Abstract
Background
Human onchocerciasis remains a public health problem in Ghana. Mass drug administration (MDA) with ivermectin (IVM) has reduced disease morbidity and prevalence, but the transmission of onchocerciasis remains ongoing in several endemic foci. We investigated parasite transmission in some endemic communities in Ghana that had received > 18 rounds of annual MDA with IVM and determined the species composition of black fly (Simulium damnosum) vectors in these areas.
Methods
Adult female black flies were collected using human landing catches and identified as either forest or savanna species using morpho-taxonomic keys. The adult flies underwent dissection to determine their parity and detect any O. volvulus larvae, followed by the calculation of entomological indices. Simulium damnosum s.l. larvae were collected and preserved in freshly prepared Carnoy’s fixative and were later used for cytotaxonomic studies.
Results
A total of 9,983 adult flies were caught: 6,569 and 3,414 in the rainy and dry seasons respectively. Black fly biting activities over the study period showed bimodal or trimodal patterns. The highest monthly biting rate (MBR) of 10,578.75 bites/person/month was recorded in July in Beposo, while the highest monthly transmission potential of 100.69 infective bites/person/month was recorded in Asubende in August. Morphological analysis of 2,032 flies showed that 99.8% (2,028) of the flies were savanna species, with only 4 (0.2%) adult flies being of the forest species. Cytogenetic studies on 114 black fly larvae revealed three cytospecies (Simulium damnosum s.s., S. sirbanum and S. sanctipauli) in the study area.
Conclusions
The present studies confirmed an ongoing transmission of onchocerciasis in the study communities except Abua-1. It also provides further information on biting behaviors and onchocerciasis transmission indices in the study communities. Further, our data confirmed the savanna species (S. damnosum s.s. and S. sirbanum) of the S. damnosum s.l. to be the major vectors of onchocerciasis in the study areas, with only an occasional influx of forest cytotypes.
Graphical Abstract

Similar content being viewed by others
Background
Onchocerciasis is the second leading cause of infectious blindness in the world, only preceded by blinding trachoma [1,2,3]. The disease is a major obstacle to socio-economic development in Africa and other parts of the world [4, 5], being endemic in 30 countries in Africa, one country (Yemen) in the Arabian Peninsula and a few foci in the Americas [3, 6]. The adult stage of Onchocerca volvulus, the causative agent of the disease, have an average lifespan of 10 years and are found in the subcutaneous tissues of the host [7]. The adult female worms produce millions of microfilariae that migrate to the skin and eyes, where they cause dermal [7] and ocular [3] pathologies associated with the disease. Microfilariae in the skin are ingested during blood meals by adult female black fly vectors belonging to the Simulium damnosum complex, and later transmitted to human hosts as infective L3 larvae during another blood meals [8].
In West Africa, nine members of the S. damnosum species complex have been identified: S. damnosum s.s., S. dieguerense, S. konkourense, S. leonense, S. sanctipauli, S. sirbanum, S. soubrense, S. squamosum and S. yahense [9,10,11,12]. In Ghana, six members of this complex have been reported, and these are: S. damnosum s.s., S. sirbanum, S. sanctipauli, S. soubrense, S. squamosum and S. yahense [13, 14]. Several subcomplexes make up the S. damnosum complex, including the S. damnosum subcomplex, S. squamosum subcomplex, S. sanctipauli subcomplex (all of which are common in Ghana) and the S. kibwezi subcomplex, S. sanje subcomplex and S. ketaketa subcomplex (which are alien to the Ghanaian ecosystem).
The distribution of members of the S. damnosum complex in West Africa is reportedly associated with the savanna-forest dichotomic characteristics of the prevailing bioclimatic zones as well as with the river system types in West Africa. All black fly species described in West Africa to date are vectors of O. volvulus, albeit to varying degrees of vectorial capacity. For example, transmission of O. volvulus is maintained by members of S. damnosum s.l. (S. damnosum s.l.) in West Africa, by members of the S. neavei group in East and Central Africa and in the Americas by vectors belonging to six distinct species complexes [12, 15,16,17].
In West Africa, the epidemiological patterns are determined predominantly by the existence of two different strains of the Onchocerca parasite in which savanna and forest species of Simulium transmit their ecologically co-adapted savanna and forest strains of the Onchocerca worms respectively, even though this assertion has been strongly debated [16, 18, 19]. Thus the savanna cytospecies, S. damnosum s.s. and S. sirbanum will transmit the more pathologic savanna strain of O. volvulus while S. sanctipauli, the Beffa form of S. soubrense, S. squamosum (of which C and E forms exist) and S. yahense transmit the benign forest strain of O. volvulus [14]. Given the complex species structure within the genus Simulium, their different vectorial capacities, the unique associations with different geographical and ecological settings and the important role of vectors in maintaining disease transmission, it has become crucial to develop field tools for easily and accurately assigning species to their appropriate taxonomic units.
Since the work of Theobald [20] and Blacklock [21] in describing and incriminating onchocerciasis vectors respectively, there have been continual efforts to develop more robust morphometric tools for easy and accurate identification of adult members of the Simuliidae. In recent times, however, there have been some challenges with using only the morphological identification technique [22, 23]. For example, the West African S. damnosum complex can be readily recognized by morphological characters at the larval and pupal stages but not at adult stage, but in Guatemala, adult females of S. ochraceum, S. metallicum and S. callidum can easily be distinguished even by color differences, whereas their immature stages are more difficult to separate morphologically [22]. Other challenges include the enigmatic nature of the constituent species; differences in behavior and ecological requirements; variations in habitat preferences; and genetic introgression [23]. Reliable identification of the members of the S. damnosum complex now requires a combination of both morpho-taxonomy and other tools [24]. Larval cytotaxonomy have been found to be a more reliable technique for vector species discrimination. It clarifies and widens our knowledge of the systematics [11, 25,26,27,28,29] and evolutionary biology [30,31,32] of the Simuliidae using micro-morphological features of the giant polytene chromosomes of late-stage larvae. It utilizes differences in banding patterns and inversions of polytene chromosomes in providing sufficient inter- and intra-specific variations within and among fly populations [33,34,35,36,37].
