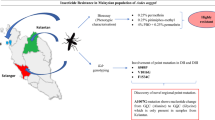
Abstract
Background
Vector control based on indoor residual spraying (IRS) is one of the main components of the visceral leishmaniasis (VL) elimination programme in India. Dichlorodiphenyltrichloroethane (DDT) was used for IRS until 2015 and was later replaced by the synthetic pyrethroid alpha-cypermethrin. Both classes of insecticides share the same target site, the voltage-gated sodium channel (Vgsc). As high levels of resistance to DDT have been documented in the local sand fly vector, Phlebotomus argentipes, it is possible that mutations in the Vgsc gene could provide resistance to alpha-cypermethrin, affecting current IRS pyrethroid-based vector control.
Methods
This study aimed to compare frequencies of knockdown resistance (kdr) mutations in Vgsc between two sprayed and two unsprayed villages in Bihar state, India, which had the highest VL burden of the four endemic states. Across four villages, 350 female P. argentipes were collected as part of a 2019 molecular xenomonitoring study. DNA was extracted and used for sequence analysis of the IIS6 fragment of the Vgsc gene to assess the presence of kdr mutations.
Results
Mutations were identified at various positions, most frequently at codon 1014, a common site known to be associated with insecticide resistance in mosquitoes and sand flies. Significant inter-village variation was observed, with sand flies from Dharampur, an unsprayed village, showing a significantly higher proportion of wild-type alleles (55.8%) compared with the three other villages (8.5–14.3%). The allele differences observed across the four villages may result from selection pressure caused by previous exposure to DDT.
Conclusions
While DDT resistance has been reported in Bihar, P. argentipes is still susceptible to pyrethroids. However, the presence of kdr mutations in sand flies could present a threat to IRS used for VL control in endemic villages in India. Continuous surveillance of vector bionomics and insecticide resistance, using bioassays and target genotyping, is required to inform India’s vector control strategies and to ensure the VL elimination target is reached and sustained.
Graphical Abstract

Similar content being viewed by others
Background
Vector-borne diseases (VBDs) account for around 17% of the estimated global burden of all infectious diseases, disproportionately affecting poorer populations in tropical and subtropical areas [1]. Visceral leishmaniasis (VL), caused by parasites of the Leishmania donovani complex, and transmitted by phlebotomine sand flies, is responsible for an estimated 30,000 cases each year with high fatality rates if untreated. In 2023, 80 countries were endemic for VL, with most cases occurring in three eco-epidemiological hotspots, namely East Africa (72% of total cases), Brazil (13%) and the Indian subcontinent (8%) [2]. Significant progress in controlling VL has been made, especially in the Indian subcontinent, where an elimination initiative was launched in 2005. Bangladesh already met the elimination threshold (< 1 detected VL case per 10,000 population per year at the upazila level) in 2017 and has sustained elimination since then. In 2022, in India and Nepal the VL programme target was reached in 99% of endemic implementation units (blocks in India and districts in Nepal) [2,3,4,5,6,7].
In India, VL remains endemic across four states: Bihar, Jharkhand, Uttar Pradesh and West Bengal. It caused 818 cases and 3 deaths in 2022, with most cases still occurring in Bihar [8]. The only VL vector in India is Phlebotomus argentipes, considered a primarily endophilic and endophagic species [9, 10]. Hence, vector control by indoor residual spraying (IRS) has been one of the main components of VL elimination programme, exploiting sand fly bionomics and also building on the past experience of malaria control IRS additionally resulting in VL control [11]. However, reports from Bihar indicate this species may be more exophilic and exophagic than previously thought and is an opportunistic feeder [12,13,14], indicating that IRS might need to be supplemented with other vector control tools [13, 15].
The accelerated plan for VL elimination in India recommends two rounds of IRS in endemic villages [16]. DDT was used for IRS until 2015 when it was replaced by the synthetic pyrethroid alpha-cypermethrin [17] because of the widespread DDT resistance of P. argentipes documented in many regions, including the state of Bihar [18,19,20,21,22,23]. This vector appears to remain susceptible to pyrethroids, with only minimal possible resistance detected to alpha-cypermethrin, based on mortality ranging between 97.6 and 100% [21, 24].
