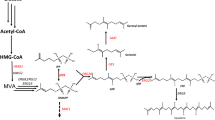
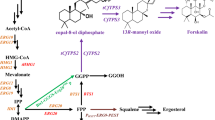
Abstract
Monogalactosyldiacylglycerol (MGDG), a predominant photosynthetic membrane lipid derived from plants and microalgae, has important applications in feed additives, medicine, and other fields. The low content and various structural stereoselectivity differences of MGDG in plants limited the biological extraction or chemical synthesis of MGDG, resulting in a supply shortage of monogalactosyldiacylglycerol with a growing demand. Herein, we established Saccharomyces cerevisiae as a cell factory for efficient de novo production of monogalactosyldiacylglycerol for the first time. Heterologous production of monogalactosyldiacylglycerol was achieved by overexpression of codon-optimized monogalactosyldiacylglycerol synthase gene MGD1, the key Kennedy pathway genes (i.e. GAT1, ICT1, and PAH1), and multi-copy integration of the MGD1 expression cassette. The final engineered strain (MG-8) was capable of producing monogalactosyldiacylglycerol with titers as high as 16.58 nmol/mg DCW in a shake flask and 103.2 nmol/mg DCW in a 5 L fed-batch fermenter, respectively. This is the first report of heterologous biosynthesis of monogalactosyldiacylglycerol in microorganisms, which will provide a favorable reference for study on heterologous production of monogalactosyldiacylglycerol in yeasts.
Graphical Abstract

Similar content being viewed by others
Introduction
Monogalactosyldiacylglycerol (MGDG) has been proven to have multiple physiological active functions. Various monogalactosyldiacylglycerols from cyanobacteria have been found to inhibit the enzymatic activity of HIV-1 reverse transcription [1]. The MGDG from Spirulina has demonstrated a range of functional activities, including antioxidant and anti-allergic properties that promote wound regeneration in zebra fish [2]. Four types of MGDGs isolated from Sargassum thunbergii extract exhibited moderate antifungal effects on Candida albicans [3]. The MGDG isolated from Petalonia binghamiae was also characterized as a potent inhibitor of the activities of mammalian DNA polymerase α [4]. Moreover, the MGDGs obtained from Sargassum muticum inhibited the bacteria Shewanella putrefaciens and Polaribacter irgensii and the fungi Halosphaeriopsis mediosetigera and Asteromyces cruciatus with the inhibitory activity was reported for a concentration of 0.75 mg/L [5]. Natural MGDG mainly comes from thylakoid membrane lipids in plants and microalgae [6, 7], which impede the application of microalgae as sources of MGDG based on the relatively low fermentation biomass. Compared with general industrial microorganisms (such as bacteria, yeast, etc.), most microalgae can only be cultured through traditional photoautotrophs [8, 9], and only a few microalgae can achieve the approximately 0.4 g/L of biomass after 12 h with under different culture conditions [10]. In view of the current preparation technology for MGDGs is complex, with high production costs and low yields, which cannot meet the needs of its market-oriented development. The establishment of novel and economical biotechnological processes for large-scale production of MGDG is in high demand. Hence, there is a growing interest in microbial production of MGDG as a novel sustainable industrial model to effectively alleviate the growing demand.
With the breakthroughs in microbial synthetic biology technologies, the biosynthesis of MGDG in microbial cell factories is expected to be an effective strategy to take the place of the traditional plant extraction and chemical synthesis. Among these microorganisms, Saccharomyces cerevisiae as a model microorganism has been used in the wide variety of food and drug related industries owing to its clear understanding of genetic background, regulatory networks, and metabolic pathways. With the rapid development of synthetic biology, it has been successfully engineered as a cell factory for the production of various chemicals [11], such as polyunsaturated fatty acids [12, 13], terpenes [14, 15], organic acids [16, 17] and other products [18, 19]. However, there has been no report on the biosynthesis of MGDG in S. cerevisiae so far.
In this study, we conducted the MGDG heterologous biosynthesis in S. cerevisiae by introducing the MGDG synthase gene MGD1 and the key genes of the Kennedy pathway (Fig. 1). The biosynthesis of MGDG was achieved by integrating the MGD1 expression cassette into the chromosome of S. cerevisiae. Subsequently, the Kennedy pathway genes were overexpressed and the copy number of MGD1 was fine-tuned to further improve MGDG biosynthesis. Finally, the final engineered strain of MG-8 achieved a MGDG titer of 103.2 nmol/mg DCW in a 5 L fed-batch fermentation. The stability and high-level production of these engineered strains promise further exploration for industrial applications of MGDG.
