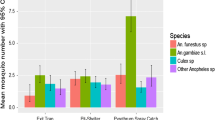
Abstract
Background
Malaria is a major public health problem in Angola, with Anopheles gambiae sensu lato (s.l.) and An. funestus s.l. being the primary vectors. This study aimed to clarify the information gaps concerning local Anopheles mosquito populations. Our objectives were to assess their abundance, geographical dispersion, and blood-feeding patterns. We also investigated their insecticide resistance. Molecular methods were used to identify sibling species, determine the origin of blood meals, measure Plasmodium falciparum infection rates, and detect the presence of knockdown resistance (kdr) mutations.
Methods
Adult mosquitoes were collected indoors using CDC light traps from nine randomly selected households at two sentinel sites with distinct ecological characteristics. The samples were collected from 1 February to 30 June 2022. Anopheles mosquitoes were morphologically identified and subjected to molecular identification. Unfed Anopheles females were tested for the presence of P. falciparum DNA in head and thorax, and engorged females were screened for the source of the blood meals. Additionally, members of An. gambiae complex were genotyped for the presence of the L1014F and L1014S kdr mutations.
Results
In total, 2226 adult mosquitoes were collected, including 733 Anopheles females. Molecular identification revealed the presence of Anopheles coluzzii, An. gambiae senso stricto (s.s.), An. arabiensis, and An. funestus s.s. Notably, there was the first record of An. coluzzii/An. gambiae s.s. hybrid and An. vaneedeni in Benguela Province. Plasmodium falciparum infection rates for An. coluzzii at the urban sentinel site and An. funestus s.s. at the rural site were 23.1% and 5.7%, respectively. The L1014F kdr mutation was discovered in both resistant and susceptible An. coluzzii mosquitoes, while the L1014S mutation was detected in An. gambiae s.s. for the first time in Benguela Province. No kdr mutations were found in An. arabiensis.
Conclusions
This study provides valuable insights into the molecular characteristics of malaria vectors from the province of Benguela, emphasising the need for continuous surveillance of local Anopheles populations regarding the establishment of both kdr mutations for tailoring vector control interventions.
Graphical abstract

Similar content being viewed by others
Background
Malaria remains a major public health challenge, with an estimated 249 million cases and 608,000 deaths worldwide in 2022 [1]. In sub-Saharan Africa, the primary vectors responsible for malaria transmission are Anopheles gambiae senso stricto (s.s.), An. coluzzii, An. arabiensis, and An. funestus s.s. [2]. In Angola, malaria is endemic and mostly caused by Plasmodium falciparum [3]. Additionally, cases caused by Plasmodium malariae have been recorded in the province of Benguela [4]. In Angola, malaria transmission is complex with vectors occupying different eco-epidemiological areas. Anopheles gambiae s.s., An. coluzzii, An. arabiensis, and An. funestus are considered the primary vectors in Angola [3, 5,6,7]. Insecticide-based vector control strategies, such as the use of insecticide-treated nets (ITN) and indoor residual spraying (IRS), have significantly contributed to the reduction of the malaria burden [8]. However, the emergence and spread of insecticide resistance in these main vectors [9, 10] are threating the effectiveness of these interventions. Two main types of mechanism have been identified to be involved in insecticide resistance: target-site resistance, which involves mutations in the target proteins of insecticides, and metabolic resistance, which involves increased detoxification of insecticides [11]. One well-known target site resistance is the knockdown resistance (kdr) caused by mutations in the voltage-gated sodium channel gene, which compromises its binding to pyrethroid insecticides [12]. The identification of Anopheles species and their susceptibility statute to insecticides are essential for effective planning of vector control strategies. Molecular techniques have improved the accuracy of species identification within the An. gambiae complex and Anopheles funestus group [13, 14]. Furthermore, molecular methods, such as polymerase chain reaction (PCR) assays, have been developed to detect kdr mutations, enabling the assessment of the frequency and distribution of these resistance-associated mutations in field populations [15]. Despite the crucial role of vector control in reducing malaria transmission, little published evidence on Angola malaria vector abundance, behaviour, and insecticide susceptibility has been published in the past 20 years [16,17,18,19,20]. This study aimed to describe the local populations of Anopheles mosquito species in two districts of Benguela, with a focus on characterising the vector abundance, behaviour, and insecticide susceptibility of these populations. Using molecular methods to investigate the presence of knockdown resistance (kdr), P. falciparum infectivity rates, and blood meal sources, the outcomes will aid in bridging knowledge gaps concerning malaria vectors in Benguela Province.