DDT and alpha-cypermethrin target the voltage-gated sodium channel (Vgsc). Knockdown resistance mutations (kdr) within the Vgsc are a major mechanism of resistance against DDT and pyrethroids in many insects, including mosquitoes and sand flies [25, 26]. Wild-type Vgsc is normally a leucine residue (Leu or L), with L1014F and L1014S being the most common mutations in insects, where Leu changes to phenylalanine (Phe or F) or serine (Ser or S) [25, 27, 28]. Both L1014F and L1014S have been detected in sand flies in Bihar previously [28] and were also already detected in Sri Lanka [29]. Apart from codon 1014, other codons associated with insecticide resistance were detected in Aedes aegypti mosquitoes (codons 1011 and 1016) and in other insect orders besides Diptera such as Lepidoptera and Blattodea (codon 1020) [28].
A better understanding of insecticide resistance in sand fly populations is needed to improve the efficiency of vector control and achieve sustained elimination of VL. Rotation of insecticides to prevent emergence of insecticide resistance, together with susceptibility testing to inform insecticide choice, has already been recommended [15]. The aim of the present study was to compare the frequencies of kdr mutations in Vgsc, particularly at codon 1014, but also codons 1011 and 1016, between the two VL endemic sprayed and two unsprayed villages in Bihar, India.
Methods
Sand fly collections
Phlebotomus argentipes females were sampled in a previous study, comparing CDC light traps, mechanical vacuum aspirators and Prokopack aspirator collection methods. The study was performed in May–June 2019 in four villages in Bihar: Rampur Jagdish and Bishambharpur in Saran District where VL was endemic and Dharampur and Ruchanpura in Nalanda District with no VL transmission [30] (Fig. 1).
Rampur Jagdish was sprayed in May 2018 and May and September of 2019, while Bishambharpur was sprayed in April 2018 and April and September of 2019. Dharampur and Ruchanpura were not sprayed.
Genotyping of kdr mutations
Three hundred fifty P. argentipes females were used for sequence analysis of the IIS6 fragment of the Vgsc gene. DNA from the samples was extracted using Qiagen DNeasy Blood & Tissue Kits following the manufacturer’s protocol [30] and quantified using a NanoDrop (Thermofisher). The Vgsc primers (Vssc8F: 5ʹ ± AATGTGGGATTGCATGCTGG ± 3ʹ, Vssc1bR: 5ʹ ± CGTATCATTGTCTGCAGTTGGT ± 3ʹ) described by Gomes et al. [28] were used to amplify a genomic DNA fragment from VGSC domain II, segment 6, which includes codons 1011, 1014 and 1016.
A volume of 2 µl of sample DNA (around 1–5 ng of DNA) was used per reaction. Polymerase chain reaction (PCR) was carried out in a total volume of 25 µl by combining 5 µl of 5 × Q5 reaction buffer (NEB), 1.25 µl of each forward and reverse primer (10 µM stock), 0.5 µl of 10 mM dNTPs, 14.75 µl of nuclease free water and 0.25 µl of Q5 DNA polymerase enzyme (NEB), as per protocol (New England Biolabs Inc.). Thermocycling conditions followed those described by Gomes et al. [28] (initial denaturation step of 5 min at 95 °C, followed by 30 cycles each of 96 °C for 30 s, 56 °C for 30 s and 72 °C for 30 s, and a final extension step of 72 °C for 5 min), using G- Storm Thermal Cycler PCR machine.
The quality of PCR products (5 µl each) was checked using 1% agarose gel electrophoresis in TAE buffer, stained with Sybr Safe (ThermoFisher) and visualised on a BIO-RAD ChemiDoc MP Imaging System. Bands visualised at around 400 base pairs indicated that the fragment was correctly amplified.
Sanger sequencing
The PCR products were sent for Sanger sequencing (Genewiz, Takeley, Essex, UK), obtaining forward and reverse sequence data for each sample. Genetic sequence data were visualised, trimmed and edited using SnapGene Viewer (version 5.3.2, 2022). Both forward and reverse sequences for each sample were analysed. Sequences from FASTA format files were aligned using Aliview software (version 1.23) and mutations recorded. BLASTn tool from the National Centre for Biotechnology Information (NCBI) was used to confirm the taxonomic identification. Databases to which sequences were compared were sourced from GenBank (accession nos. KY114615, KY114616, KY114617, KY114618, KY114619) [28].