In this study, we sought to assess the status of O. volvulus transmission in the study communities and explore the the black fly species composition sustaining transmission of onchocerciasis in the study areas. Considering that the disease is endemic in nine of the ten regions of the country during the study period, with an at-risk population of 3.2 million people [38], this study is crucial. Moreso, because all our study communities had undergone annual IVM MDA for >18 years and are now under the biannual IVM treatment strategy.
Methods
Study areas
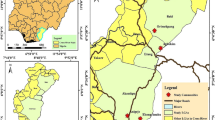
The studies were conducted in four first-line onchocerciasis endemic communities located at least 5 km away from each other along the Pru River basin which is the major breeding site in the area. The names and Geographical Positioning System (GPS) location of the communities are: Abua-I (07°58′N, 000°53′W), Asubende (08°01′N, 00°58′W), Beposo (08°01′N, 000°57′W) and Mantukwa (08°01′N, 001°00′W), all located in the Pru district of the Brong-Ahafo region, Ghana. The Pru district lies between latitudes 07°50′N and 08°22′N and longitudes 00°30′W and 01°26′W, with a land area of 2,195 km2. It shares boundaries with Salaga district to the North, Atebubu-Amantin and Nkoranza districts to the South, Sene district to the East and Kintampo South and Kintampo North districts to the West (Fig. 1). The district lies within the Guinea-savanna bioclimatic zones of West Africa with two major seasonal variations in weather: the rainy and dry seasons.
Collection of man-biting adult black flies
Six sets of entomological surveys were conducted, three in the rainy season and three in the dry season in each of the four communities. The rainy season (May-October) data were collected in the months of July, August and October (2012) while data from the months of January, February and March represented the dry season (November 2012-April 2013). For each month, flies were collected for four consecutive days in each community at designated black fly catching points located along the banks of the Pru River using human landing method [39]. Two consented collectors from each community were trained for vector collection. They worked alternately in each community between 06:00 and 18:00 GMT. Each seated vector collector wore protective clothing which allowed only their legs to be exposed. To minimize the effects of skin colour among the collectors, dark-skined persons were selected from among members of each local endemic community. Care was also taken to capture the black flies before they could obtain a blood meal. Female adult flies were caught individually into plastic catching tubes. Flies collected on hourly basis were labeled to indicate the hour, date, and place of capture. Flies were kept alive by wrapping the collection tubes containing the flies with moist cotton wool and placed in a cool environment, and later transported in ice chest to a makeshift field laboratory for further analysis.
Larval collection
Larval prospection for cytological studies was conducted in two communities: Asubende and Mantukwa because rapids and exposed rocky surfaces that supported fly breeding as at the time of these studies were found only in these communities. Prospection involved careful inspection of substrates such as overhanging and submerged plants, rocks, and twigs in the breeding sites for samples of preimaginal (aquatic) black fly stages. Larvae were preserved in freshly prepared Carnoy’s fixative (one part glacial acetic acid in three parts absolute ethanol) contained in universal bottles and transported in ice to a makeshift field laboratory and stored at 4ºC for future karyotyping. This aspect of our study was to serve as a confirmatory study for the finding from our preliminary morphological assessments to ascertain the species mix in the study area.
Morphological identification and dissection of adult black flies
Adult flies were distinguished morphologically into savanna and forest species using color variations of the antennae, scutella setae, arculus, wing tuft and the ninth abdominal tergite setae in line with previously published morphological taxonomic keys [40, 41]. Savanna flies were characterized by pale colors of the first three antennal segments, scutella satae, the wing tufts and the 9th abdominal tergite satae whereas their forest counterparts show darker colors of the aforementioned features. All flies collected were anaesthetized individually with chloroform vapor to immobilize them before they were dissected. Each fly was placed dorso-ventrally on a microscope slide in a drop of physiological saline and dissected from the abdomen to assess their parity status. Parous flies were identified exclusively by the presence of ovarian relics; retained eggs, pale coloured Malphigian tubules, absence of fat bodies and elasticity of the ovariole follicles were also used to infer parity [42, 43]. A parous fly, one that had taken at least one blood meal and had completed at least one gonotrophic cycle, resulted in the presence of follicular relics below the maturing oocyte [42, 43], whereas nulliparous flies had not yet taken a blood meal and had not completed any gonotropic cycle as indicated by the tightly coiled ovarian tracheal systems, absence of follicular relics. The head, thorax and abdomen of all parous flies were then dissected separately and the number of all third stage infective L3 and other developmental stages (L2 and L1) were recorded. Data obtained from adult black flies dissection were used to estimate entomological parameters such as parity and transmission potentials.
Preparation and staining of Simulium larvae
Polytene chromosome slides were prepared using the methods previously described by Dunbar and Vajime [44] with minor modifications. The larvae were stained in lacto-acetic orcein for a period between 3 and 12 hours. In cases where the larvae had been stored for more than 3 months, they were placed in 1M HCl for 1 minute and washed 3 times in distilled water before staining. Full karyotyping and cytospecies identifications were based on the criteria of Boakye [11], Boakye et al. [12] and Post et al. [45]. The chromosome complements were read under a compound microscope to visualize the banding patterns, inversion breakpoints and new inversions used for the identification of each larva following previously described criteria [11, 12, 45].
Estimation of entomological indices
The monthly biting rates (MBR) were measured as the theoretical number of Simulium bites received by a person who remained at a catching site during the 12 hours of the daylight for one complete month in a community. Similarly, the monthly transmission potential (MTP) was estimated as the total number of infective L3 larvae inoculated into one person in 1 month at a fly catching point during the 12 hours of the daylight period in a community [22, 46].