Methods
Study areas
The study was conducted from 1 February to 30 June 2022 at two sentinel sites in the province of Benguela. Bela Vista (Fig. 1A, urban site, 12°37′27.8ʺS, 13°22′23.4ʺE) is an urban neighbourhood located in the city of Benguela at an average altitude of 20 m. The climate is characterised by a dry season (May to September) and a rainy season that extends from October to April with an average rainfall of 12.9 mm (October) to 33.9 mm (March). The average temperature varies from 30 °C (March/April) to 19 °C (July/August). Cavaco River (Fig. 1C, 12°34′10.0ʺS 13°25′11.5ʺE) is located at the urban north border of the city of Benguela. It is used by the local population during the wet and dry season to collect water for different uses. During the dry season, the local population dig wells in the dry riverbed to facilitate water collection. Alto Catumbela in the district of Ganda (Fig. 1B, rural site, 12°56′15.3ʺS 14°45′19.1ʺE) is a rural community located 210 km east of Bela Vista at an altitude of 1244 m. The predominant activity in this area is agriculture and cattle breeding. Families who own cattle keep them in corrals or fenced off near their houses. Temperatures range from 31 °C (September) to 10 °C (June/July). The rainy season lasts from October to April, with a monthly rainfall average of 37 mm in April to 130.6 mm in December. In both locations, during field work, we observed that households use ITN, aerosol insecticides, and mosquito-repelling coils to protect themselves against mosquito bites.
Adult sampling methods
Adult mosquitoes were collected indoors from the Bela Vista and Alto Catumbela sentinel sites. At each sentinel site, nine houses were randomly selected at least 50 m apart. The selected houses had cement or mud walls, painted or unpainted, with or without ceilings, and roofs made of sheet metal or traditional materials. CDC light traps (CDC-LT; Model 512; John W. Hock Company, Gainesville, FL, USA) were used overnight to collect mosquitoes for 3 consecutive nights in three different houses each night. The CDC-LTs were installed circa 150 cm above the ground at the foot end of the sleeping area where people were sleeping under a mosquito net. Mosquitoes were captured between 18:00 and 07:00. Non-anopheline species were discarded after counting. In households where permission was granted for the collection of adult mosquitoes indoors, the collection procedure was communicated to the household head, who provided their consent.
Larva collections and WHO susceptibility test
To test the insecticide susceptibility of mosquitoes, the WHO tube test was performed using papers impregnated with alpha-cypermethrin at a diagnostic concentration of 0.05% [21]. The tests were performed using F0 generation, 2–5-day-old females collected as larvae in June 2022 from shallow wells in the dry riverbed of Cavaco River (Fig. 1C, 12°34′10.0ʺS 13°25′11.5ʺE). Collected larvae were brought to the field insectary in Benguela and reared to adult stage. Before the start of the test, a preliminary morphological identification was carried out on live adult Anopheles mosquitoes. Mosquitoes identified as Anopheles gambiae s.l. were sorted for WHO tube tests. At the end of the test, a confirmatory morphological identification was carried out to ensure that only Anopheles gambiae s.l. were included in the mortality analysis. Any other mosquito species found were excluded from the final count. The population was classified as “resistant” if mortality < 90%, “possible resistant” if mortality < 98%, and “susceptible” if mortality ≥ 98%. After the assay, mosquitoes were individually stored in labelled microtubes containing silica gel for further molecular processing.