Mutation profiling
Samples that were Leu homozygotes (wild-type) or Leu/Ser or Leu/Phe heterozygotes could be classified as organochlorine (i.e. DDT) susceptible, whereas those with kdr genotypes (Ser/Ser and Phe/Phe homozygotes or Ser/Phe heterozygotes) could potentially be classified as resistant, in line with previous findings by Gomes et al. [28] where allele frequencies in female sand flies that survived or were killed by insecticide exposure were compared.
Frequencies of alleles that could be identified as Leu, Ser or Phe from the genetic sequence data were calculated based on the total number of alleles in each village. Chi-square tests were used to assess differences between sand flies in allele and genotype frequencies at Vgsc codon 1014 per village. For smaller sample sizes, Fisher’s exact tests were used to obtain exact P values.
Accession numbers
All sequences were submitted to the European Nucleotide Archive (ENA) (project number PRJEB74041; accession nos. ERR12764362–ERR12764733).
Results
The sequence analysis of the IIS6 fragment of the Vgsc gene was carried out for 350 samples of P. argentipes collected in sprayed and unsprayed villages in Bihar. Three hundred ten samples (out of 350) were of sufficient quality to be included in further data analysis: 77 from Dharampur and 42 from Ruchanpura, the two unsprayed villages from Nalanda; 85 from Rampur Jagdish and 106 from Bishambharpur, the two sprayed villages from Saran. Mutations were identified at various positions within the IIS6 fragment, most frequently at codon 1014, causing replacement of the wild type leucine codon (TTA) with serine, L1014S (TCA/TCT/TCC) or leucine with phenylalanine, L1014F (TTC and TTT). The most frequent were mutations resulting in the L1014S codon (48.5%), followed by wild type L1014 codon (39.5%). Additionally, mutations causing replacement L1014F were present in almost 12% of specimens (Table 1).
Significant inter-village variation in allele and genotype frequencies was observed. Sand flies from Dharampur, an unsprayed village, had a significantly higher proportion of wild-type homozygotes (55.8%) compared to the three other villages (8.5–14.3%), whereas almost 30% of sand flies in this location were Leu/Ser heterozygotes (Tables 2 and 3; Fig. 2) (allelic inter-village comparison \(\chi_{6}^{2}\) = 105.7, P = 1.6 × 10–20; genotypic comparison of wild-type homozygotes vs. Leu heterozygotes vs. other genotypes, \(\chi_{6}^{2}\) = 99.8, P = 2.8 × 10–19). The other three villages, including unsprayed Ruchanpura, had a high frequency of samples with L1014S mutation and a mixture of different heterozygotes.
A map showing locations of villages where samples were collected, together with Vgsc-1014 allele frequencies (pie chart colours represent different alleles: Leu = blue, Ser = orange, Phe = grey). Base layer available at World Topo Map (MapServer) (arcgisonline.com). Map generated using Epi Info 7 software
There was also significant allelic and genotypic variation between the unsprayed Nalanda and sprayed Saran districts (Tables 2 and 3, Fig. 2) (allelic \(\chi_{3}^{2}\) = 62.3, P = 2.98 × 10–14; genotypic \(\chi_{4}^{2}\) = 42.5, P = 1.3 × 10–8). Whereas 41% of samples from Nalanda were of wild-type genotypes (Leu/Leu) and further 29% were heterozygotes with one susceptible Leu allele, most samples from Saran (68.6%) entirely lacked the susceptible allele.
Discussion
Knowledge on insecticide resistance mechanisms in phlebotomine sand flies is limited [30], even though vector control is a key component of leishmaniasis control and elimination in the absence of a vaccine and where there are difficulties related to diagnosis and treatment [6, 30, 31]. Our report on the presence of kdr mutations in the Vgsc gene in P. argentipes sand flies from Bihar, India, adds to a very limited number of kdr reports from the Indian subcontinent [28, 29, 32, 33].