Metabolic engineering of S. cerevisiae for biosynthesis of MGDG. MGDG was synthesized from the Kennedy pathway intermediate diacylglycerol (DAG) by MGDG synthase (MGD1). Genes overexpressed and knockout in the present study were shown in red and purple, respectively. ZWF1 glucose 6-phosphate dehydrogenase, ACC1 acetyl-CoA carboxylase, GAT1 glycerol-3-phosphate acyltransferase, ICT1 lysophosphatidic acid acyltransferase, PAH1 phosphatidic acid phosphatase, MGD1 MGDG synthase, FAS fatty acid synthase, DGA1 diacylglycerol acyltransferase, G3P glycero-3-phosphate, LPA lysophosphatidic acid, PA phosphatidic acid, DAG diacylglycerol, TAG triacylglycerol, PEP phosphoenolpyruvate, GA3P 3-phosphoglyceraldehyde, DHAP dihydroxyacetone phosphate
Materials and methods
Strains, medium and culture condition
E. coli DH5α was employed for plasmid construction and cultured in LB medium with 100 mg/L ampicillin. S. cerevisiae CEN.PK113-7D (MATɑ, MAL2-8c SUC2) was used as the chassis for MGDG biosynthesis and routinely cultured in YPD medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose). Recombinant S. cerevisiae strains were screened in YPD solid medium containing 100 mg/L Zeocin, 200 mg/L Hygromycin B, and/or 200 mg/L G418.
Plasmid construction
The codon-optimized MGDG synthase gene MGD1 from cucumber was synthesized by Jinkairui Biological Engineering Co., Ltd. (Wuhan, China). The key Kennedy pathway genes including glycerol-3-phosphate acyltransferase (GAT1), lysophosphatidic acid acyltransferase (ICT1), and phosphatidic acid phosphatase (PAH1) were amplified from the chromosome of the S. cerevisiae and subsequently cloned into the donor plasmids using Gibson assembly methods. Benchling CRISPR tool (https://benchling.com) was used to design gRNAs, which were cloned into BsaI digested PSCM-gRNA. All the DNA polymerase, T4 DNA ligases, and restriction enzymes were purchased from New England Biolabs. Primers were synthesized by Tsingke Biotech Co., Ltd. (Wuhan, China). All the plasmids used in this study are listed in Supplementary Table S1. All the primers used for plasmid construction and genome integration were listed in Supplementary Table S2. The coding sequences of heterologous genes were listed in Supplementary Table S3.
Strain construction
The integration of MGDG biosynthetic pathway genes into the chromosome of S. cerevisiae was performed through the CRISPR/Cas9 method. The gene expression cassettes together with the homology arms and the corresponding gRNA plasmids were co-transformed into the Cas9-expressing strain (MG-0). The transformation of S. cerevisiae was performed using the LiAc method [20]. In this study, all the constructed strains were listed in Table 1 and the corresponding integration sites were listed in Supplementary Table S4.
Fed-batch fermentation in a 5 L bioreactor
An engineered single colony strain was inoculated into a test tube containing 5 mL of YPD medium, incubated for 12 h at 30℃ and 250 rpm, and then transferred to 250 mL shaker flask containing 80 mL YPD with 6% inoculum in the same culture conditions. Subsequently, the secondary seed cultures were inoculated into 5 L bioreactors containing 2.5 L of YPD. The dissolved oxygen concentration was maintained at 30% by adjusting stirring rates (200 ~ 700 rpm). The initial glucose concentration was at 20 g/L, and the pH was maintained at 5.5 using 3 M NaOH. The feed medium (500 mL) was composed of 400 g/L glucose, 18 g/L KH2PO4, 7 g/L K2SO4, 0.56 g/L Na2SO3, 20 mL/L trace element A (comprising 5.75 g/L ZnSO4·7H2O, 0.47 g/L CoCl2·6H2O, 0.32 g/L MnCl2·4H2O, 0.48 g/L NaMoO4·2H2O, 2.8 g/L FeSO4·7H2O, 2.9 g/L CaCl2·2H2O, and 80 mL 0.5 M EDTA, pH 8.0), 24 mL/L trace element B (comprising 0.05 g/L biotin, 25 g/L myoinositol, 1 g/L thiamine HCl, 1 g/L pyridoxal HCl, 1 g/L calcium pantothenate, 1 g/L nicotinic acid, and 0.02 g/L p-aminobenzoic acid), 1.0 g FeSO4·7H2O. The glucose concentration was maintained at 0 ~ 2 g/L. Samples were taken from the bioreactor to determine the glucose concentration, cell densities and MGDG titer at regular intervals.