Data analysis
The statistical differences in monthly relative abundances of members of the S. damnosum s.l. were estimated using the two-way analysis of variance (ANOVA) in a GENSTAT statistical software version 11 at the 5% level of significance. The difference in infection rate as well as the relationship between season and fly infectivity rates were also estimated using the Chi-square (χ2) test.
Results
Relative abundance of adult female black flies
A total of 9,983 female adult black flies were caught in the four communities during the entire study period, 6,569 (65.8% of total) were collected during the rainy season months of July, August and October while 3,414 (34.2% of total) were caught during the dry season months (January–March) (Table 1). The highest (2,870 flies) and lowest (876 flies) were captured in the months of July and January, respectively. Among the four study communities, the highest number of flies collected during the rainy season was recorded at Beposo, and the highest number of flies caught during the dry season was recorded at Asubende; however, when total collection was considered, more flies were recorded at Asubende than at Beposo. In contrast, the lowest number of flies captured in both seasons was recorded at Abua-I. A comparison of the relative abundances of black flies caught during the entire study period showed that a significantly higher number of flies were captured during the rainy season compared to the dry season (F = 0.021, df = 1, P < 0.05) (Table 1).
Diurnal biting rate
Hourly variations in diurnal pattern of fly biting activities were observed across the four communities during the study period. In general, fly biting activities showed a characteristic bimodal pattern, with a morning peak occurring at different hours of the day, but mostly between 09:00 and 10:00 GMT in the mornings, and an evening peak between 16:00 and 17:00 GMT. Depending on the weather and other ecological conditions, a third peak was observed between 11:00 and 13:00 GMT or even multiple peaks of biting activities (see Fig. 2). In communities like Abua-1, Beposo and Mantukwa, the biting peaks were less distinct, while in the Asubende community multiple peaks were recorded, with distinct early morning peaks between 9:00 and 10:00 GMT and evening peak between 15:00 and 16:00 GMT (Figs. 2, 3).
There were three peaks of biting activities in the month of July: between 09:00 and 10:00, between 11:00 and 12:00 and between 15:00 and 17:00 GMT. Two peaks of biting activity were recorded in August: between 09:00 and 10:00 and 13:00 and 14:00 GMT. Only one peak of biting activity was recorded in October: between 15:00 and 16:00 GMT. Fly activities in January peaked between 09:00 and 10:00 and between 16:00 and 17:00 GMT. A pronounced fluctuation in fly biting activities was recorded in February, with peak biting intensities occurring between 07:00 and 08:00, between 09:00 and 10:00, between 11:00 and 12:00 and between 13:00 and 14:00, and two distinct peaks occurring between 16:00 and 17:00 GMT. Fly biting activities in March also exhibited a bimodal pattern, with peaks between 07:00 and 08:00 and between 15:00 and 16:00 GMT. The minimum number of flies biting humans was recorded between 06:00 and 08:00 GMT while the maximum catches occurred between 16:00 and 18:00 GMT. The hourly and seasonal variations in fly numbers in the four study communities are shown in Figures 2, 4 and 5. The diurnal biting activities of black flies among the wet months as well as those between the dry months were not significantly different from each other (F = 0.058, df = 2, P > 0.05). However, the difference in diurnal biting activities between the dry months and the wet months was found to be significantly different (F = 0.04, df = 2, P < 0.05).
Monthly biting rates
The MBR of black flies in each community were estimated for each month during the study period (Fig. 6). The highest MBR of 10,578.8 bites/person/month was recorded during the rainy month of July in Beposo, and the lowest MBR of 147.3 bites/person/month was recorded during the dry month of January at Abua-I. A comparison of MBR among the communities showed that Asubende, Beposo and Mantukwa had significantly higher MBR than Abua-1 and that Asubende and Beposo had significantly higher MBR than Mantukwa (F = 0.01, df = 3, P < 0.05); however, there was no significant statistical difference in MBR between Asubende and Beposo (F = 0.063, df = 1, P > 0.05).
A comparison of MBR in the rainy and dry seasons showed that MBR were significantly higher during the rainy months than during the dry months (F = 0.023, df = 2, P < 0.05). Among the rainy months, the MBR was significantly higher in July than in October, but not August. However, there was no significant difference in MBR among the dry months (F = 0.053, df = 2, P > 0.05).
Parity rates
All of the 9,983 flies collected from the four study communities for the purpose of this study were dissected for parity. A total of 4,197 (42.0%) flies were parous. All parous flies were further dissected to determine onchocerciasis infection rate. Among the parous flies dissected, 2,670 (63.6%) were collected during the rainy season and 1,527 (36.4%) were caught in the dry season. According to study site, the highest proportion (43.2%) of parous flies was collected in the Asubende community, and the lowest proportion (3.3%) was collected in the Abua-1 community; 33.0% and 20.4% of the parous flies were collected in the Beposo and Mantukwa communities, respectively. A seasonal consideration of parity rate indicated that the proportion of parous flies collected was highest in the dry season, especially in January when > 50% of flies dissected in all communities were parous. In the wet month of August however, a > 50% parity rate was recorded only in Asubende and Beposo, with the other communities (Mantukwa and Abua-1) having a < 50% parity rate in the same month. Analysis of the samples collected in October and March showed that only Asubende recorded a > 50% parity rate. During the months of July and February, all study communities reported parity rates of < 50% (Fig. 7).
Monthly transmission potential
Of a total of 2670 (63.6%) parous flies collected in the rainy season that were dissected, 45 (1.7%) were infected with various developmental stages of Onchocerca larvae which were morphologically indistinguishable from O. volvulus, resulting in an infection rate of 1.7%. Of 45 infected flies, 27 harbored infective larvae (L3) in the heads (Table 2).
The overall Onchocerca infection rates during the months of the dry season were very low as compared with the wet season. Of the 1,527 flies dissected in the dry season, only five (0.3% of flies caught) were infected and only two flies had infective L3 in the head. Our results showed different levels of O. volvulus transmission in the study communities, with the highest transmission potential of 101 infective bites/person/month recorded in Asubende and no. transmission of O. volvulus recorded in the Abua-1 throughout the study period. Onchocerciasis transmission was generally low during the dry season, occurring only in Asubende and Beposo in the months of January and February, respectively, with transmission potentials of 7.8 and 21.0 infective bites/person/month (Fig. 8).