Like in mosquitoes, insecticide resistance in sand flies can be caused by target site mutations, such as kdr mutations in the Vgsc gene, or it can be metabolic, where resistant insects have higher levels or more efficient forms of the enzymes that cause degradation or sequestration of insecticides; thus far, no reports on cuticular or behavioural resistance mechanisms in sand flies are available [34]. Furthermore, fewer studies report on metabolic resistance.
Spatial and temporal variation in kdr allele frequencies has been described in Anopheles [35, 36] and Aedes mosquitoes [37] as well as sand flies [33]. A high frequency of kdr mutations, both L1014S and L104F, was observed in this study, similar to previously reported data [28, 33]. There was significant inter-village variation in allele frequencies: sand flies from Dharampur, an unsprayed village, had more wild-type Leu alleles than other villages—both unsprayed Ruchanpura and especially the two villages from Saran where IRS takes place. These observed differences across the four villages might have resulted at least partially from selection pressure caused by insecticide exposure in the past, as other studies have shown evidence for links between past IRS exposure and kdr frequencies [33]. Kdr mutations L1014S and L1014F are associated with insecticide resistance—specifically resistance to organochlorines such as DDT and to pyrethroids—in many vectors [38, 39], including sand flies [25, 27, 40]. DDT has been used for IRS in India since the 1950s and was replaced by the pyrethroid alpha-cypermethrin in 2015. While DDT resistance has been reported in Bihar, P. argentipes is still susceptible to pyrethroids [18, 19, 22, 28, 41]. DDT resistance can also be caused by glutathione S-transferases (GSTs) [42]. The involvement of this metabolic resistance mechanism was already observed in P. argentipes in Bihar [43] and also in P. argentipes populations from Sri Lanka [28]. Furthermore, pyrethroid resistance has already been detected in sprayed and also unsprayed villages in Nepal [44].
We compared our findings with other data from this area [28] where a lot of variation in the frequency of genotypes between different districts was also observed. Dharampur (Nalanda District), a village from this study with the largest proportion of wild-type sand flies, is close to Dhanarua (Patna District), an area with the largest proportion of wild-type sand flies in the study carried out by Gomes et al. [28]. In both studies, areas north of the river Ganges (Saran District and Vaishali District) had much lower frequencies of genotypes with at least one Leu allele compared to areas south of Ganges (Nalanda District and Patna District). Incidence of VL used to be high especially in districts north of the Ganges River, such as Vaishali and Saran, where IRS has been regularly carried out [45,46,47].
Data on insecticide resistance on the Indian subcontinent obtained from bioassays have been reviewed by Dhiman and Yadav [48]. The presence of kdr mutations in P. argentipes in Bihar, India, linking the presence of mutations to phenotypic data, was first described in 2017 [28]. While both L1014S and L1014F confer resistance to DDT, it is thought that L1014F confers stronger resistance to DDT than L1014S, and only two mutant alleles (Phe/Phe, Phe/Ser and Ser/Ser) are thought to produce a resistant phenotype [28]. Phenylalanine frequencies as reported from Vaishali and also Patna Districts [28] were much higher than those we observed in Saran in our study (53.9% and 29.0% vs. 13.1%).
The main limitations of our study are the lack of bioassay data and having no knowledge of the resistant phenotypes present in the area. Repeated sampling and testing would help show if and how kdr frequencies are changing and whether resistance is increasing, including resistance to pyrethroids. Repeated sampling across the years in eight sentinel sites in VL-endemic areas in northeastern India showed some temporal variations in the frequencies of the kdr genotypes, resulting in both increases and decreases between the years [33].
Conclusions
The presence of kdr in sand flies could present a threat to IRS used for VL control in endemic villages in India. Use of different insecticides, such as carbamates, organophosphates or neonicotinoids, as well as alternative or supplemental methods of control, should be explored for the purpose of insecticide resistance management and potential integration of vector control activities for VL and other vector-borne diseases [34, 49, 50]. Further research on vector bionomics and insecticide resistance will be required to inform India’s vector control strategies and ensure the VL elimination target is reached and sustained—including pairing of bioassay data to confirm the resistance phenotype of sand fly populations with genotypic data and transcription levels of metabolic enzymes that cause resistance to insecticides.