Sample processing
Anopheles mosquito species identification
Prior to DNA extraction, female Anopheles mosquitoes were identified to species using morphological keys [22, 23]. Morphological identification was performed by trained entomologists. Abdomens from captured female Anopheles mosquitoes were categorised as unfed or fed. Samples were stored individually in labelled microtubes containing silica gel for molecular analysis at room temperature. Genomic DNA was extracted according to Collins et al. [24]. DNA was used for identification of sibling species, the presence of P. falciparum, blood meal source, and presence of kdr mutations. Molecular sibling species identification was conducted on randomly selected morphological identified members of the An. gambiae s.l. and An. funestus s.l. For An. gambiae s.l., molecular identification was conducted by targeting species-specific polymorphisms at the intergenic spacer (IGS) of the ribosomal DNA [25] and by a PCR assay targeting the SINE 200X6.1 retrotransposon insertion [26]. Specimens were identified as An. coluzzii, An. gambiae s.s., An. coluzzii/An. gambiae s.s. hybrids, Anopheles melas, or An. arabiensis if they had coincident species-specific patterns for both markers. For the An. funestus s.l. members, which include An. funestus s.s., Anopheles parensis, An. rivulorum, An. leesoni, An. rivulorum-like, and An. vaneedeni, molecular identification was conducted using the protocol described by Koekemoer et al. [14]. All PCR assays contained negative controls (no DNA template) and positive controls, consisting of samples of known specimen molecular identification. Anopheles gambiae s.l. and An. funestus s.l. PCR species-specific identifications giving a negative result and randomly selected specimens of less common species of Anopheles mosquitoes were submitted to Cytochrome Oxidase I (COI) barcode sequencing for species identification, following Folmer et al. [27]. Comparison between morphological and molecular identification was performed to assess the accuracy of the morphological identification. All COI sequences have been submitted to NCBI and are available online under GenBank accession numbers OR839824–OR839854.
Identification of the blood meal source
The origin of blood meals in blood-fed Anopheles mosquitoes was performed using a multiplex PCR [28], targeting human, cow, dog, goat, and pig DNA. Positive and negative controls were included in all reactions.
Detection of Plasmodium falciparum DNA in Anopheles mosquitoes
Molecular procedure describe by Demas et al. [29] was used to detect the presence of P. falciparum DNA. Since this technique does not allow to distinguish between infected and infectious mosquitoes, only heads and thoraxes of unfed mosquitoes were used. Preference was given to mosquitoes molecularly identified as An. gambiae s.s., An. coluzzii, An. arabiensis, and An. funestus s.s.
Kdr mutation detection
Kdr mutations were surveyed in An. gambiae s.l. exposed and not exposed to insecticides, following the well-established protocols targeting kdr-West (L1014F) and kdr-East (L1014S) mutations [12, 15].
Data analysis
The accuracy of morphological identification was determined as the ratio between molecular species confirmations and the total number of morphological identifications subject to molecular identification by species. Human blood index (HBI) was calculated as the number of mosquitoes fed on humans (including mixed blood meal origins) by the total number of blood-fed anopheline mosquitoes analysed. Infection rate (IR%) was estimated as the proportion of unfed Anopheles females positive for P. falciparum DNA. To assess the association between kdr genotypes and resistance phenotypes, in An. gambiae complex, Fisher’s exact test was calculated with contingency tables using GraphPad Prism (version 8.0.1).