Morphological identification of adult black flies
A preliminary investigation was conducted using a random sample of 2,032 flies collected from the four study communities to ascertain whether members of the forest and savanna species of the S. damnosum complex coexist in the study communities. The morphological identificationof forest and savanna species was based on a combination of color variations. The color of the fore coxae was not included in the analysis since some populations of S. squamosum share this feature in common with members of both S. damnosum s.s. and S. sirbanum, especially in the eastern parts of the former Onchocerciasis control program (OCP) area in West Africa. However, the color of the first three antennal segments, the color of the wing tuft and the color of the ninth abdominal tergite setae were used to separate forest species from their savanna counterparts. In all, 2.028 flies were scored with pale wing tuft, pale antennae and pale ninth abdominal tergite setae, which are characteristics of the savanna species of the S. damnosum complex, and were therefore designated as savanna flies. In comparison, only four flies were characterized by the dark colors of all these aforementioned anatomical parts and were thus identified as forest flies.
Cytotaxonomic identification
Given the determination of the presence of forest cytospecies in our study area, we employed cytological tools to further confirm whether these forest flies were inhabiting this savanna area or were just on a mere host-seeking expedition in the study area. The polytene chromosomes of a total of 656 Simulium larvae collected from breeding sites located at Asubende and Mantukwa were stained and karyotyped for cytological studies. However, only 114 larvae produced readable chromosomes, among which 73 were sampled in the rainy season (43 from Asubende and 30 from Mantukwa). Twenty-nine of the samples collected from Asubende were identified as S. damnosum s.s. based on the diagnostic inversion IIL-C/C or IIL-C/C.8 and the rest were identified by the inversions IIL-C.8/C.8, IIL-C.8.3/C.8.3 or IIL-C.8/C.8.3 as S. sirbanum. In the Mantukwa area, 19 samples were identified as S. damnosum s.s. while 11 were identified as S. sirbanum. Thus, all larvae karyotyped in the wet season were savanna flies and there were no records of forest species in the wet season. During the dry season, however, there was no fly breeding at Mantukwa presumably because the flow rate of the rapids at the breeding sites was insufficient to support fly breeding. Therefore, all 41 larval samples karyotyped were collected from the Asubende breeding site, of which 24 were identified as S. damnosum s.s. and 16 as S. sirbanum, all based on the aforementioned diagnostic inversions. A single specimen was identified from Asubende as S. sanctipauli sensu lato (S. sanctipauli s.l.) in the dry season.
Some of the inversions recorded in this study include IS-3, IS-2, IS-18, IS-22, IIL-C/C, IIL-C.8/C.8, IIL-C/C.8, IIL-4, IIIL-2, among others. Members of the S. damnosum s.s. were identified by the diagnostic inversion IIL-C/C or IIL-C/C.8; S. sirbanum was identified by the inversions IIL-C.8/C.8, IIL-C.8.3/C.8.3 or IIL-C.8/C.8.3. The single larva of S. sanctipauli s.l. found was diagnosed by the presence of the inversion IIL-4 (Additional file 1: Figures S1–S10).
Discussion
The dual focus of this study is the transmission status of O. volvulus and Simulium species composition in some selected communities in Ghana. Such data is crucial in view of the onchocerciasis endemicity of the study areas specifically, and Ghana in general [38]. Moreso, all our study communities had received annual MDA with ivermectin for about two decades and are now under the biannual IVM treatment strategy with an average of about 80% treatment coverage, making it necessary to assess the impacts of these efforts on onchocerciasis transmission in these areas.
Our results showed seasonal variation in the relative abundance of flies caught. Fly populations in three of the study communities were significantly higher during the rainy season than the dry season. However, fly populations in Abua-1 were stable in both seasons, possibly because fly numbers remained small in this site and did not show much variation. The distribution and population density of black flies in the study areas varied considerably across seasons, possibly influenced by an increase in the number of breeding sites in the wet season as river volumes increased and submerged more trailing vegetations, logs, rocks and any other substrates that may later serve as points of attachment for immature black flies. In Abua-1 where the fly population appeared to be stable in both seasons, a cursory observation of the river system within the Abua-1 catchment area showed neither sufficient rapids nor rocky surfaces, both of which are essential to support breeding, possibly explaining, at least in part, our observations in the area. Our reports of seasonal variations in fly biting rates in relation to water level and ecological conditions are in agreement with the reports of Le Berre [47]. The flies collected from Abua-1 might have been foraging for blood meals but not necessarily breeding there.
Generally, in Asubende, Beposo and Mantukwa, the flies exhibited bimodal diurnal biting patterns peaking at different times of the day but mostly in the morning between 9:00 and 10:00 GMT and in the evening between 16:00 and 17:00 GMT. These periods of intense biting correspond to the periods when most individuals in the communities are actively engaged in their farming and fishing activities and are maximally exposed to the bites of black flies. This has significant epidemiological implications for disease transmission. This situation was worse in all the communities during the rainy season when fly populations were high, as also reported by other studies [48, 49], showing that more individuals were likely to acquire infections during the rainy season in most rural endemic communities.
The proportion of parous black flies peaked during the rainy season compared to the dry season, suggesting that parasite transmission may be more pronounced during periods of increased rainfall. The increased parous rate in the rainy season increases the risk of onchocerciasis transmission in the areas as more flies are likely to live long enough to transmit the disease. This finding is consistent with that of Boatin et al. [50] in the southern rainforest onchocerciasis focus of Nigeria, but in contrast to those of the WHO [51] in southwestern Nigeria and [24] in parts of the Brong-Ahafo region of Ghana.