Analytical methods
MGDG analyses were carried out by a triple quadrupole MS/MS (Xevo TQ-S, Waters, USA) with electrospray ionization (ESI) source coupled with an Acquity Ultra-Performance Liquid Chromatography (UPLC) system (Waters, USA). The ESI/MS analysis was performed according to previously described methods [10, 21]. 5 g of dried yeast cells were thoroughly ground with liquid nitrogen for breaking the yeast wall, followed by adding 1 mL chloroform/methanol (1:1, v/v) and then were mixed with internal standards (ISTD, MGDG 18:0–18:0) for quantification. All the lipid standards were purchased from Avanti Polar Lipids Ltd. (USA). All experiments were repeated three times on different biological samples.
The content of glucose was determined by an SBA-90 biosensor (Shandong Academy of Sciences, Jinan, China). Cell densities (OD600) were measured using a UV-2802 spectrophotometer (Lonico Instrument Co. Ltd., Shanghai, China).
Results
Construction of MGDG heterologous synthetic pathway in S. cerevisiae strain
S. cerevisiae strain possesses the native Kennedy pathway that can supply the precursor diacylglycerol DAG, but it is not able to synthesize MGDG due to the lack of MGDG synthase. To enable the production of MGDG, the codon optimized MGDG synthase gene (MGD1) from cucumber [22] was introduced into S. cerevisiae. In this study, a constitutive promoter TEF1p was tested to drive the expression of MGD1, resulting in 2.42 nmol/mg DCW of MGDG in S. cerevisiae MG-1 (Fig. 2B). Compared with the MGDG standard substance, the fermentation product of MG-1 showed two new peaks at 4.09 and 4.55 min (Fig. 2A), respectively. The MGDGs in S. cerevisiae MG-1 were further identified as MGDG (32:2) and MGDG (34:2) by MS analysis (Figure. S1). Subsequently, we knocked out the diacylglycerol acyltransferase gene (DGA1) for more flux from Kennedy pathway to synthesize MGDG. The titer of MGDG in MG-2 was increased by 1.33-fold to 5.65 nmol/mg DCW (Fig. 2B).
Construction of MGDG producing S. cerevisiae strain. A MRM chromatograms for MGDG in strain MG-1 and MGDG standards. Production of MGDG with the MGD1 gene driven by a constitutive TEF1 promoter in MG-1 strain. The retention time of the MGDG (32:2) and MGDG (34:2) peak was in 4.09 and 4.55 min, respectively. B Improvement of MGDG accumulation via blocking the competitive pathway (ΔDGA1) in MG-1 strain. The fermentation products were detected by HPLC–MS and the results represented the average ± s. d. of biological triplicates (n = 3)
Overexpression of Kennedy pathway genes and enhancing the supply of NADPH to increase the production of MGDG
To improve the production of MGDG, overexpression of Kennedy pathway genes is beneficial for the production of DAG. Therefore, GAT1, ICT1, and PAH1 expression cassettes were integrated into the genome of MG-2 strain to obtain engineered strains MG-3, MG-4 and MG-5, respectively (Table 1). When GAT1 was overexpressed, the titer of MGDG in MG-3 was increased by 2.20-fold to ~ 7.758 nmol/mg DCW. When ICT1 and PAH1 were overexpressed, the titer of MGDG in strain MG-5 was increased to 10.58 nmol/mg DCW, about 3.37-fold higher than that of MG-1 (Fig. 3).