Results
Anopheles mosquito species composition
From February to June 2022, 2226 adult mosquitoes were collected using indoor CDC-LT. Among the collected mosquitoes, 748 (33.6%; 733 females and 15 males) were identified as anopheline mosquitoes and 1478 (66.4%; 1148 females and 330 males) were classified as non-anopheline mosquitoes. Out of the total Anopheles females collected as adults, 622 (84.9%) were morphologically identified to species or complex of species, while 111 (15.1%) could not be identified because of the absence of anatomical parts. Of the larvae collected from Cavaco River and reared in an insectary to an adult stage, 60 were identified as An. gambiae s.l. The district of Ganda contributed 89.9% (n = 713) while Benguela District contributed 10.1% (n = 80) of the female Anopheles mosquitoes collected and morphologically identified (Table 1). A subsample of 155 An. gambiae s.l. and 375 An. funestus s.l. were molecularly processed for species identification. The molecular identification of the 155 An. gambiae s.l. indicated the presence of An. arabiensis (n = 26), An. coluzzii (n = 75), An. gambiae s.s. (n = 49), and An. coluzzii/An. gambiae s.s. hybrid (n = 2); three showed no amplification. From the 375 An. funestus s.l. mosquitos, 360 were identified as An. funestus s.s. and two as An. vaneedeni, and 13 specimens showed no amplification (Table 2). From those that did not show amplification on species-specific molecular identification (n = 16), five An. funestus s.l. and two An. gambiae s.l., as well as 34 anophelines of other Anopheles species, were identified by COI barcode analysis (Table 3). The accuracy of the morphological identification was determined by crossing morphological and molecular identification. This reveled an overall accuracy of 94.5% (533/504). Anopheles pretoriensis, An. coustani s.l., and An. maculipalpis had 100% accuracy, followed by An. gambiae s.l. (98.1%), An. funestus s.l. (97.3%), and An. squamosus (85.7%). Morphological identifications failed on An. obscurus, An. rhodesiensis s.l., An. ruarinus, An. argenteolobatus, and An. tenebrosus (Table 4). Molecular species identification revealed a total of 11 species, including the hybrids (Table 5). Overall, more adult Anopheles mosquitoes were caught in the rainy season (n = 388) than in the dry season (n = 164) (Table 5). Overall, in the dry season, the predominant species collected was An. funestus s.s. followed by An. coluzzii, An. arabiensis. and An. gambiae s.s. (Table 5). In the rainy season, An. funestus s.s. was the predominant species recorded followed by An. gambiae s.s., An. coluzzii, and An. arabiensis. In the rainy season, An. squamosus was also registered in relatively high numbers (n = 11; 2.3%) (Table 5). The two specimens of the hybrid An. coluzzii/An. gambiae s.s. were registered in the dry season, in both Cavaco River and Alto Catumbela collection sites, the former as a larva and the latter as an adult (Table 5). In terms of geographical distribution, there were clear differences, with An. coluzzii being found in Bela Vista and Cavaco River sentinel sites (Table 5).
WHO susceptibility test
In the insecticide susceptibility assays conducted on An. gambiae s.l. from Benguela using 0.05% alpha-cypermethrin, a mortality rate of 57.6% was observed. Notably, only 60 An. gambiae s.l. females were exposed to impregnated filter papers. This sample size was not considered robust according to WHO standards. Nonetheless, these preliminary results suggested that this population may exhibit phenotypic resistance to alpha-cypermethrin. Molecular identification revealed that this population was An. coluzzii (n = 59) and An. coluzzii/An. gambiae s.s. (n = 1) (Table 2).
Origin of Anopheles blood meals
The origin of blood meals of Anopheles mosquitoes collected indoors using CDC-LT showed that humans were the main host (71%) in both sites (Table 6). In Bela Vista, An. coluzzii was the only species screened with a human blood index (HBI) of 0.67. In Alto Catumbela, where four species were tested, the highest HBI was registered for An. funestus s.s. (0.84) followed by An. vaneedeni and An. arabiensis (both with 0.50) and An. gambiae s.s. (0.25). Anopheles arabiensis, An. funestus, and, An. vaneedeni collected indoors had also fed on cows.
Plasmodium falciparum infection rate
A total of 371 female anopheline mosquitoes were screened for the presence of P. falciparum DNA (Table 7). Infection rate varied depending on the species and season of collections. No P. falciparum DNA was found in An. arabiensis. In the Bela Vista sentinel site, An. coluzzii was the only species screened with an overall infection rate of 23.1%. Overall, at Alto Catumbela, An. funestus s.s. had a higher P. falciparum infection rate of 5.7% compared to the 2.4% rate in An. gambiae s.s. Despite these differences, statistical analysis revealed no significant differences between the two rates (Fisher’s exact test, P > 0.05). When comparing the infection rate within the rainy and dry season in Alto Catumbela for An. funestus group and An. gambiae complex, no significant differences were observed between the two (Fisher’s exact test, P > 0.05).