Active parasite transmission occurred in the Asubende, Mantukwa and Beposo communities where we reported several flies harboring the infective L3 in their heads. The assessment of fly infectivity rates (one of the key determinants of interruption of O. volvulus transmission) showed that the highest fly infectivity rate was recorded in Asubende, followed by Mantukwa and Beposo. No infective flies were recorded from Abua-1 throughout the study. The significant infectivity rates of onchocerciasis vectors recorded in three of the communities provides strong evidence of on-going transmission. The WHO stipulated that a vector infectivity rate of < 1 L3 per 1000 parous flies is required to interrupt transmission of O. volvulus [23, 52]. Therefore, the vector infectivity rates in all three communities (Asubende, Beposo and Mantukwa) greatly exceeded the WHO’s recommended threshold for transmission interruption by more than fivefolds. Also, there have been reports of significantly persistent microfilaridemia as well as reports on suboptimal responses to IVM treatment in some of these endemic foci [53,54,55]. These findings have been further reinforced by the more recent findings of Frempong et al. [56].
Our results, when compared with those of other published studies, seem to suggest the interruption of O. volvulus transmission at Abua-1, but we urge extreme caution when interpreting this information because the data on fly numbers reported here were insufficient to draw statistically meaningful conclusions on Abua-1; molecular confirmatory studies are required to draw reliable conclusions. It must be recognized that even our reported biting rates for Abua-1 were below the threshold biting rates at which infection transmission may not be detected [57]. Earlier surveys conducted in Asubende in 2005 recorded infectivity rates of 1.82 infective larvae/1000 parous flies [38]. However, in the present study we found a fourfolds increase in fly infectivity rate—at 8.9 infective flies/1000 parous flies in the same community and this trend is worrisome given the continual biannual distribution of IVM in the area. The gradual build-up of fly infection rates in Asubende and the continued transmission of onchocerciasis in three out of the four study communities despite continuous MDA with ivermectin may be attributed to a number of factors, including a gradual increase in the number of human reservoirs of infection as a result of consistent noncompliance of individuals to IVM treatment; possible presence of other Onchocerca species like O. ochengi which are capable of infecting and developing in the S. damnosum s.l., leading to overestimation of O. volvulus transmission potentials; earlier than expected skin repopulation by O. volvulus from individuals responding sub-optimally to IVM treatment; and immigration of individual human hosts with high microfilaridemia from untreated hyper-endemic communities into already treated areas.
In line with the WHO’s Roadmaps for Neglected Tropical Diseases (NTDs), Ghana has currently changed its treatment strategy from annual to biannual treatment to meet the current goal of elimination of infection in selected African countries [58]. This involves mass distribution of IVM at intervals of 6 months in selected hyper-endemic and meso-endemic communities aimed at effectively reducing the prevalence and intensity of infection to levels where the infection is not self-sustaining, ultimately leading to the interruption and elimination of O. volvulus infection. To attain these targets, there is the need to improve and strengthen existing logistic support systems for community volunteers, who are the lifeline for the community directed treatment with ivermectin (CDTi). This will ensure that community treatments are carried out at stipulated time periods to maximize treatment compliance and improve treatment coverage [59]. Since maximum parasite transmission were reported in the months of July and August, it will be extremely essential to schedule the timings of IVM treatments to take advantage of the impact of IVM on transmission. Ivermectin MDA should be conducted just before these periods of peak parasite transmission to ensure maximum effect on parasite transmission intensity. Alternative strategies, such as testing for onchocerciasis and treating those still infected with anti-Wolbachia drugs (such as doxycycline), and vector control strategies, such as the ‘Slash and Clear’ technique targeting larvae in the breeding site, could be employed to facilitate onchocerciasis elimination.
Accurate vector identification is an essential tool in the planning, execution, management and evaluation of vector control programs. Previous information on the species diversity of members of the S. damnosum complex involved in the transmission of O. volvulus in the Asubende catchment area is available [24], but the data in this study are not based on cytotaxonomic analysis, which remains the most reliable means of species identification in the Simuliidae [11]. Moreover, following our initial report of forest species in the study area, further cytological descriptions of members of the S. damnosum complex breeding at Asubende and Mantukwa were conducted using the criteria provided for West Africa simuliid species [11, 12]. Similar guides have been published for other parts of Africa [28, 60]. The members of the S. damnosum complex sampled from the study areas conformed to the descriptions by Boakye [11], Boakye et al. [12] and Post et al. [45]. Simulium. damnosum s.s. and S. sirbanum are reported to have a widespread distribution across Ghana, especially in areas close to the study areas [14].
We report here that the savanna members (S. damnosum s.s. and S. sirbanum) of the S. damnosum subcomplex are the dominant species in the study areas. This result is in agreement with earlier findings [50, 52, 61], all of which showed that S. sirbanum and S. damnosum s.s. are the most widely distributed members of the S. damnosum complex in the former OCP area of West Africa. We also report for the first time, at least up to the time of this study, the occurrence of forest cytospecies in the savanna setting, which we confirmed by cytotaxonomic analysis. In West Africa (Ghana and Togo), there have been reports of savanna members of the S. damnosum s.l. invading forest areas that have undergone environmental degradation, but there had not been any such report of the forest species moving into savanna areas until our study. However, the circumstances surrounding the migration of forest flies into savanna areas have not been established [57] in the forest settings. It is not clear whether the presence of S. sanctipauli s.l. as recorded in Asubende has a long history or represents a recent invasion probably in search of suitable breeding sites. The confirmation of the presence of forest black fly species in Asubende by cytotaxonomy points to a likely establishment of this group of flies in the area. This is in agreement with reports from earlier studies of [35, 62] who also reported that S. squamosum, S. yahense and the Beffa form of S. soubrense coexisted with S. damnosum s.s. and S. sirbanum in some parts of Nigeria, although the forest species dominated in the forest bioclimes while the savanna species dominated in the savanna enclaves. It is possible that the forest fly (S. sanctipauli) had actually migrated to this area as there has never been any such record from this site, although Boakye et al. [14] did report the presence of S. sanctipauli from relatively nearby rivers. Such findings could be of significant epidemiology importance to onchocerciasis elimination in Ghana [61].