Overexpression of Kennedy pathway genes in S. cerevisiae. The Kennedy pathway genes, GAT1, ICT1, and PAH1 were overexpressed to enhance the metabolic fluxes towards DAG and ACC1 was overexpressed to enhance the supply of malonyl-CoA. " + " represented gene overexpression in the chromosome of S. cerevisiae. The results represented the average ± s.d. of biological triplicates (n = 3)
The NADPH from pentose phosphate pathway is required for fatty acid biosynthesis, in which NADPH is used to reduce the acetyl group to the acyl chain of fatty acids [23] and enhanced production of isoprenoids [24]. Glucose-6-phosphate dehydrogenase (ZWF1) is the rate-limiting enzyme in the pentose phosphate pathway [25, 26], and overexpression of ZWF1 should benefit to improve the accumulation of MGDG. Simultaneous overexpression of acetyl-CoA carboxylase (ACC1) and ZWF1 (MG-6) further increased the production of MGDG to 12.59 nmol/mg DCW, which was about 4.2-fold higher than that of MG-1 (Fig. 3).
Increasing the copy number of MGD1 to further enhance MGDG biosynthesis
To further improve the production of MGDG, S. cerevisiae strains with multiple copies of MGD1 expression cassette were constructed. The integration of an additional copy of MGD1 gene into MG-6 and MG-7 was carried out, respectively. The production of MGDG in MG-7 and MG-8 was approximately 0.23- and 0.31-fold higher than that of the single-copy strains MG-6, indicating the significance of the copy number of MGD1 expression cassette on the production of MGDG. Therefore, another MGD1 copy was integrated to construct MG-9, harboring four copies of MGD1 expression cassette. While the production of MGDG in MG-9 was the same level in that of MG-8, indicating that three copies of MGD1 expression cassette was optimal for MGDG production in S. cerevisiae (Fig. 4).
Multi-copy integration of MGD1 to enhance MGDG biosynthesis in S. cerevisiae. " + " represented gene overexpression and the corresponding number represented the copy number of MGD1 expression cassette integrated into the chromosome of S. cerevisiae. The results represented the average ± s.d. of biological triplicates (n = 3). The significance of the differences was tested using a paired t-test, **p < 0.01
Fed-batch fermentation for high-level production of MGDG
Fed-batch fermentation of the optimal strain MG-8 strain was carried out in a 5 L bioreactor. The initial glucose concentration was 20 g/L, after which glucose was added in the fermenter to keep the glucose concentration at a low level (0 ~ 2 g/L). After fermentation for 120 h, the yeast cell densities (OD600) reached 72.3 in the fed-batch fermenter and the highest production of MGDG was achieved to 103.2 nmol/mg DCW (Fig. 5), representing the highest titer of MGDG at present.
Fermentation profiles of the engineered S. cerevisiae strain in 5 L bioreactors. The fed-batch fermentation was performed using the engineered S. cerevisiae strain MG-8. Samples were taken to measure OD600, glucose concentration and MGDG titer. The results represented the average ± s.d. of biological triplicates (n = 3)
Discussion
MGDG is the most abundant lipids in nature with a wide range of applications. While traditional preparation methods, such as plant or microalgae extraction and chemical synthesis, suffer from low yield and high cost [27]. At present, the common extraction and purification process for MGDGs is as follows: acetone extraction for microalgae and plant products combined extraction solution and silica gel separation column with different proportions of organic solvents (chloroform: methanol) for segmented elution and separation, in which this preparation not only involves multiple steps, low yield, and high cost, but also facilitates a large of toxic organic solvents. These residual toxic organic solvents in the product are not suitable for use in pharmaceutical materials and the preparation of ingredients, cosmetics, and food additives. Industrial production of MGDG using microbial cell factories has attracted increasing attention. In this study, S. cerevisiae was engineered for the production of MGDG for the first time.
Metabolic engineering is an effective way to increase target production. In this study, metabolic flux of Kennedy pathway was increased by overexpression of GAT1, ICT1, and PAH1. Although the Kennedy pathway provides the precursor DAG for MGDG synthesis, it also competes with synthesis pathways of TAG and other essential substances for basic cellular functions. Hence, more procedures could be taken to manipulate more flux from Kennedy pathway to synthesize MGDG. For example, the competing pathway genes should be removed or to down-regulated for redirecting the metabolic fluxes towards MGDG biosynthesis. In addition, the other UDP-galactose biosynthesis pathway such as UDP-glucose pyrophosphorylase (UGP) and UDP-glucose 4-epimerase (UGE) genes should be overexpressed and preferably optimized to further enhance MGDG biosynthesis in S. cerevisiae. Most microalgae can only be cultured through traditional photoautotrophs with the low biomass, which limited the development of the industrial application of microalgae as sources of MGDG. For some oil-producing yeasts such as Yarrowia lipolytica, it is widely used in various food and drug industries based on its superior characteristics. With the rapid development of gene editing technologies, it has been successfully developed as chassis host cells for the production of various fine chemicals [28, 29], including fatty acid derivatives [30], polyunsaturated fatty acids [31] and some specific high-value-added lipid compounds for the dietary supplement and feed industries [32]. However, there is no report on the engineering of Y. lipolytica for producing MGDG so far. Hence, oleaginous yeasts with the abundant acetyl-CoA properties have an incomparable advantage in production of MGDG in the future.