Availability of data and materials
All data supporting the findings of this study are available within the paper, while DNA sequences have been deposited in the European Nucleotide Archive (ENA), with the primary accession code (project number) PRJEB74041.
References
World Health Organization. Global vector control response 2017–2030. Geneva: World Health Organization; 2017.
Ruiz-Postigo JA, Jain S, Madjou S, Agua JFV, Maia-Elkhoury AN, Valadas S, et al. Global leishmaniasis surveillance, 2022: assessing trends over the past 10 years. World Health Organization—Weekly epidemiological record. 2023;40:471–88.
Reithinger R. Xenodiagnosis leads the way: elimination of visceral leishmaniasis from the Indian subcontinent is feasible and sustainable. Lancet Microbe. 2021;2:e2–3.
World Health Organization. Process of validation of elimination of kala-azar as a public health problem in South-East Asia. New Delhi: World Health Organization, Regional Office for South-East Asia; 2016.
Singh OP, Sundar S. Visceral leishmaniasis elimination in India: progress and the road ahead. Expert Rev Anti Infect Ther. 2022;20:1381–8.
Garlapati R, Iniguez E, Serafim TD, Mishra PK, Rooj B, Sinha B, et al. Towards a sustainable vector-control strategy in the post kala-azar elimination era. Front Cell Infect Microbiol. 2021;11:641632.
World Health Organization. Regional Strategic Framework for accelerating and sustaining elimination of kala-azar in the South-East Asia Region: 2022–2026. New Delhi: World Health Organization, Regional Office for South-East Asia; 2022. Licence: CC BY-NC-SA 3.0 IGO.
National Center for Vector Borne Diseases Control, Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. Kala-Azar Situation in India. 2023. https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=467&lid=3750. Accessed 25 Mar 2024.
Gidwani K, Picado A, Rijal S, Singh SP, Roy L, Volfova V, et al. Serological markers of sand fly exposure to evaluate insecticidal nets against visceral leishmaniasis in India and Nepal: a cluster-randomized trial. PLoS Negl Trop Dis. 2011;5:e1296.
Rahman SJ, Menon PK, Rajagopal R, Mathur KK. Behaviour of Phlebotomus argentipes in the foothills of Nilgiris (Tamil Nadu), South India. J Commun Dis. 1986;18:35–44.
World Health Organization. Regional strategic framework for Elimination of Kala-azar from the South-East Asia Region (2005–2015). New Delhi: World Health Organization, Regional Office for South-East Asia; 2005.
Chowdhury R, Kumar V, Mondal D, Das ML, Das P, Dash AP, et al. Implication of vector characteristics of Phlebotomus argentipes in the kala-azar elimination programme in the Indian sub-continent. Pathog Glob Health. 2016;110:87–96.
Poche DM, Garlapati RB, Mukherjee S, Torres-Poche Z, Hasker E, Rahman T, et al. Bionomics of Phlebotomus argentipes in villages in Bihar, India with insights into efficacy of IRS-based control measures. PLoS Negl Trop Dis. 2018;12:e0006168.
Kumar V, Shankar L, Rama A, Kesari S, Dinesh DS, Bhunia GS, et al. Analysing host preference behavior of Phlebotomus argentipes (Diptera: Psychodidae) under the impact of indoor residual spray. Int J Trop Dis Health. 2015;7:69–79.
World Health Organization. Regional strategic framework for elimination of kala-azar from the South-East Asia Region (2011–2015). New Delhi: World Health Organization, Regional Office for South-East Asia; 2012.
National Vector Borne Disease Control Programme. Accelerated plan for kala-azar elimination. Delhi, India. 2017.
World Health Organization. Kala-Azar elimination programme: report of a WHO consultation of partners, Geneva, Switzerland, 10–11 February 2015. Geneva: World Health Organization; 2015.
Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4:e859.
Kumar V, Shankar L, Kesari S, Bhunia GS, Dinesh DS, Mandal R, et al. Insecticide susceptibility of Phlebotomus argentipes & assessment of vector control in two districts of West Bengal, India. Indian J Med Res. 2015;142:211–5.