Knockdown resistance mutations in Anopheles gambiae s.l.
In Bela Vista, a total of 71 An. coluzzii mosquitoes were analysed for the presence of L1014F and L1014S mutations; among these, 16 were not exposed to insecticides. The genotypic frequency of L1014F in these mosquitoes showed a dominance of the resistant allele genotype, with a frequency of 0.94. In addition, the allele frequencies of L1014F in susceptible or resistant mosquitoes were similar (Fisher exact test, P > 0.05). When considering both phenotypes and unexposed mosquitoes, the frequency of the mutant allele was 0.71. At Alto Catumbela site, 76 mosquitoes, comprising An. arabiensis and An. gambiae s.s., were analysed. The An. arabiensis group, consisting of 26 mosquitoes, did not exhibit the L1014F mutation, whereas the An. gambiae s.s., with 49 mosquitoes, predominantly exhibited the mutant allele with an allele frequency of 0.90. Allele 1014S was reported for the first time to our knowledge in An. gambiae s.s. from the province of Benguela, only in heterozygosity with the 1014F allele. In total, across both sentinel sites, 148 mosquitoes were analysed. The overall frequency of 1014F mutation was 0.65, while the 1014S mutation was found to be 0.01 (Table 8).
Discussion
This study significantly advances our understanding of the Anopheles spp. populations in urban and rural settings in Benguela Province, highlighting key aspects of malaria transmission. Our research confirms the presence of An. coluzzii, An. gambiae s.s., An. arabiensis, and An. funestus s.s., consistent with prior studies in Angola and sub-Saharan Africa [2, 5,6,7]. The relatively low number of An. gambiae complex members collected indoors may suggest a behavioural change due to ITN presence as previously reported in other locations [30, 31]. We confirm the presence of An. coustani s.l., An. squamosus, An. pretoriensis, and the first report to our knowledge of An. vaneedeni in Alto Catumbela sentinel site. These species were found infected with P. falciparum in other sub-Saharan Africa countries [32,33,34,35]. These results highlight the importance of continued investigation into the roles that less studied species play in the transmission of malaria in the province. Anopheles coluzzii/An. gambiae s.s. hybrids were reported from Cavaco River and Alto Catumbela sentinel site during the dry season. The use of molecular methods revealed a significant accuracy in morphological identification done in the field, especially for the An. funestus group (97.3%) and An. gambiae complex (98.1%), the primary malaria vectors in Angola. This emphasises the importance of skilled technical staff and robust surveillance networks in malaria control strategies. In Bela Vista, we observed an overall P. falciparum infection rate of 23.1% in An. coluzzii, surpassing the 1.9% rate reported by Cuamba et al. [5]. This discrepancy may not be solely attributed to variations in sample sizes or collection methods. Environmental factors, such as the region’s unique ecological and climatic conditions, may enhance breeding and survival rates of Anopheles mosquitoes, particularly An. coluzzii and An. funestus s.s. Additionally, changes in human behaviour and vector control measures, alongside potential genetic variations in Plasmodium strains and varying immunity levels within the human population, could contribute to the observed high infection rates. The consistency of P. falciparum infection rates among An. funestus s.s. populations across seasons further underscores the species’ role in indoor malaria transmission within the Alto Catumbela region, highlighting the complex interplay of factors influencing malaria dynamics. This observation aligns with findings from other provinces, further emphasising its role in malaria transmission [6, 36]. Our study’s novel discovery of the West African kdr-resistance allele 1014F in An. coluzzii and An. gambiae s.s. confirms the presence of pyrethroid resistance in these populations. Interestingly, the first detection to our knowledge of the East African kdr-resistance allele 1014S in An. gambiae s.s. highlights the emergence of this mutation in Benguela Province, showing the importance of continuous monitoring of the setting of insecticide resistances in Anopheles gambiae s.s. populations. This finding suggests an emerging challenge in insecticide resistance, previously unrecorded in this region [5, 6, 26, 37]. Multiple factors might be responsible for this resistance, including the selective pressure exerted by ITN. The absence of kdr mutations in An. arabiensis indicates potential susceptibility to pyrethroids, warranting further investigation. However, our study has limitations. The geographic scope, limited to two sentinel sites, and the method of collection might not fully represent the broader Anopheles population dynamics. Future research should explore genetic diversity and resistance patterns more comprehensively. The detection of mutations involved in pyrethroid resistance was limited to L1014F and L1014S in An. gambiae s.l. Other potential mechanisms of insecticide resistance, such as metabolic resistance, were not yet explored in Angola. Future studies should investigate additional resistance mechanisms to provide a more comprehensive understanding of insecticide resistance across malaria vector populations. Additionally, a deeper investigation into the vectors’ feeding behaviours is necessary for a complete understanding of malaria transmission dynamics.