In summary, we successfully constructed MGDG synthetic pathway in S. cerevisiae, achieving heterologous biosynthesis of MGDG by co-expressing MGDG synthase MGD1 and the Kennedy pathway rate limiting enzymes GAT1, ICT1, and PAH1. The other cell hosts such as the C. reinhardtii, the total content of MGDGs ranged from 0.23 to 36.42 nmol/mg DCW during the logarithmic phase under low light (LL) [10]. Through metabolic engineering and bioprocess optimization, the titer of MGDG reached as high as 16.58 nmol/mg DCW in a shake flask and 103.2 nmol/mg DCW in a 5 L fed-batch fermenter, respectively. Owing to the fermentation by fermenter is more conducive to improve the product’s yield due to better control of dissolved oxygen, rotation speed, PH and other fermentation conditions, similar examples have been reported in many literatures [33, 34]. In the future, it is worth trying to optimize the fermentation conditions to further increase the yield of MGDG. This study provides a new way for de novo heterologous biosynthesis of MGDG in S. cerevisiae.
Data availability
No datasets were generated or analysed during the current study.
References
Reshef V, Mizrachi F, Maretzki T, et al. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J Nat Prod. 1997;60:1251–1260.
Yang X, Li Y, Li Y, et al. Solid matrix-supported supercritical CO2 enhances extraction of gamma-linolenic acid from the cyanobacterium Arthrospira (Spirulina) platensis and bioactivity evaluation of the molecule in Zebrafish. Mar Drugs. 2019. https://doi.org/10.3390/md17040203.
Kim YH, Kim EH, Lee C, et al. Two new monogalactosyl diacylglycerols from brown alga Sargassum thunbergii. Lipids. 2007;42:395–399.
Mizushina Y, Sugiyama Y, Yoshida H, et al. Galactosyldiacylglycerol, a mammalian DNA polymerase alpha-specific inhibitor from a sea alga, Petalonia bingbamiae. Biol Pharm Bull. 2001;24:982–987.
Plouguerné E, Ioannou E, Georgantea P, et al. Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar Biotechnol (NY). 2010;12:52–61.
Block MA, Dorne AJ, Joyard J, Douce R. Preparation and characterization of membrane-fractions enriched in outer and inner envelope membranes from spinach-chloroplasts. J Biol Chem. 1983;258:3273–3280.
Iwai M, Yamada-Oshima Y, Asami K, et al. Recycling of the major thylakoid lipid MGDG and its role in lipid homeostasis in Chlamydomonas reinhardtii. Plant Physiol. 2021;187:1341–1356.
Riccio G, De Luca D, Lauritano C. Monogalactosyldiacylglycerol and sulfolipid synthesis in microalgae. Mar Drugs. 2020. https://doi.org/10.3390/md18050237.
Plouguerné E, da Gama BA, Pereira RC, Barreto-Bergter E. Glycolipids from seaweeds and their potential biotechnological applications. Front Cell Infect Microbiol. 2014;4:174.
Zang Z, Li Y, Hu Q, Han D. Unraveling enhanced membrane lipid biosynthesis in Chlamydomonas reinhardtii starchless mutant sta6 by using an electrospray ionization mass spectrometry-based lipidomics method. J Oceanol Limnol. 2020;38:783–794.
Li M, Borodina I. Application of synthetic biology for production of chemicals in yeast Saccharomyces cerevisiae. Fems Yeast Res. 2015. https://doi.org/10.1111/1567-1364.12213.
Yazawa H, Iwahashi H, Kamisaka Y, et al. Improvement of polyunsaturated fatty acids synthesis by the coexpression of CYB5 with desaturase genes in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2010;87:2185–2193.