Conclusions
The results of this study confirm the active transmission of onchocerciasis in the study communities, with the exception of the Abua-1 community. Our study also provides further information on the biting behaviors and onchocerciasis transmission indices of the S. damnosum complex among communities located along the Pru river basin, including Abua-1, Asubende, Beposo and Mantukwa, and shows a clearer trend in the current situation in the study communities, especially Asubende. This information generated will help provide the basis for continuous monitoring and evaluation of the impact of ongoing control programs on disease transmission in these areas. MTPs in all but one of the communities were greater than the WHO tolerable limits for interruption of O. volvulus transmission in hyperendemic areas. Since there has been a new paradigm shift targeted at eliminating O. volvulus infection from among selected African countries, including Ghana, effective evaluation of the impact of IVM MDA and monitoring for persistent infection transmission should be sustained to ensure that transmission levels are brought below transmission breakpoints to facilitate the achievement of the goals of onchocerciasis elimination. Further, our data have confirmed the species S. damnosum complex inhabiting the study area as savanna (S. damnosum s.s. and S. sirbanum) species as the principal vectors of onchocerciasis in the Pru river catchment area although there is the occasional influx of forest cytotypes alien to this area.
Availability of data and materials
All data related to this work have been included in the manuscript and associated files.
Abbreviations
- GMT:
-
Greenwich Mean Time
- GPS:
-
Geographical Positioning System
- HLC:
-
Human landing collection
- IVM:
-
Ivermectin
- L1 :
-
First-stage larvae of Onchocerca volvulus
- L2 :
-
Second-stage larvae of Onchocerca volvulus
- L3 :
-
Third-stage larvae of Onchocerca volvulus
- MBR:
-
Monthly biting rate
- MDA:
-
Mass drug administration
- MTP:
-
Monthly transmission potential
- NTDs:
-
Neglected tropical diseases
- OCP:
-
Onchocerciasis control program
References
Sommer A. Avoidable blindness. Aust N Z J Ophthalmol. 1988;16:31–5.
Thylefors B, Negrel AD, Pararajasegaram R. Epidemiologic aspects of global blindness prevention. Curr Opin Ophthalmol. 1992;3:824–34.
World Health Organization. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. Geneva: World Health Organization; 2010. p. 1–26.
Burnham GM. Onchocerciasis. Lancet. 1998;351:1341–6.
Basanez M-G, Pion SD, Churcher TS, Lutz BP, Little MP, Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:371.
Etya’ale D. Eliminating onchocerciasis as a public health problem: the beginning of the end. Br J Ophthalmol. 2002;86:844–6.
Murdoch ME, Hay RJ, Mackenzie CD, Williams JF, Ghalib HW, Cousens S, et al. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br J Dermatol. 1993;129:260–9.
Chikezie FM, Ezihe EK, Uzoigwe NR, Opara KN, Igbe MA, Okonkwo NJ, et al. A concise entomological evaluation of onchocerciasis transmission in Ahani-Achi community in Oji River Local Government Area of Enugu State, Nigeria. Int J Curr Res Rev. 2015;7:56–62.
Vajime CG, Dunbar RW. Chromosomal identification of eight species of the subgenus Edwardsellum near and including Simulium (Edwardsellum) damnosum Theobald (Diptera: Simuliidae). Tropenmed Parasitol. 1975;26:111–38.
Post RJ. The cytotaxonomy of Simulium sanctipauli and Simulium soubrense (Diptera: Simuliidae). Genetica. 1986;69:191–207.
Boakye DA. A pictorial guide to the chromosomal identification of members of the Simulium damnosum Theobald complex in West Africa with particular reference to the onchocerciasis control programme area. Trop Med Parasitol. 1993;44:223–40.
Boakye DA, Post RJ, Mosha FW, Sortees DP, Baker RHA. Cytotaxonomic revision of the Simulium sanctipauli subcomplex (Diptera: Simuliidae) in Guinea and the adjacent countries including descriptions of two new species. Bull Entomol Res. 1993;83:171–86.
WHO. Ten years of onchocerciasis control programme in West Africa: review of the works of onchocerciasis control programme in the Volta River basin area from 1974–1984. OCP/GVA/85.1B; 1985. https://iris.who.int/handle/10665/61819. Accessed 17 Nov 2011.
Boakye DA, Back C, Fiasorgbor GK, Sib APP, Coulibaly Y. Sibling species distributions of the Simulium damnosum complex in the West African onchocerciasis control programme area during the decade 1984–93, following intensive larviciding since 1974. Med Vet Entomol. 1998;12:345–58.
WHO. Strategies for ivermectin distribution through primary health care systems: report of the Meeting on Strategies for Ivermectin Distribution through Primary Health Care Systems, Geneva, 22-25 April 1991. WHO/PBL. 91.24; 1991. p. 14–24. https://iris.who.int/handle/10665/58164. Accessed 12 Nov 2011.
Basanez M-G, Churcher TS, Grillet ME. Onchocerca-Simulium interaction in the population and evolutionary biology of Onchocerca volvulus. Adv Parasitol. 2009;68:263–313.
Chikezie FM, Uzoigwe NR, Opara KN, Ezihe EK. Seasonal variations in biting density and infectivity of Simulium damnosum complex in Ezeagu and Oji-River Local Government Areas of Enugu State, Nigeria. Ann Res Rev Biol. 2018;24:1–10.
Cheke RA, Garms R. Indices of onchocerciasis transmission by different members of the Simulium damnosum complex conflict with the paradigm of forest and savanna parasite strains. Acta Trop. 2013;125:42–53. https://doi.org/10.1016/j.actatropica.2012.09.002.
Chikezie FM, Opara KN, Ubulom PME, Yaro CA, Al-Akeel RK, Osei-Atweneboana MY, et al. Onchocerciasis transmission status in some endemic communities of Cross River State, Nigeria after two decades of mass drug administration with ivermectin. Sci Rep. 2023;13:5413. https://doi.org/10.1038/s41598-023-31446-6.