Singh R, Kumar P. Susceptibility of the sandfly Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) to insecticides in endemic areas of visceral leishmaniasis in Bihar, India. Jpn J Infect Dis. 2015;68:33–7.
Deb R, Singh RP, Mishra PK, Hitchins L, Reid E, Barwa AM, et al. Impact of IRS: four-years of entomological surveillance of the Indian Visceral Leishmaniases elimination programme. PLoS Negl Trop Dis. 2021;15:e0009101.
Coleman M, Foster GM, Deb R, Pratap Singh R, Ismail HM, Shivam P, et al. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci USA. 2015;112:8573–8.
Singh R, Das RK, Sharma SK. Resistance of sandflies to DDT in Kala-azar endemic districts of Bihar, India. Bull World Health Organ. 2001;79:793.
Dinesh DS, Hassan F, Kumar V, Kesari S, Topno RK, Yadav RS. Insecticide susceptibility of Phlebotomus argentipes sandflies, vectors of visceral leishmaniasis in India. Trop Med Int Health. 2021;26:823–8.
Davies TG, Field LM, Usherwood PN, Williamson MS. A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species. Insect Mol Biol. 2007;16:361–75.
Field LM, Emyr Davies TG, O’Reilly AO, Williamson MS, Wallace BA. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur Biophys J. 2017;46:675–9.
Rinkevich FD, Du Y, Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol. 2013;106:93–100.
Gomes B, Purkait B, Deb RM, Rama A, Singh RP, Foster GM, et al. Knockdown resistance mutations predict DDT resistance and pyrethroid tolerance in the visceral leishmaniasis vector Phlebotomus argentipes. PLoS Negl Trop Dis. 2017;11:e0005504.
Pathirage DRK, Karunaratne S, Senanayake SC, Karunaweera ND. Insecticide susceptibility of the sand fly leishmaniasis vector Phlebotomus argentipes in Sri Lanka. Parasit Vectors. 2020;13:246.
McIntyre-Nolan S, Kumar V, Mark-Carew M, Kumar K, Nightingale ES, Dalla Libera Marchiori G, et al. Comparison of collection methods for Phlebotomus argentipes sand flies to use in a molecular xenomonitoring system for the surveillance of visceral leishmaniasis. PLoS Negl Trop Dis. 2023;17:e0011200.
World Health Organization. Operational manual on leishmaniasis vector control, surveillance, monitoring and evaluation. Geneva: WHO; 2022.
Sarkar SR, Kuroki A, Ozbel Y, Osada Y, Omachi S, Shyamal PK, et al. First detection of voltage-gated sodium channel mutations in Phlebotomus argentipes collected from Bangladesh. J Vector Borne Dis. 2021;58:368–73.
Reid E, Deb RM, Ali A, Singh RP, Mishra PK, Shepherd J, et al. Molecular surveillance of insecticide resistance in Phlebotomus argentipes targeted by indoor residual spraying for visceral leishmaniasis elimination in India. PLoS Negl Trop Dis. 2023;17:e0011734.
Balaska S, Fotakis EA, Chaskopoulou A, Vontas J. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:e0009586.
Das S, Maquina M, Phillips K, Cuamba N, Marrenjo D, Saute F, et al. Fine-scale spatial distribution of deltamethrin resistance and population structure of Anopheles funestus and Anopheles arabiensis populations in Southern Mozambique. Malar J. 2023;22:94.
Mathias DK, Ochomo E, Atieli F, Ombok M, Bayoh MN, Olang G, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malar J. 2011;10:10.
Solis-Santoyo F, Rodriguez AD, Penilla-Navarro RP, Sanchez D, Castillo-Vera A, Lopez-Solis AD, et al. Insecticide resistance in Aedes aegypti from Tapachula, Mexico: spatial variation and response to historical insecticide use. PLoS Negl Trop Dis. 2021;15:e0009746.
Silva AP, Santos JM, Martins AJ. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids—a review. Parasit Vectors. 2014;7:450.
Du Y, Nomura Y, Zhorov BS, Dong K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects. 2016;7:60.