Conclusion
In conclusion, our findings provide crucial insights into the malaria vector populations in Angola, with significant implications for public health policy, vital for tailoring vector control strategies, ensuring their continued efficacy. This study not only fills a critical knowledge gap but also lays the groundwork for enhancing malaria entomological surveillance and control efforts in Angola.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon request. Sequences have been submitted to NCBI Genbank database.
Abbreviations
- COI:
-
Cytochrome c oxidase subunit
- PCR:
-
Polymerase chain reaction
- HBI:
-
Human blood index
- ITN:
-
Insecticide-treated nets
- KDR:
-
Knockdown resistance
- IRS:
-
Indoor residual spraying
References
World Health Organization. World Malaria Report 2023. Geneve; 2023.
Coetzee M, Koekemoer LL. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu Rev Entomol. 2013;58:393–412.
Ministry of Health Angola. National Malaria Control Program. Annual report 2019. Luanda; 2019.
Manguin S, Foumane V, Toto J, Martinaud F, Santos M, Carnevale P. First observations of Plasmodium malariae and its evolution during the parasitological evaluation of the long-term village scale malaria vector control program in the Balombo area of Angola. Res Sq. 2021;
Cuamba N, Kwang SC, Townson H. Malaria vectors in Angola: distribution of species and molecular forms of the Anopheles gambiae complex, their pyrethroid insecticide knockdown resistance (kdr) status and Plasmodium falciparum sporozoite rates. Malar J. 2006;5:1–6.
Ribeiro H, Ramos H. Research on the mosquitoes of Angola. VI—the genus Anopheles Meigen, 1818 (Diptera, Culicidae) Check-list with new records, key to the females and larvae, distribution and bioecological note. Sep Garcia de Orta, Sér Zool. 1975;4:31–8.
Calzetta M, Santolamazza F, Carrara GC, Cani PJ, Fortes F, Angela M, et al. Distribution and chromosomal characterization of the Anopheles gambiae complex in Angola. Am J Trop Med Hyg. 2008;78:169–75.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health. 2021;9:e1325–31.
Ranson H, Lissenden N. Insecticide resistance in african Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–8.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Coetzee M, Hunt RH, Wilkerson R, Torre Della A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–74.
Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–11.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Carnevale P, Toto J, Besnard P, Adelaide M, Santos D, Fortes F, et al. Spatio-temporal variations of Anopheles coluzzii and An. gambiae and their Plasmodium infectivity rates in Lobito, Angola. J Vector Ecol. 2015;40:172–9.
Brosseau L, Drame PM, Besnard P, Toto JC, Foumane V, Le Mire J, et al. Human antibody response to Anopheles saliva for comparing the efficacy of three malaria vector control methods in Balombo, Angola. PLoS ONE. 2012;7:e44189.
Carnevale P, Toto JC, Foumane V, Carnevale G, Stahl D. Plasmodial prevalence with respect to gender in 6 villages around Balombo town, Benguela Province, Angola, before malaria vector control implementation. Int J Trop Dis Health. 2022;43:19–42.
Choi K, Townson H. Evidence for X-linked introgression between molecular forms of Anopheles gambiae from Angola. Med Vet Entomol. 2012;26:218–27.
Tavares W, Morais J, Martins JF, Scalsky RJ, Stabler TC, Medeiros MM, et al. Malaria in Angola: recent progress, challenges and future opportunities using parasite demography studies. Malar J. 2022;21:396.