Gibbins EG. Natural malaria infection of house frequenting Anopheles mosquitoes in Uganda. Ann Trop Med Parasitol. 1932;26:239.
Blacklock DB. The development of Onchocerca volvulus inincriminating. Ann Trop Med Parasitol. 1926;20:1–48.
WHO. Vector control series. Simulium. Advanced level training and information guide. WHO/VBC/92.992; 1991. p. 1–120. https://iris.who.int/bitstream/handle/10665/59007. Accessed 09 Jan 2013.
WHO. Onchocerciasis and its control. Report of a WHO Expert Committee on Onchocerciasis Control. World Health Organization technical report series. 1995;852:1–104. https://iris.who.int/handle/10665/37346. Accessed 27 Dec 2012.
Lamberton PHL, Cheke RA, Walker M, Winskill P, Osei-Ateweneboana MY, et al. Onchocerciasis transmission in Ghana: biting and parous rates of host-seeking sibling species of the Simulium damnosum complex. Parasit Vectors. 2014;7:511.
Rothfels KH. Cytotaxonomy of black flies (Simuliidae). Annu Rev Entomol. 1979;24:507–39.
Vajime CG. Cytotaxonomy of sirba form populations of the Simulium damnosum complex in West Africa: amendments to sex chromosomes and sibling status. Trop Med Parasitol. 1989;40:464–7.
Millest AL. Identification of members of the Simulium ochraceum complex in the three onchocerciasis foci in Mexico. Med Vet Entomol. 1992;6:23–8.
Arteaga LT, Munoz DHP. New cytotype in the Simulium metallicum complex (Diptera: Simuliidae) from Cundinamarca, Colombia. J Med Entomol. 1999;36:133–40.
Vajime CG, Tambala PA, Kruger A, Post RJ. The cytotaxonomy of Simulium damnosum s.l. (Diptera: Simuliidae) from the Thyolo onchocerciasis focus in Malawi and description of a new member of the complex. Ann Trop Med Parasitol. 2000;94:279–90.
Bedo DG. Cytogenetics and evolution of Simulium ornatipes Skuse (Diptera: Simuliidae) I. Sibling speciation. Chromosoma. 1977;64:37–65.
Rothfels KH. Chromosomal variability and speciation in black flies. Symp R Entomol Soc Lond. 1980;10:207–24.
Rothfels KH. Speciation in black flies. Genome. 1989;32:500–9.
Hirai H, Procunier WS, Ochoa JO, Uemoto K. A cytogenetic analysis of the Simulium ochraceum species complex (Diptera: Simuliidae) in Central America. Genome. 1994;37:36–53.
Maegga BTA, Cupp EW. Cytotaxonomy of the Simulium damnosum complex and description of new cytotypes in the Tukuyu focus southwest Tanzania. Trop Med Parasitol. 1994;45:125–9.
Mafuyai HB, Post RJ, Vajime CG, Molyneux DH. Cytotaxonomic identification of the Simulium damnosum complex (Diptera: Simuliidae) from Nigeria. Trop Med Int Health. 1996;1:779–85.
Charalambous M, Shelley AJ, Herzog MM, Dias AP. Four new cytotypes of the onchocerciasis vector black fly Simulium guianense in Brazil. Med Vet Entomol. 1996;10:111–20.
Dunbar RW. Four sibling species included in Simulium damnosum Theobald (Diptera; Simuliidae) from Uganda. Nature. 1966;209:597–9.
Taylor MJ, Awadzi K, Basanez M-G, Biritwum N, Boakye DA, Boatin B, et al. Onchocerciasis control: vision for the future from a Ghanaian perspective. Parasit Vectors. 2009;2:7–9.
Walsh JF. Sampling simuliid black flies. In: Youdeowei A, Service M, editors. Pest and vector management in the tropics. London: Longman; 1983. p. 93–9.
Wilson MD, Post RJ, Gomulski LM. Multivariate morphotaxonomy in the identification of adult females of the Simulium damnosum Theobald complex (Diptera: Simuliidae) in the onchocerciasis control programme area of West Africa. Ann Trop Med Parasitol. 1993;87:65–82.
Uzoigwe NR, Amuga GA, Chikezie FM, Onweluzor EI, Uzoigwe NC. Biting density and microfilariae infection of Simulium damnosum complex around the Mada River, Nasarawa State, North Central Nigeria. Niger J Parasitol. 2015;36:108–12.
Cupp EW, Collins RC. The gonotrophic cycle of Simulium ochraceum. Am J Trop Med Hyg. 1979;281:422–6.
Mokry JE. A method of estimating the age of field collected female Simulium damnosum (Diptera-Simulidae). Trop Med Parasitol. 1980;31:121–4.
Dunbar RW, Vajime CG. The Simulium (Edwardsellum) damnosum complex. A report of cytotaxonomic studies to April 1972. Geneva: WHO. Mimeographed document WHO/ONCHO/72.100. 1972. p. 13.
Post RJ, Onyenwe E, Somiari SAE, Mafuyai HB, Crainey JL, Ubachukwu PO. A guide to the Simulium damnosum complex (Diptera: Simuliidae) in Nigeria, with a cytotaxonomic key for the identification of the sibling species. Ann Trop Med Parasitol. 2011;105:277–97.
Walsh JF, Davis JB, Le Berre R. Standardization of criteria for assessing the effect of Simulium control in the onchocerciasis control programme. Trans R Soc Trop Med Hyg. 1978;72:675–6.
Le Berre R. Contribution à l’ étude biologique et écologique de Simulium damnosum Theobald: Diptera, Dimuliidae. Memoires 17. Paris: ORSTOM. 1966.
Opara KN, Usip LP, Akpabio EE. Transmission dynamics of onchocerciasis in rural communities of Akwa Ibom State, Nigeria. J Vect Borne Dis. 2008;45:225–30.