Balaska S, Calzolari M, Grisendi A, Scremin M, Dottori M, Mavridis K, et al. Monitoring of insecticide resistance mutations and pathogen circulation in sand flies from Emilia-Romagna, a Leishmaniasis endemic region of Northern Italy. Viruses. 2023;15:148.
Singh RK, Mittal PK, Dhiman RC. Insecticide susceptibility status of Phlebotomus argentipes, a vector of visceral leishmaniasis in different foci in three states of India. J Vector Borne Dis. 2012;49:254–7.
Hassan F, Singh KP, Ali V, Behera S, Shivam P, Das P, et al. Detection and functional characterization of sigma class GST in Phlebotomus argentipes and its role in stress tolerance and DDT resistance. Sci Rep. 2019;9:19636.
Hassan F, Dinesh DS, Purkait B, Kumari S, Kumar V, Das P. Bio-chemical characterization of detoxifying enzymes in DDT resistant field isolates of Phlebotomus argentipes in Bihar, India. Int J Med Pharm Sci. 2015;5:23–32.
Roy L, Uranw S, Cloots K, Smekens T, Kiran U, Pyakurel UR, et al. Susceptibility status of the wild-caught Phlebotomus argentipes (Diptera: Psychodidae: Phlebotominae), the sand fly vector of visceral leishmaniasis, to different insecticides in Nepal. PLoS Negl Trop Dis. 2022;16:e0010304.
Das P, Samuels S, Desjeux P, Mittal A, Topno R, Siddiqui NA, et al. Annual incidence of visceral leishmaniasis in an endemic area of Bihar, India. Trop Med Int Health. 2010;15:4–11.
Kesari S, Bhunia GS, Chatterjee N, Kumar V, Mandal R, Das P. Appraisal of Phlebotomus argentipes habitat suitability using a remotely sensed index in the kala-azar endemic focus of Bihar, India. Mem Inst Oswaldo Cruz. 2013;108:197–204.
Bhunia GS, Kesari S, Chatterjee N, Kumar V, Das P. Spatial and temporal variation and hotspot detection of kala-azar disease in Vaishali district (Bihar). India BMC Infect Dis. 2013;13:64.
Dhiman RC, Yadav RS. Insecticide resistance in phlebotomine sandflies in Southeast Asia with emphasis on the Indian subcontinent. Infect Dis Poverty. 2016;5:106.
Muller GC, Schlein Y. Different methods of using attractive sugar baits (ATSB) for the control of Phlebotomus papatasi. J Vector Ecol. 2011;36:S64-70.
Banda GT, Deribe K, Davey G. How can we better integrate the prevention, treatment, control and elimination of neglected tropical diseases with other health interventions? A systematic review. BMJ Glob Health. 2021;6:e006968.
Acknowledgements
The research team thank all householders in Nalanda and Saran who permitted access to their homes; Health Ministry Screening Committee, India, for providing permission for the study; the National Centre for Vector Borne Disease Control (formerly the Vector Borne Disease Control Programme) for their support and advice in placement of the research; field workers and project administration staff who participated in this research.
Funding
This study was supported by the Bill and Melinda Gates Foundation through the SPEAK India consortium (OPP1183986).
Author information
Authors and Affiliations
Contributions
MC and PD conceptualised the work. SC, MC and MK conceived the study. KK, AK, VK, MM-C participated in data collection. CH, SC, MK, MM-C, EC analysed the samples and participated in data interpretation. MK, CH, SC drafted the manuscript. MM-C, EC and MK prepared the figures. MC, MK, SC, MM-C and EC contributed to manuscript review and revision. VK and PD contributed to resource provision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics committee of the Government of India Health Ministry’s Screening Committee (Ref: 2017-4126), Rajendra Memorial Research Institute of Medical Sciences (Ref: 39/RMRI/EC/2017) and the London School of Hygiene & Tropical Medicine (Ref: 1463).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kristan, M., Hazelgrove, C., Kumar, K. et al. Knockdown resistance mutations in Phlebotomus argentipes sand flies in Bihar, India. Parasites Vectors 17, 334 (2024). https://doi.org/10.1186/s13071-024-06424-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06424-0