Shi T, Yu A, Li M, et al. Identification of a novel C22-Δ4-producing docosahexaenoic acid (DHA) specific polyunsaturated fatty acid desaturase gene from Isochrysis galbana and its expression in Saccharomyces cerevisiae. Biotech Lett. 2012;34:2265–2274.
Paramasivan K, Mutturi S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit Rev Biotechnol. 2017;37:974–989.
Carrau FM, Medina K, Boido E, et al. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett. 2005;243:107–115.
Jang B-K, Ju Y, Jeong D, et al. L-lactic acid production using engineered Saccharomyces cerevisiae with improved organic acid tolerance. J Fungi. 2021. https://doi.org/10.3390/jof7110928.
Raab AM, Gebhardt G, Bolotina N, et al. Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng. 2010;12:518–525.
Leonard E, Yan YJ, Lim KH, Koffas MAG. Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 2005;71:8241–8248.
Zhang T, Sun L, Xin Y, et al. A vaccine grade of yeast Saccharomyces cerevisiae expressing mammalian myostatin. BMC Biotechnol. 2012. https://doi.org/10.1186/1472-6750-12-97.
Gietz RD, Woods RA. Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods Mol Biol. 2006;313:107–120.
Yoon K, Han D, Li Y, et al. Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell. 2012;24:3708–3724.
Shimojima M, Ohta H, Iwamatsu A, et al. Cloning of the gene for monogalactosyldiacylglycerol synthase and its evolutionary origin. Proc Natl Acad Sci U S A. 1997;94:333–337.
Wasylenko TM, Ahn WS, Stephanopoulos G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab Eng. 2015;30:27–39.
Kwak S, Yun EJ, Lane S, et al. Redirection of the glycolytic flux enhances isoprenoid production in Saccharomyces cerevisiae. Biotechnol J. 2020. https://doi.org/10.1002/biot.201900173.
Zhao X, Shi F, Zhan W. Overexpression of ZWF1 and POS5 improves carotenoid biosynthesis in recombinant Saccharomyces cerevisiae. Lett Appl Microbiol. 2015;61:354–360.
Feng R, Li J, Zhang A. Improving isobutanol titers in Saccharomyces cerevisiae with over-expressing NADPH-specific glucose-6-phosphate dehydrogenase (Zwf1). Ann Microbiol. 2017;67:785–791.
Kaufmann B, Christen P. Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal. 2002;13:105–113.
Larroude M, Rossignol T, Nicaud JM, Ledesma-Amaro R. Synthetic biology tools for engineering Yarrowia lipolytica. Biotechnol Adv. 2018;36:2150–2164.
Groenewald M, Boekhout T, Neuvéglise C, et al. Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit Rev Microbiol. 2014;40:187–206.
Liu H, Song Y, Fan X, et al. Yarrowia lipolytica as an oleaginous platform for the production of value-added fatty acid-based bioproducts. Front Microbiol. 2020;11: 608662.
Ledesma-Amaro R, Nicaud JM. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res. 2016;61:40–50.
Sun ML, Madzak C, Liu HH, et al. Engineering Yarrowia lipolytica for efficient γ-linolenic acid production. Biochem Eng J. 2017;117:172–180.
Wu F, Wang S, Zhou D, et al. Metabolic engineering of Escherichia coli for high-level production of the biodegradable polyester monomer 2-pyrone-4,6-dicarboxylic acid. Metab Eng. 2024;83:52–60.
Zhang Q, Yu S, Lyu Y, et al. Systematically engineered fatty acid catabolite pathway for the production of (2S)-naringenin in Saccharomyces cerevisiae. ACS Synth Biol. 2021;10:1166–1175.
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (2022M712772).
Author information
Authors and Affiliations
Contributions
Xiaosong Gu, Changxin Luo and Jintao Cheng designed the study, analyzed the data, and wrote the paper. Xiaosong Gu and Yumei Shi conducted the experiments. All the authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13068_2024_2560_MOESM1_ESM.doc
Supplementary Material 1. Experimental results, plasmids constructed in this study, and primers used, list of gene coding sequences, and integration sites
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, X., Shi, Y., Luo, C. et al. Establishment of Saccharomyces cerevisiae as a cell factory for efficient de novo production of monogalactosyldiacylglycerol. Biotechnol Biofuels 17, 111 (2024). https://doi.org/10.1186/s13068-024-02560-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-024-02560-y