Renz A. Studies on the dynamics of transmission of onchocerciasis in a Sudan savanna area of North Cameroon III. Infection rate of the simulium vectors and Onchocerca volvulus transmission potentials. Ann Trop Med Parasitol. 1987;81:239–52.
Boatin BA, Hougard JM, Alley ES, Akpoboua LK, Yaméogo L. The impact of Mectizan on the transmission of onchocerciasis. Ann Trop Med Parasitol. 1998;92:46–60.
WHO. Onchocerciasis control programme in West Africa, progress report of the World Health Organization for 2000. Geneva: WHO. JPC21.2; 2000. https://iris.who.int/bitstream/handle/10665/311409/311409. Accessed 28 May 2013.
Krueger A. Guide to black flies of the Simulium damnosum complex in Eastern and Southern Africa. Med Vet Entomol. 2006;20:60–75.
Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–9.
Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT, Lazdins-Helds JK, et al. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:359–70.
Crosskey RW. Observations on the bionomics of adult Simulium damnosum (Theobald) (Diptera: Simuliiadae) in northern Nigeria. Ann Trop Med Parasitol. 1955;49:142–53.
Frempong KK, Walker M, Cheke RA, Tetevi EJ, Gyan ET, Owusu EO, et al. Does increasing treatment frequency address suboptimal responses to ivermectin for the control and elimination of river blindness? Clin Infect Dis. 2016;62:1338–48. https://doi.org/10.1093/cid/ciw144.
Garms R, Vajime CG. The ecology and distribution of the species of the Simulium damnosum complex in different bioclimatic zones of Liberia and Guinea. Tropenmed Parasitol. 1975;26:375–80.
Vagime CG, Quillevere D. The distribution of the Simulium (Edwardsellum) damnosum complex in West Africa with particular reference to the onchocerciasis control programme area. Trop Med Parasitol. 1978;29:473–82.
Quillevere D, Guillet P, Sechan Y. La repartition geographique des especes du complexe Simulium damnosum dans la zone du projet Senegambie (ICP/MPD/007). Cahiers ORSTOM Serie Entomologie medicate et Parasitologie. 1982;19:303–9.
Wilson MD, Cheke RA, Flasse SPJ, Grist S, Osei-Atweneboana MY, Tetteh-Kumah A, et al. Deforestation and spatio-temporal distribution of forest members of the Simulium damnosum species complex in southern Ghana and Southwestern Togo. Trans R Soc Trop Med Hyg. 2002;96:632–9.
Duke BO. Human onchocerciasis: an overview of the disease. Acta Leiden. 1990;59:9–24.
Chikezie FM, Opara KN, Ubulom PME, Osei-Atweneboana MY. Simulium vectors of the lower Cross River Basin: a morphometric and cytotaxonomic identification. Int J Entomol Res. 2022;7:72–80.
Acknowledgements
We wish to acknowledge the technical support of Mr. Alhassan Ahmed of the Ghana Health Services, Techiman, Ghana as well as all Chiefs and people in our study communities and the Neglected Tropical Diseases Directorate of the Ghana Health Services, Accra, Ghana.
Funding
This work was funded by Deutscher Akademischer Austusch Dienst (DAAD) and further supported by funding from European Foundation Initiative for Neglected Tropical Diseases (EFINTDs) through Professor M. Y. Osei-Atweneboana and Professor Mark Taylor. The DAAD and EFINTDs had no role in the design of the study; collection, analysis and interpretation of data; and in the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
FMC, MYO-A, DAB initiated and drafted the proposal. FMC and FBV collected and analyzed field data. MYO-A, DAB supervised the field work. FMC, MYO-A, DAB, TM analyzed data, FMC and MYO-A drafted the manuscript, FMC, MYO-A, DAB, TM, FBV SA reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the ethical review committee of the Ghana Health Service with identification number GHS-ERC:04/3/11. All fly collectors who had not been treated in the past 6 months preceding the study were treated by the community-based ivermectin distributor and properly documented before and after their involvement in the study. Written informed consent were obtained from all vector collectors before the commencement of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure S1. Chromosome II showing IIS-st/st, IIL-C.8.3/C.8.3 and IS/st inversions typical of S. sirbanum. C = centromere, B = balbiani ring, db = double bubble. Figure S2. Chromosome II showing IIL-C.8/C.8 homozygous inversion typical of S. sirbanum. C = centromere, B = balbiani ring, db = double bubble. Figure S3. Chromosome II showing IIL-C/C homozygous inversion typical of S. damnosum s.s. C = centromere, B = balbiani ring, db = double bubble. Figure S4. Chromosome I showing IS-3 heterozygous inversion and IS-2 homozygous inversions typical of S. damnosum s.s./S. sirbanum. C = centromere, NO = nuclear organizer. Figure S5. Short arm of chromosome I of S. sirbanum. C = centromere, NO = nuclear organizer. Figure S6. Chromosome III showing IIIL-2/2 and IIL-27 homozygous inversions typical of S. damnosum s.s./S. sirbanum/S. dieguerense. C = centromere. Figure S7. Chromosome III showing IIIL-2/2 & IIL-27 homozygous inversions typical of S. damnosum s.s./S. sirbanum/S. dieguerense. C = centromere. Figure S8. Chromosome III showing IIIL-2/2.7 homozygous inversions typical of S. damnosum s.s./S. sirbanum C = centromere. Figure S9. Chromosome II showing IIL-4 homozygous inversion typical of members of the S. sanctipauli s.l. C = centromere, B = balbiani ring, db = double bubble. Figure S10. Chromosome II showing IIL-C.8/C.8.64 and IS/st inversions typical of S. sirbanum C = centromere, B = balbiani ring, db = double bubble.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chikezie, F.M., Veriegh, F.B.D., Armoo, S. et al. Ongoing transmission of onchocerciasis in the Pru District of Ghana after two decades of mass drug administration with ivermectin and comparative identification of members of the Simulium damnosum complex using cytological and morphological techniques. Parasites Vectors 17, 394 (2024). https://doi.org/10.1186/s13071-024-06333-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06333-2