. J Bacteriol. 2008. https://doi.org/10.1128/JB.00848-08.
Bao T, Zhang X, Zhao X, Rao Z, Yang T, Yang S. Regulation of the NADH pool and NADH/NADPH ratio redistributes acetoin and 2, 3-butanediol proportion in Bacillus subtilis. Biotechnol J. 2015;10:1298–306.
Wang M, Fu J, Zhang X, Chen T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotech Lett. 2012;34:1877–85.
Jia X, Peng X, Liu Y, Han Y. Conversion of cellulose and hemicellulose of biomass simultaneously to acetoin by thermophilic simultaneous saccharification and fermentation. Biotechnol Biofuels. 2017;10:1–11.
Acknowledgements
None.
Funding
This research was supported by grants from the National Key Research and Development Program of China (No. 2020YFA0908300), the National Natural Science Foundation of China (No. 32171471), the Natural Science Foundation of Jiangsu Province (BK20210464), the Key Research and Development Program of Ningxia Hui Autonomous Region (No. 2020BFH02011), the 111 Project (111-2-06), and the Jiangsu Province “Collaborative Innovation Center for Advanced Industrial Fermentation” industry development program.
Author information
Authors and Affiliations
Contributions
ZMR and XZ designed the research; QW performed the major experiments and wrote the manuscript draft; and QW, KXR, RMH, RQL, TB, XWP, MJX, and TWY analyzed the data and drafted and revised the manuscript. QW and XZ contributed equally. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to the publication of this manuscript.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The recombinant strains, recombinant plasmids, and primers were used in this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Q., Zhang, X., Ren, K. et al. Acetoin production from lignocellulosic biomass hydrolysates with a modular metabolic engineering system in Bacillus subtilis. Biotechnol Biofuels 15, 87 (2022). https://doi.org/10.1186/s13068-022-02185-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-022-02185-z