World Health Organization. Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO tube tests. Geneve; 2022.
Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera : Culicidae). Malar J. 2020;19:1–20.
Ribeiro H, Ramos HD. Guia ilustrado para a identificação dos mosquitos de Angola (Diptera. Culicidae). 1st ed. Lisboa: Boletim da Sociedade Portuguesa de Entomologia; 1995.
Collins FH, Mehaffey PC, Rasmussen MO, Brandling-Bennett AD, Odera JS, Finnerty V. Comparison of DNA-Probe and isoenzyme methods for differentiating Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae). J Med Entomol. 1988;25:116–20.
Wilkins EE, Howell PI, Benedict MQ. PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar J. 2006;5:1–7.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Della TA. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:1–10.
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek RC. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–9.
Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;72:336–42.
Demas A, Oberstaller J, Debarry J, Lucchi NW, Srinivasamoorthy G, Sumari D, et al. Applied genomics: data mining reveals species-specific malaria diagnostic targets more sensitive than 18S rRNA. J Clin Microbiol. 2011;49:2411–8.
Sanou A, Nelli L, Guelbéogo WM, Cissé F, Tapsoba M, Ouédraogo P, et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci Rep. 2021;11.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:1–10.
Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 2011;11:1173.
Ciubotariu II, Jones CM, Kobayashi T, Bobanga T, Muleba M, Pringle JC, et al. Genetic diversity of Anopheles coustani (Diptera: Culicidae) in malaria transmission foci in Southern and Central Africa. J Med Entomol. 2020;57:1782.
Gebhardt ME, Searle KM, Kobayashi T, Shields TM, Hamapumbu H, Simubali L, et al. Understudied Anophelines contribute to malaria transmission in a low-transmission setting in the Choma District, Southern Province, Zambia. Am J Trop Med Hyg. 2022;106:1406–13.
Burke A, Dandalo L, Munhenga G, Dahan-Moss Y, Mbokazi F, Ngxongo S, et al. A new malaria vector mosquito in South Africa. Sci Rep. 2017;7:1–5.
Alves G, Jolomba V, Feliciano A, Cani P, Martins JF, Marques C, et al. Entomological surveillance of Anopheles mosquitoes in the province of Cuando-Cubango, Angola, 2020–2021. Annual Meeting of the American Society of Tropical Medicine and Hygiene. 2021.
Janeira F, Vicente JL, Kanganje Y, Moreno M, Do Rosário VE, Cravo P, et al. A primer-introduced restriction analysis-polymerase chain reaction method to detect knockdown resistance mutations in Anopheles gambiae. J Med Entomol. 2008;45:237–41.
Acknowledgements
The authors thank the Ministry of Health (MoH), National Malaria Control Programme and the Provincial and Municipal health authorities for their contribution and support. We acknowledge the excellence work of MoH malaria municipal and provincial supervisors and The Mentor Initiative entomological supervisors Pedro Mbambi and Veronica Jolomba for all the support given during the field work.
Funding
Funding for the programme implemented by The Mentor Initiative and from which this report arises was provided by The Global Fund, NMF3 Angola grant. The funding agency had no role in the design and conduct of the original programme; collection, management, analysis, and interpretation of the data, including the entomological data reported here; preparation, review, or approval of this manuscript; or the decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
GA and SL designed the study. GA supervised all fieldwork. GA and AT identified morphological the mosquito samples. GS carried out the molecular processing. GA and GS carried out data analysis. GA, GS, and SL wrote the paper. RP developed the map of collection sites. CP, LG, and JFM provided on site logistical and technical assistance. All authors reviewed the original manuscript and agreed to the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Instituto Nacional de Investigação em Saúde de Angola (INIS). Reference of the approval: no. 12C.E/MINSA.INIS/2022.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alves, G., Troco, A.D., Seixas, G. et al. Molecular and entomological surveillance of malaria vectors in urban and rural communities of Benguela Province, Angola. Parasites Vectors 17, 112 (2024). https://doi.org/10.1186/s13071-024-06214-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06214-8